Open Access Article
Open Access ArticleEfficient and chemoselective hydrogenation of aldehydes catalyzed by well-defined PN3–pincer manganese(II) catalyst precursors: an application in furfural conversion†
Sandeep Suryabhan
Gholap‡
a,
Abdullah Al
Dakhil‡
ab,
Priyanka
Chakraborty
a,
Huaifeng
Li
a,
Indranil
Dutta
 a,
Pradip K.
Das
a and
Kuo-Wei
Huang
a,
Pradip K.
Das
a and
Kuo-Wei
Huang
 *a
*a
aKAUST Catalysis Center and Division of Physical Sciences and Engineering, King Abdullah University of Science and Technology, Thuwal 23955-6900, Saudi Arabia. E-mail: hkw@kaust.edu.sa
bDepartment of Chemistry, College of Science, Imam Mohammad Ibn Saud Islamic University, Riyadh 11432-5701, Saudi Arabia
First published on 15th October 2021
Abstract
Well-defined and air-stable PN3–pincer manganese(II) complexes were synthesized and used for the hydrogenation of aldehydes into alcohols under mild conditions using MeOH as a solvent. This protocol is applicable for a wide range of aldehydes containing various functional groups. Importantly, α,β-unsaturated aldehydes, including ynals, are hydrogenated with the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond/C
C double bond/C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C triple bond intact. Our methodology was demonstrated for the conversion of biomass derived feedstocks such as furfural and 5-formylfurfural to furfuryl alcohol and 5-(hydroxymethyl)furfuryl alcohol respectively.
C triple bond intact. Our methodology was demonstrated for the conversion of biomass derived feedstocks such as furfural and 5-formylfurfural to furfuryl alcohol and 5-(hydroxymethyl)furfuryl alcohol respectively.
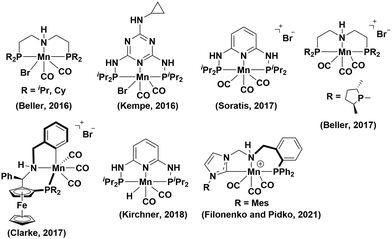
The conversion of carbonyl compounds to alcohols is a widely used synthetic tool in organic synthesis.1,2 The catalytic hydrogenation with hydrogen (H2) is an attractive way to avoid waste formation due to the use of stoichiometric reductants such as lithium aluminum hydride, sodium borohydride and lithium borohydride, silanes, and boranes.3 Homogenous catalytic systems offer advantages in terms of selectivity and activity over their heterogeneous counterparts.4 While a number of complexes containing precious metals, such as Ru, Rh, and Ir, show excellent selectivity towards hydrogenation of aldehydes over other sensitive functionalities such as ketones and/or alkenes,5 the search for more economical and efficient methods by using catalysts derived from earth-abundant metals, for example, Fe, Mn and Co, is of great interest. In this regard, several Mn-based catalysts have been reported for the hydrogenation of aldehydes, ketones, and esters (Fig. 1);6,7 however, the catalyst preparation typically requires expensive Mn(I) precursors, such as Mn(CO)5Br, and harsh reaction conditions are often employed, such as high catalyst loadings, use of strong bases as additives, and high temperature. In 2016, Beller and co-workers reported a well-defined PNP–Mn pincer complex for the hydrogenation of aldehydes and ketones.7a Kempe et al. reported a triazine-based PN3P–Mn pincer system for hydrogenation of ketones, showing quantitative conversion in a short reaction time.7b Thereafter, Sortais, Beller, and Clark reported various Mn pincer complexes for the reduction of both ketones and aldehydes.7f–h In 2018, Kirchner reported an efficient PN3P–Mn(I) pincer complex for hydrogenation of aldehydes over ketone and alkene functionalities.7n More recently, Filonenko and Pidko demonstrated the use of Mn(I)–CNP precatalyst for the hydrogenation of ketones, aldehydes, esters and imines.7o The further development of selective hydrogenation catalysts from readily available and abundant metal complexes offers a promising and cost-effective protocol for practical applications.
