Open Access Article
Open Access ArticleLow-dimensional magnetism in calcium nitridonickelate(II) Ca2NiN2†
Simon D.
Kloß
 * and
J. Paul
Attfield
* and
J. Paul
Attfield
 *
*
Centre for Science at Extreme Conditions, University of Edinburgh, Edinburgh EH9 3FD, UK. E-mail: v1skloss@ed.ac.uk; j.p.attfield@ed.ac.uk
First published on 13th September 2021
Abstract
Calcium nitridonickelate(II) Ca2NiN2 has been prepared through a high-temperature and high-pressure azide-mediated redox reaction, demonstrating that this method can stabilise nitrides of late transition metals in relatively high oxidation states. Ca2NiN2 crystallizes in the Na2HgO2 structure type and displays low-dimensional antiferromagnetic ordering of Ni2+ spins.
Multinary 3d transition metal nitrides have been extensively investigated with ambient and medium pressure (<1 GPa) synthesis methods for their diverse physical and electronic properties, their structural chemistry, and their close relation to the well-studied oxometallates.1–7 However, access to nitrogen-rich transition metal nitrides is limited by the low energy of nitride formation8–10 and especially for later transition metals such as Co, Ni and Cu, the obtainable nitrides are usually nitrogen-poor and metallic: while in molecular chemistry low-coordinate NiII imido complexes and a transient NiIV nitrido complex have been investigated,11,12 currently reported solid-state nitridonickelates such as MNiN (M = Ca, Sr, Ba), Ba2Ni2/3+3N2, and Li3Sr3Ni3/4+4N4,13–17 feature low nitrogen to metal ratios and mean Ni oxidation states of 1+ or lower. LiNiN is the only well-characterized compound with Ni(II)18 as a proposed Na2HgO2-type; the reported Sr2NiIIN2 was later shown to be a cyano-nitridonickelate(0) i.e. Sr2[(NC)NiN].19,20
A higher nitrogen content could lead to opening of a band gap enabling semiconductivity, light-absorption for solar energy conversion applications, and emergence of localized-electron properties such as charge order and magnetism as found in oxo-metallates.21–25 However, although recent high-throughput calculations have predicted many new nitride systems highlighting the great potential for materials discovery, employable synthesis routes for nitrogen rich compounds remain scarce.9,26 Applying high pressures in the gigapascal range is favorable for stabilizing nitrides against loss of dinitrogen but starting materials like transition metals or their binary nitrides often need to be nitrided in situ. This was demonstrated with N2-loaded diamond anvil cell syntheses of binary materials such as metal diazenides and pernitrides like NiN2 or PtN2, polynitrides like FeN4, and pentazolate salts like CsN5.27–30 Much less work has been done in multianvil large-volume presses but recently it was discovered that sodium azide can be used as a N2 source, as demonstrated in the syntheses of rocksalt-type Mg0.4Fe0.6N and the highly oxidized nitridoferrate(IV) Ca4FeN4.31–33 Large-volume-presses offer the advantage over DACs that larger sample quantities for physical properties measurements can be prepared and multinary systems can more easily be studied.
Here we adapt the azide-route for the preparation of a new Na2HgO2-type nitridonickelate with Ni in the 2+ state, which is very unusual in this class of materials. Ca2NiN2 was obtained at 900 °C and 8 GPa following eqn (1) with a 10 mol% excess of Ca3N2 as a black moisture sensitive (lifetime in air several minutes) microcrystalline powder (Fig. S1, ESI†). The excess of Ca3N2 and NaN3 is required to suppress the formation of byproduct CaNiN, which also forms when raising the temperature or lowering the pressure.13
| 6Ca3N2 + 9Ni + 4NaN3 → 9Ca2NiN2 + 4Na + 3N2 | (1) |
 | ||
| Fig. 1 (a) Rietveld refinement of Ca2NiN2 with datapoints as black crosses, Rietveld fit as red line, difference curve as grey line and tick marks of Ca2NiN2 and the Na byproduct shown above. The weight percent ratio of Ca2NiN2 and Na was refined to 97/3, which is in the range expected from stoichiometry, ideally 94/6. The diffuse background at low Q-values is probably due to amorphization of excess Ca3N2. (b–d) Structure of Ca2NiN2 with Ca as blue, Ni as orange and N as white ellipsoids at a 90% probability level. CaN5 square pyramids are shown in blue and NCa5Ni octahedra in grey. (e) Energy level diagram for d8 Ni in linear coordination and simple σ-bonding adapted from literature.11 | ||
The crystal structure of Ca2NiN2 (space group I4/mmm, Z = 2, a = 3.57206(2), c = 12.19453(10) Å, V = 155.719(5) Å3) was determined from powder diffraction data by charge flipping in P![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) n2 and subsequent refinement (Fig. 1a) in I4/mmm. Details and results of the refinement are in Tables S2 and S3 (ESI†).
n2 and subsequent refinement (Fig. 1a) in I4/mmm. Details and results of the refinement are in Tables S2 and S3 (ESI†).
