Role of fluorine on the structure and second-harmonic-generation property of inorganic selenites and tellurites
Abstract
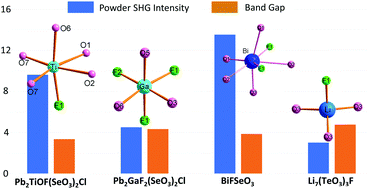
Fluorine, as the most electronegative element, can replace the oxygen ligands of functional groups under given conditions. These fluoride groups are more or less different from the pure oxide groups in composition, symmetry, polarizability, transmittancy, etc. The rational use of these differences is expected to improve the probability of noncentrosymmetric structures and the comprehensive performance of second-harmonic-generation (SHG) materials. In this feature article, we introduce the recent developments in fluoride selenite and tellurite SHG materials together with highlighting our contributions, including Se(IV) and Te(IV) compounds with (i) d0 transition metal oxyfluoride octahedron, (ii) IIIA metal oxyfluoride octahedron, (iii) fluoride lone pair cation polyhedron, and (iv) other fluoride polyhedron. The future perspectives of fluoride selenite and tellurite SHG materials are also discussed.
- This article is part of the themed collection: 10th Anniversary of the Youth Innovation Promotion Association of the Chinese Academy of Science


 Please wait while we load your content...
Please wait while we load your content...