Upon reduction biomass-derived feedstocks, such as furfural (FAL) and 5-formylfurfural (FFAL) containing the aldehyde functionality, can be converted into several renewable commodities.8 FAL is widely available in the fine chemical industry,9 and the conversion of FAL to furfuryl alcohol (FOL) and other value-added chemicals is of great interest.10 The catalytic hydrogenation of FAL to FOL has been accomplished mostly using heterogeneous catalyst systems such as Cu/Cr, Ni, Cu Co, Pt, and Pd supported catalysts;11 however, these catalysts create some concerns about toxicity. On the other hand, the use of homogeneous catalysts for FAL conversion has been limited. For the first time, Paganelli et al. reported a reduction of FAL to FOL with H2 using a recyclable dihydrothioacetic acid-modified Rh system.12 Thereafter, Li and co-workers reported RuCl2(PPh3)3/CH3COOH catalyst to prepare FOL from FAL.13 Similarly, Ru–tris(2,2,6,6-tetramethyl-3,5-heptanedionate)3 and NHC-based Ru-catalyst were demonstrated for the hydrogenation of FAL.14 Recently, Mika reported a bidentate phosphine modified Ru-based catalytic system for the efficient conversion of FAL to FOL,15 and more recently, Nielsen reported well-defined Ru and Ir PNP pincer complexes with low catalyst loadings for the hydrogenation of furanic aldehydes.16 However, the use of expensive catalysts has limited their practical applications. Thus far, non-noble homogenous catalyst systems for FAL to FOL have not been reported. In contrast to the significant achievements in the applications of novel Mn(I) pincer complexes as discussed earlier, the use of Mn(II)-based pincer complexes in the organic transformation is limited,7c,17 even though MnX2 (X = Cl, Br) ($8.6 per molMn) is considerably more economical than Mn(CO)5Br ($13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250 per molMn) as a catalyst precursor.
250 per molMn) as a catalyst precursor.
Previously, our group developed a class of pincer complexes based on the phosphino aminobipyridine (PN3(P) pincer) ligand system and demonstrated the unique and enhanced reactivities in the direct coupling of amines to imines and ester hydrogenation.18 In this communication, we describe an experimental investigation of the chemoselective hydrogenation of aldehydes with hydrogen (H2) using air-stable Mn(II) PN3–pincer complexes (Scheme 1a). Extension of this protocol was applied to the selective conversion of FAL to FOL and FFAL to 5-(hydroxymethyl)furfuryl alcohol (HMFOL), respectively.
 | ||
| Scheme 1 In this work: (a) hydrogenation of aldehydes and (b) conversion of furfural (FAL) to furfuryl alcohol (FOL) and 5-(formylfurfural (FFAL) to 5-(hydroxymethyl)furfuryl alcohol (HMFOL). | ||
The Mn(II) based pincer complexes Mn1 and Mn2 were synthesized by the reaction of MnX2 (X = Cl, Br) with the tBu2PNH-BPy (bis-tert-butylphosphine-2,2′-bipyridine-6-amine) pincer ligand in THF at room temperature (Scheme 2). Upon crystallization in a solution of THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acetonitrile (1
acetonitrile (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
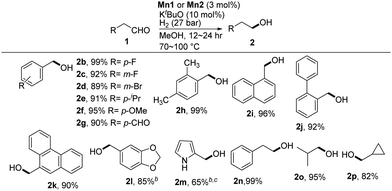
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), the crystals of Mn1 and Mn2 were fully characterized by single-crystal XRD analysis (Fig. 2) and elemental analysis (see the ESI†). The catalytic performance of Mn1 and Mn2 was first investigated for the hydrogenation of 3-methylbenzaldehyde as a model substrate to find the optimal hydrogenation reaction conditions (Table S1, ESI†). Changing the solvent to MeOH leads to an enhanced yield of product 2a with full conversion (Table S1, ESI†). The performance of Mn2 was tested, which showed its lower activity as compared to Mn1 (Table S1, entries 4 and 5, ESI†). At 130 °C, Mn2 exhibited a higher reactivity in MeOH (Table S1, entry 6, ESI†). MeOH was thus chosen as the optimal solvent for the conversion. Performing the reaction at 110 °C using Mn1 resulted in full conversion to 2a (Table S1, entry 7, ESI†). Decreasing the temperature to 70 °C still offered an excellent yield of 2a (Table S1, entries 8–10). So, an excellent yield of 3-methylbenzyl alcohol was obtained using Mn1 (3 mol%) and H2 (27 bar) in MeOH (Scheme 3).