Ca2NiN2 crystallizes in the Na2HgO2-structure type and features linear [NiIIN2]4− complex anions as well as layers of edge-sharing quadratic pyramids of CaN5 (Fig. 1b) while the nitrogen atoms are coordinated by five Ca and one Ni atom forming layers of edge-sharing NCa5Ni octahedra. In solid state chemistry, two-fold coordination of transition metals has for example been observed in Na2HgO2-type compounds such as M2NiO2 (M = K, Rb and Cs) and A2ZnN2 (A = Ca, Sr, Ba), and nitridometallates of Co, Ni and Cu with either linear chains, kinked chains, or [MN2]-dumbbells such as AMN-type compounds, (BaCa4[MN2]2) (M = Co, Cu) and Sr39Co12N31.34–40 The interatomic distances in the CaN5 square pyramid (Fig. 1c) are equal in the equatorial plane and both Ca–N distances (Fig. 1d) are in the range observed in similar compounds like CaNiN, Ca2ZnN2, Ca2FeN2, Ca4FeN4, Ca5Co2N4, and Ca3N2 (dCa–N = 2.40–2.82 Å).13,32,36,41–43 The Ni–N distance observed in Ca2NiN2 is smaller than in compounds containing infinite N–Ni–N chains like CaNiIN (dNi–N = 1.792(1) Å) and BaNiIN (dNi–N = 1.8281(1), 1.781(1) Å), which is probably owed to the higher oxidation state of Ni and the terminal Ni–N bonds.13,17
Bond valence sum (BVS) calculations were performed for Ca and Ni for a coordination sphere of 4 Å using bond valence parameters reported by O’Keeffe (Table S3, ESI†). The bond valence sum for Ni VNi,N = 2.03 corroborates the presence of NiII, while the bond valence sum for Ca is slightly lower with VCa–N = 1.80.44 This is probably owed to the covalent bonding in nitrides and metal–metal interactions and has also been observed in related compounds such as CaGaN (VGa–N = 0.9), Ca4FeN4 (VCa–N = 1.85, 1.65), and the isotypic Ca2ZnN2 (VCa–N = 1.82).32,35,45,46
Magnetic susceptibility measurements (Fig. 2) carried out at 30 kOe in the temperature range from 2.5 to 300 K showed a broad maximum at ca. 200 K and a decrease towards lower temperatures. The higher temperature region of the susceptibility is indicative of short-range low-dimensional antiferromagnetic spin interactions, which also has been observed in related nitridometallates such as Ca3CrN3 and Ba4Mn3N6.23,47 The derivative dχ/dT (Fig. S2, ESI†) shows a maximum at TN = 74 K indicating the possible onset of long-range magnetic ordering. Neutron diffraction data will be needed to confirm this.
 | ||
| Fig. 2 Magnetic molar susceptibility of Ca2NiN2 measured in a field of 30 kOe with black circles as datapoints and combined Bonner–Fisher and Curie-tail fit as orange line. | ||
The susceptibility data were fit according to χmol = χC + χBF with a Bonner–Fisher-type function χBF and a Curie function χC to account for an impurity producing a Curie tail at low temperatures (see methods for details, ESI†).48 The resulting fit (Fig. 2) follows the data well, with a deviation below ∼75 K close to the maximum in dχ/dT at 74 K (Fig. S2, ESI†). The fitted paramagnetic moment of μeff = 2.19 μB is reduced from the ideal S = 1 spin-only value of 2.83 μB, most likely due to mixing with excited states through spin–orbit coupling. The fitted exchange coupling of J = −157 K confirms strong antiferromagnetic coupling within the ab-planes. Both the μeff and J values are consistent with localised Ni2+ moment behavior. The impurity phase has an effective moment of 0.11 μB, equivalent to 0.4% of a S = ½ byproduct, which is below the detection limit of powder diffraction. The low-dimensional magnetic behavior reflects the arrangement of Ni-atoms in square nets with interatomic distances of dNi–Ni = 3.572(1) Å, which are separated by layers of CaN5 square-pyramids. Field-dependent magnetization data at 300 and 2.5 K (Fig. S2, ESI†) shows a linear dependence in accordance with paramagnetic or antiferromagnetic behavior.
