Fe-Catalyzed dicarbofunctionalization of electron-rich alkenes with Grignard reagents and (fluoro)alkyl halides†
Abstract
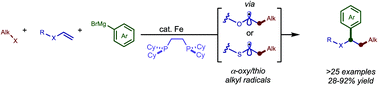
An iron-catalyzed regioselective dicarbofunctionalization of electron-rich alkenes is described. In particular, aryl- and alkyl vinyl ethers are used as effective linchpins to couple alkyl or (fluoro)alkyl halides and sp2-hybridized Grignard nucleophiles. Preliminary results demonstrate the ability to engage thioethers as linchpins and control enantioselectivity in these transformations, an area which is largely unexplored in iron-catalyzed three-component cross-coupling reactions.
- This article is part of the themed collection: 2021 Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...