1), the crystals of Mn1 and Mn2 were fully characterized by single-crystal XRD analysis (Fig. 2) and elemental analysis (see the ESI†). The catalytic performance of Mn1 and Mn2 was first investigated for the hydrogenation of 3-methylbenzaldehyde as a model substrate to find the optimal hydrogenation reaction conditions (Table S1, ESI†). Changing the solvent to MeOH leads to an enhanced yield of product 2a with full conversion (Table S1, ESI†). The performance of Mn2 was tested, which showed its lower activity as compared to Mn1 (Table S1, entries 4 and 5, ESI†). At 130 °C, Mn2 exhibited a higher reactivity in MeOH (Table S1, entry 6, ESI†). MeOH was thus chosen as the optimal solvent for the conversion. Performing the reaction at 110 °C using Mn1 resulted in full conversion to 2a (Table S1, entry 7, ESI†). Decreasing the temperature to 70 °C still offered an excellent yield of 2a (Table S1, entries 8–10). So, an excellent yield of 3-methylbenzyl alcohol was obtained using Mn1 (3 mol%) and H2 (27 bar) in MeOH (Scheme 3).
 | ||
| Fig. 2 Crystal structures of complexes Mn1 and Mn2. All atoms have been shown with 30% probability ellipsoids. All hydrogen atoms except for N–H have been omitted for clarity. For Mn1 and Mn2, details of selected bond distances (Å) and angles (degrees) are shown (Fig. S1 and S2, ESI†). | ||
 | ||
Scheme 3 Hydrogenation of 3-methylbenzaldehyde. a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) For the reaction, 3-methylbenzaldehyde (0.83 mmol), Mn1 (3 mol%, 0.024), KtBuO (0.083 mmol) and H2 (27 bar) were used. b For the reaction, 3-methylbenzaldehyde (0.83 mmol), Mn1 (3 mol%, 0.024), KtBuO (0.083 mmol) and H2 (27 bar) were used. b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Isolated yield. Isolated yield. | ||
With the optimized reaction conditions in hand, various aldehyde substrates were examined. The aldehyde substrates bearing halogens such as fluorine (F) and bromine (Br) gave the alcohol products in excellent yields (Scheme 4, products 2b–2d). Investigation of the aldehydes bearing different para substituents on the phenyl ring, such as isopropyl (iPr) and methoxy (OMe), showed higher yields of hydrogenation products (Scheme 4, products 2d and 2e). When terephthaldehyde was employed as a substrate, both aldehyde (–CHO) groups were reduced to afford 1,4-benzenedimethanol in 90% yield (Scheme 4, product 2g). A dimethyl substituted aromatic aldehyde also underwent hydrogenation to give an excellent yield of the alcohol product (Scheme 4, product 2h). Biphenyl and naphthalene containing aldehydes were also converted to alcohol products in high yields (Scheme 4, products 2i and 2j). Heteroaromatic rings did not interfere with the hydrogenation reactions (Scheme 4, products 2l and 2m). Both acyclic and cyclic aldehydes worked efficiently to give alcohol products in high yields (Scheme 4, products 2o and 2p).
Encouraged by the excellent results obtained with aromatic aldehydes, we further examined more challenging α,β-unsaturated substrates. Selective hydrogenation of such aldehydes is important because double bonds are often reduced along with the aldehyde functionality.15 Using our PN3 Mn(II) catalytic system, only the aldehyde group was hydrogenated selectively, with conjugated and nonconjugated C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bonds untouched (Scheme 5, products 4a–4c). The aldehyde substrate containing two double bonds, for example, 3,7-dimethyl-2,6-octadienal (ethyl citral), was hydrogenated smoothly to give the alcohol product in good yield without affecting the double bonds (Scheme 5, 4d). We also examined alkyne conjugated aldehydes (ynals). Previously, PNP Mn(II) pincer complex catalyzed semi-hydrogenation of alkynes with ammonia borane was reported.17a Surprisingly, the hydrogenation of pent-2-ynal and 3-phenylpropiolaldehyde gave pent-2-yn-1-ol and 3-phenylprop-2-ynol in 80% and 78% yields, respectively, without affecting the alkyne functionality (Scheme 5, 6a and 6b).