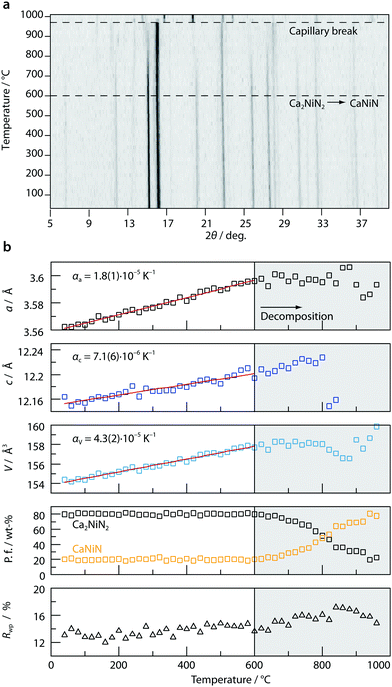
The high-temperature behavior of Ca2NiN2 was investigated with temperature-dependent powder X-ray diffraction (Fig. 3) and reveals Vegard-type behavior of the lattice parameters up to 600 °C with thermal expansion coefficients of αa = 1.8(1) × 10−5 K−1 and αc = 7.1(6) × 10−6 K−1. The larger value of αa reflects greater amplitudes of thermal vibration perpendicular to the NiN2 dumbbells which are aligned in the c-direction. Above this temperature Ca2NiN2 starts to decompose through elimination of ½ N2 and 1 Ca per formula unit resulting in crystalline CaNiN.13 Ca/Ca3N2 could not unambiguously be identified in the powder patterns and might also be amorphous. The decomposition was monitored through phase fractions, which show gradual decomposition over a wider temperature range, which might be owed to the relatively fast data collection of 30 min per step. At 960 °C the capillary presumably broke as CaNiN, which was reported to be stable up to 1100 °C, decomposed into CaO and metallic Ni.6
In conclusion, the preparation of Ca2NiN2 with Ni in oxidation state +II, which is very unusual for nitridometallates, is demonstrated through the azide-mediated nitridation of metallic Ni under high-pressure conditions. Ca2NiN2 crystallizes in the Na2HgO2 structure type with linear coordination of Ni. While in molecular chemistry low metal coordination environments are stabilized through sterically demanding ligands, the stabilization here is probably due to covalent Ni–N multiple bonding and consequent short Ni–N bond distances and the electron inductive effect of the surrounding Ca2+ matrix.49 The observation of a susceptibility maximum consistent with low-dimensional antiferromagnetic ordering of Ni2+ spins in Ca2NiN2, whereas metallic CaNiN has a temperature-independent Pauli susceptibility, suggests that Ca2NiN2 is non-metallic but further measurements will be required to confirm this. Temperature-dependent powder X-ray diffraction shows that Ca2NiN2 is stable up to 600 °C before decomposing to CaNiN, which when compared to the synthesis temperature of 900 °C indicates the stabilizing influence of the high-pressure conditions. Stability estimates are particularly important for efficient planning of syntheses of compounds of noble metals like Ni and Cu, and our results highlight that nitrogen-rich compounds require very stringent temperature control which necessitates synthesis under a high nitrogen chemical potential. Discovery of Ca2NiN2 indicates that a new class of late transition metal nitrides can be synthesised, and like oxo-nickelates and -cuprates, they may have interesting correlated electron properties in proximity to a metal–insulator boundary, as illustrated by the recent discovery of superconductivity in infinite-layer nickelates LnNiO2.50
Conflicts of interest
There are no conflicts to declare.Notes and references
- R. Niewa and F. J. DiSalvo, Chem. Mater., 1998, 10, 2733 CrossRef CAS.
- H. Yamane and F. J. DiSalvo, Prog. Solid State Chem., 2018, 51, 27 CrossRef CAS.
- N. Tapia-Ruiz, M. Segalés and D. H. Gregory, Coord. Chem. Rev., 2013, 257, 1978 CrossRef CAS.
- J. M. Cameron, R. W. Hughes, Y. Zhao and D. H. Gregory, Chem. Soc. Rev., 2011, 40, 4099 RSC.
- R. Niewa and H. Jacobs, Chem. Rev., 1996, 96, 2053 CrossRef CAS PubMed.
- F. J. DiSalvo and S. J. Clarke, Curr. Opin. Solid State Mater. Sci., 1996, 2, 241 CrossRef.
- F. J. DiSalvo, Science, 1990, 247, 649 CrossRef CAS PubMed.
- D. R. Glasson and S. A. A. Jayaweera, J. Appl. Chem., 1968, 18, 65 CrossRef CAS.
- W. Sun, A. Holder, B. Orvañanos, E. Arca, A. Zakutayev, S. Lany and G. Ceder, Chem. Mater., 2017, 29, 6936 CrossRef CAS.
- A. Salamat, A. L. Hector, P. Kroll and P. F. McMillan, Coord. Chem. Rev., 2013, 257, 2063 CrossRef CAS.
- P. P. Power, Chem. Rev., 2012, 112, 3482–3507 CrossRef CAS PubMed.