C double bonds untouched (Scheme 5, products 4a–4c). The aldehyde substrate containing two double bonds, for example, 3,7-dimethyl-2,6-octadienal (ethyl citral), was hydrogenated smoothly to give the alcohol product in good yield without affecting the double bonds (Scheme 5, 4d). We also examined alkyne conjugated aldehydes (ynals). Previously, PNP Mn(II) pincer complex catalyzed semi-hydrogenation of alkynes with ammonia borane was reported.17a Surprisingly, the hydrogenation of pent-2-ynal and 3-phenylpropiolaldehyde gave pent-2-yn-1-ol and 3-phenylprop-2-ynol in 80% and 78% yields, respectively, without affecting the alkyne functionality (Scheme 5, 6a and 6b).
 | ||
Scheme 6 Hydrogenation of 5-formylfurfural (FFAL). a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Reaction condition: 5-formylfurfural (0.80 mmol), Mn2 (8.5 mg, 0.016 mmol), KtBuO (9.0 mg, 0.080 mmol) in methanol for 24 h. b Reaction condition: 5-formylfurfural (0.80 mmol), Mn2 (8.5 mg, 0.016 mmol), KtBuO (9.0 mg, 0.080 mmol) in methanol for 24 h. b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Isolated yield. Isolated yield. | ||
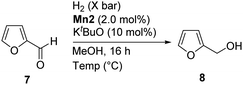
After successfully studying a wide range of aldehyde substrates, we turned our attention to exploring the hydrogenation of aldehydes that are used as feedstocks, for example, conversion of FAL to FOL and FFAL to HMFOL. The study of the effect of the Mn2 catalyst loading on the hydrogenation of FAL showed that 2.0 mol% catalyst loading gave full conversion (Table S2, ESI†). When the reaction was carried out using H2 (35 bar) at 100 °C, the product was obtained with 85% conversion with high selectivity (Table 1, entry 1). Upon increasing the reaction temperature from 120 °C to 130 °C, the full conversion of FAL was achieved within 16 h with excellent yields (Table 1, entries 2 and 3). However, when the reaction was conducted in the absence of solvent, the product conversion and selectivity both decreased. This observation suggested that methanol is necessary to achieve the full conversion of furfural (Table 1, entry 4). The effect of H2 pressure during the reaction was studied (Fig. S1, ESI†). Upon increasing the pressure from 5 bar to 35 bar, the conversion of FOL was increased to 99%. On the other hand, at H2 (50 bar), the yield was not much improved. These results suggested that 35 bar pressure of H2 is an optimum pressure for the excellent conversion of FAL to FOL.
After the excellent selectivity was achieved for the conversion of FAL to FOL, it was interesting to study the hydrogenation of feedstocks bearing two aldehyde groups such as 5-formylfurfural (FFAL). The conversion of FFAL to value-added HMFOL in one pot is an interesting approach.18b When the reaction was performed with 1.0 mol% Mn2 with H2 (20 bar) at 130 °C, a mixture of HMFOL 10 and 5-(hydroxymethyl)furfural (HMFAL) 11 was obtained with a high selectivity of 10 (Table S3, entry 1, ESI†). Upon increasing the catalyst loading to 2.0 mol% under H2 (30 bar), the conversion and selectivity of 10 improved (Table S3, entry 2, ESI†). When prolonging the reaction time to 24 h, both aldehyde groups were hydrogenated to afford HMFOL exclusively with high conversion (Table S3, entry 3, ESI†). At 48 h, under 35 bar pressure of H2, full conversion and thus an excellent yield of product 10 was obtained (Scheme 6). Finally, we studied the hydrogenation of ester and ketone substrates (see the ESI†). For both reactions, hydrogenation products were not observed, implying that this protocol is selective towards the hydrogenation of aldehyde substrates only.
In conclusion, we developed well-defined PN3 pincer Mn(II) complexes as catalyst precursors for the hydrogenation of various aldehydes into corresponding alcohols under mild reaction conditions. More importantly, α,β-unsaturated aldehydes including ynals were hydrogenated without affecting double and triple bonds with excellent selectivity. The present methodology was applied for the selective conversion of biomass-derived chemicals, such as that of FAL into FOL. Notably, by increasing the pressure of H2 to 35 bar and temperature to 130 °C, 5-formylfurfural was fully converted into 5-(hydroxymethyl)furfuryl alcohol in a quantitative yield. The fact that ester and ketone substrates resulted in no reaction indicated their excellent chemoselectivity towards aldehyde substrates. The inexpensive nature, air-stability, and robustness of the presented catalyst system may offer an opportunity for large scale applications.
This work was supported by King Abdullah University of Science and Technology (KAUST).
Conflicts of interest
The authors declare no competing financial interest.Notes and references
- A. B. Smith, J. Barbosa, W. Wong and J. L. Wood, J. Am. Chem. Soc., 1996, 118, 8316–8328 CrossRef CAS.
- Y. Kobayakawa and M. Nakada, J. Antibiot., 2014, 67, 483–485 CrossRef CAS.
- (a) M. Beller and H.-U. Blaser, Organometallics as catalysts in the fine chemical industry, Springer, 2012 CrossRef; (b) P. A. Dub and T. Ikariya, ACS Catal., 2012, 2, 1718–1741 CrossRef CAS; (c) N. B. Johnson, I. C. Lennon, P. H. Moran and J. A. Ramsden, Acc. Chem. Res., 2007, 40, 1291–1299 CrossRef CAS PubMed; (d) L. Diab, T. Šmejkal, J. Geier and B. Breit, Angew. Chem., Int. Ed., 2009, 48, 8022–8026 CrossRef CAS PubMed.
- (a) R. Noyori, Angew. Chem., Int. Ed., 2002, 41, 2008–2022 CrossRef CAS; (b) S. Baldino, S. Facchetti, A. Zanotti-Gerosa, H. G. Nedden and W. Baratta, ChemCatChem, 2016, 8, 2279–2288 CrossRef CAS; (c) L. Bonomo, L. Kermorvan and P. Dupau, ChemCatChem, 2015, 7, 907–910 CrossRef CAS.
- C. P. Casey, N. A. Strotman, S. E. Beetner, J. B. Johnson, D. C. Priebe and I. A. Guzei, Organometallics, 2006, 25, 1236–1244 CrossRef CAS.
- (a) T. Zell, Y. Ben-David and D. Milstein, Catal. Sci. Technol., 2015, 5, 822–826 RSC; (b) G. A. Filonenko, R. van Putten, E. J. Hensen and E. A. Pidko, Chem. Soc. Rev., 2018, 47, 1459–1483 RSC; (c) R. Langer, M. A. Iron, L. Konstantinovski, Y. Diskin-Posner, G. Leitus, Y. Ben-David and D. Milstein, Chem. – Eur. J., 2012, 18, 7196–7209 CrossRef CAS PubMed; (d) R. Langer, G. Leitus, Y. Ben-David and D. Milstein, Angew. Chem., Int. Ed., 2011, 50, 2120–2124 CrossRef CAS PubMed; (e) S. Fleischer, S. Zhou, K. Junge and M. Beller, Angew. Chem., Int. Ed., 2013, 52, 5120–5124 CrossRef CAS PubMed; (f) N. Gorgas, B. Stöger, L. F. Veiros, E. Pittenauer, G. N. Allmaier and K. Kirchner, Organometallics, 2014, 33, 6905–6914 CrossRef CAS PubMed; (g) N. Gorgas, B. Stöger, L. F. Veiros and K. Kirchner, ACS Catal., 2016, 6, 2664–2672 CrossRef CAS PubMed.
- (a) S. Elangovan, C. Topf, S. Fischer, H. Jiao, A. Spannenberg, W. Baumann, R. Ludwig, K. Junge and M. Beller, J. Am. Chem. Soc., 2016, 138, 8809–8814 CrossRef CAS PubMed; (b) F. Kallmeier, T. Irrgang, T. Dietel and R. Kempe, Angew. Chem., Int. Ed., 2016, 55, 11806–11809 CrossRef CAS PubMed; (c) B. Maji and M. K. Barman, Synthesis, 2017, 3377–3393 CAS; (d) F. Kallmeier and R. Kempe, Angew. Chem., Int. Ed., 2018, 57, 46–60 CrossRef CAS; (e) M. Garbe, K. Junge and M. Beller, Eur. J. Org. Chem., 2017, 4344–4362 CrossRef CAS; (f) M. B. Widegren, G. J. Harkness, A. M. Slawin, D. B. Cordes and M. L. Clarke, Angew. Chem., Int. Ed., 2017, 56, 5825–5828 CrossRef CAS PubMed; (g) A. Bruneau-Voisine, D. Wang, T. Roisnel, C. Darcel and J.-B. Sortais, Catal. Commun., 2017, 92, 1–4 CrossRef CAS; (h) M. Garbe, K. Junge, S. Walker, Z. Wei, H. Jiao, A. Spannenberg, S. Bachmann, M. Scalone and M. Beller, Angew. Chem., Int. Ed., 2017, 56, 11237–11241 CrossRef CAS PubMed; (i) M. Perez, S. Elangovan, A. Spannenberg, K. Junge and M. Beller, ChemSusChem, 2017, 10, 83–86 CrossRef CAS PubMed; (j) A. Zirakzadeh, S. R. de Aguiar, B. Stöger, M. Widhalm and K. Kirchner, ChemCatChem, 2017, 9, 1744–1748 CrossRef CAS; (k) N. A. Espinosa-Jalapa, A. Nerush, L. J. Shimon, G. Leitus, L. Avram, Y. Ben-David and D. Milstein, Chem. – Eur. J., 2017, 23, 5934–5938 CrossRef CAS PubMed; (l) R. Van Putten, E. A. Uslamin, M. Garbe, C. Liu, A. Gonzalez-de-Castro, M. Lutz, K. Junge, E. J. Hensen, M. Beller and L. Lefort, Angew. Chem., Int. Ed., 2017, 56, 7531–7534 CrossRef CAS PubMed; (m) V. Papa, J. R. Cabrero-Antonino, E. Alberico, A. Spanneberg, K. Junge, H. Junge and M. Beller, Chem. Sci., 2017, 8, 3576–3585 RSC; (n) M. Glatz, B. Stöger, D. Himmelbauer, L. F. Veiros and K. Kirchner, ACS Catal., 2018, 8, 4009–4016 CrossRef CAS PubMed; (o) W. Yang, I. Y. Chernyshov, R. K. van Schendel, M. Weber, C. Müller, G. A. Filonenko and E. A. Pidko, Nat. Commun., 2021, 12, 1–8 Search PubMed.
- (a) S. Sitthisa, W. An and D. E. Resasco, J. Catal., 2011, 284, 90–101 CrossRef CAS; (b) Y. Yang, Z. Du, Y. Huang, F. Lu, F. Wang, J. Gao and J. Xu, Green Chem., 2013, 15, 1932–1940 RSC; (c) S. Dutta, S. De, B. Saha and M. I. Alam, Catal. Sci. Technol., 2012, 2, 2025–2036 RSC.
- (a) R.-J. van Putten, J. C. Van Der Waal, E. De Jong, C. B. Rasrendra, H. J. Heeres and J. G. de Vries, Chem. Rev., 2013, 113, 1499–1597 CrossRef CAS PubMed; (b) K. Yan, G. Wu, T. Lafleur and C. Jarvis, Renewable Sustainable Energy Rev., 2014, 38, 663–676 CrossRef CAS.
- (a) M. M. Villaverde, N. M. Bertero, T. F. Garetto and A. J. Marchi, Catal. Today, 2013, 213, 87–92 CrossRef CAS; (b) J. N. Chheda, G. W. Huber and J. A. Dumesic, Angew. Chem., Int. Ed., 2007, 46, 7164–7183 CrossRef CAS PubMed; (c) Y. Song, W. Li, M. Zhang and K. Tao, Front. Chem. Eng., 2007, 1, 151–154 CrossRef CAS.
- (a) R. V. Sharma, U. Das, R. Sammynaiken and A. K. Dalai, Appl. Catal., A, 2013, 454, 127–136 CrossRef CAS; (b) K. Yan and A. Chen, Fuel, 2014, 115, 101–108 CrossRef CAS; (c) S. Wei, H. Cui, J. Wang, S. Zhuo, W. Yi, L. Wang and Z. Li, Particuology, 2011, 9, 69–74 CrossRef CAS; (d) R. Rao, A. Dandekar, R. Baker and M. Vannice, J. Catal., 1997, 171, 406–419 CrossRef CAS; (e) A. B. Merlo, V. Vetere, J. F. Ruggera and M. L. Casella, Catal. Commun., 2009, 10, 1665–1669 CrossRef CAS.
- S. Paganelli, O. Piccolo, P. Pontini, R. Tassini and V. Rathod, Catal. Today, 2015, 247, 64–69 CrossRef CAS.
- F. Huang, W. Li, Q. Lu and X. Zhu, Chem. Eng. Technol., 2010, 33, 2082–2088 CrossRef CAS.
- (a) M. D. Bhor, A. G. Panda, N. S. Nandurkar and B. M. Bhanage, Tetrahedron Lett., 2008, 49, 6475–6479 CrossRef CAS; (b) Z. Strassberger, M. Mooijman, E. Ruijter, A. H. Alberts, C. de Graaff, R. V. Orru and G. Rothenberg, Appl. Org. Chem., 2010, 24, 142–146 CAS; (c) Z. Strassberger, M. Mooijman, E. Ruijter, A. H. Alberts, A. G. Maldonado, R. V. Orru and G. Rothenberg, Adv. Synth. Catal., 2010, 352, 2201–2210 CrossRef CAS PubMed.
- J. M. Tukacs, M. Bohus, G. Dibó and L. T. Mika, RSC Adv., 2017, 7, 3331–3335 RSC.
- R. Padilla, S. Koranchalil and M. Nielsen, Green Chem., 2020, 22, 6767–6772 RSC.
- (a) A. Brzozowska, L. M. Azofra, V. Zubar, I. Atodiresei, L. Cavallo, M. Rueping and O. El-Sepelgy, ACS Catal., 2018, 8, 4103–4109 CrossRef CAS; (b) G. Zhang, H. Zeng, J. Wu, Z. Yin, S. Zheng and J. C. Fettinger, Angew. Chem., Int. Ed., 2016, 128, 14581–14584 CrossRef.
- (a) L.-P. He, T. Chen, D. Gong, Z. Lai and K.-W. Huang, Organometallics, 2012, 31, 5208–5211 CrossRef CAS; (b) T. Chen, H. Li, S. Qu, B. Zheng, L. He, Z. Lai, Z.-X. Wang and K.-W. Huang, Organometallics, 2014, 33, 4152–4155 CrossRef CAS; (c) T. o. P. Gonçalves and K.-W. Huang, J. Am. Chem. Soc., 2017, 139, 13442–13449 CrossRef PubMed; (d) H. Li, T. P. Gonçalves, D. Lupp and K.-W. Huang, ACS Catal., 2019, 9, 1619–1629 CrossRef CAS; (e) D. Lupp and K.-W. Huang, Organometallics, 2019, 39, 18–24 CrossRef; (f) H. Li, D. Lupp, P. K. Das, L. Yang, T. P. Gonçalves, M.-H. Huang, M. El Hajoui, L.-C. Liang and K.-W. Huang, ACS Catal., 2021, 11, 4071–4076 CrossRef CAS; (g) T. P. Gonçalves, I. Dutta and K.-W. Huang, Chem. Commun., 2021, 57, 3070–3082 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 2099104 and 2098470. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d1cc04808b |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2021 |