- V. Vreeken, M. A. Siegler, B. de Bruin, J. N. H. Reek, M. Lutz and J. I. van der Vlugt, Angew. Chem., Int. Ed., 2015, 54, 7055 ( Angew. Chem. , 2015 , 127 , 7161–7165 ) CrossRef CAS PubMed.
- M. Y. Chern and F. J. DiSalvo, J. Solid State Chem., 1990, 88, 459 CrossRef CAS.
- T. Yamamoto, S. Kikkawa and F. Kanamaru, J. Solid State Chem., 1995, 115, 353 CrossRef CAS.
- A. Gudat, R. Kniep and A. Rabenau, Z. Anorg. Allg. Chem., 1991, 597, 61 CrossRef CAS.
- A. Mehta, P. Höhn, W. Schnelle, V. Petzold, H. Rosner, U. Burkhardt and R. Kniep, Chem. – Eur. J., 2006, 12, 1667 CrossRef CAS PubMed.
- A. Gudat, S. Haag, R. Kniep and A. Rabenau, J. Less-Common Met., 1990, 159, L29 CrossRef CAS.
- C. H. Hu, Y. Yang and Z. Z. Zhu, Solid State Commun., 2010, 150, 669 CrossRef CAS.
- G. R. Kowach, N. E. Brese, U. M. Bolle, C. J. Warren and F. J. DiSalvo, J. Solid State Chem., 2000, 154, 542 CrossRef CAS.
- P. Höhn, M. Armbrüster, G. Auffermann, U. Burkhardt, F. Haarmann, A. Mehta and R. Kniep, Z. Anorg. Allg. Chem., 2006, 632, 2129 CrossRef.
- P. Chanhom, K. E. Fritz, L. A. Burton, J. Kloppenburg, Y. Filinchuk, A. Senyshyn, M. Wang, Z. Feng, N. Insin, J. Suntivich and G. Hautier, J. Am. Chem. Soc., 2019, 141, 10595 CrossRef CAS PubMed.
- M. Yang, A. Zakutayev, J. Vidal, X. Zhang, D. S. Ginley and F. J. DiSalvo, Energy Environ. Sci., 2013, 6, 2994 RSC.
- D. A. Vennos, M. E. Badding and F. J. DiSalvo, Inorg. Chem., 1990, 29, 4059 CrossRef CAS.
- R. Marchand and V. Lemarchand, J. Less-Common Met., 1981, 80, 157 CrossRef CAS.
- A. Zakutayev, J. Mater. Chem. A, 2016, 4, 6742 RSC.
- W. Sun, C. J. Bartel, E. Arca, S. R. Bauers, B. Matthews, B. Orvañanos, B.-R. Chen, M. F. Toney, L. T. Schelhas, W. Tumas, J. Tate, A. Zakutayev, S. Lany, A. M. Holder and G. Ceder, Nat. Mater., 2019, 18, 732 CrossRef CAS PubMed.
- K. Niwa, R. Fukui, T. Terabe, T. Kawada, D. Kato, T. Sasaki, K. Soda and M. Hasegawa, Eur. J. Inorg. Chem., 2019, 3753–3757 CrossRef CAS.
- J. C. Crowhurst, Science, 2006, 311, 1275 CrossRef CAS PubMed.
- M. Bykov, E. Bykova, G. Aprilis, K. Glazyrin, E. Koemets, I. Chuvashova, I. Kupenko, C. McCammon, M. Mezouar, V. Prakapenka, H.-P. Liermann, F. Tasnádi, A. V. Ponomareva, I. A. Abrikosov, N. Dubrovinskaia and L. Dubrovinsky, Nat. Commun., 2018, 9, 2756 CrossRef CAS PubMed.
- D. Laniel, G. Weck, G. Gaiffe, G. Garbarino and P. Loubeyre, J. Phys. Chem. Lett., 2018, 9, 1600 CrossRef CAS PubMed.
- G. Serghiou, G. Ji, N. Odling, H. J. Reichmann, J.-P. Morniroli, R. Boehler, D. J. Frost, J. P. Wright and B. Wunder, Angew. Chem., Int. Ed., 2015, 54, 15109 ( Angew. Chem. , 2015 , 127 , 15324 ) CrossRef CAS PubMed.
- S. D. Kloß, A. Haffner, P. Manuel, M. Goto, Y. Shimakawa and J. P. Attfield, Nat. Commun., 2021, 12, 571 CrossRef PubMed.
- M. Bykov, K. R. Tasca, I. G. Batyrev, D. Smith, K. Glazyrin, S. Chariton, M. Mahmood and A. F. Goncharov, Inorg. Chem., 2020, 59, 14819 CrossRef CAS PubMed.
- T. Yamamoto, S. Kikkawa and F. Kanamaru, Solid State Ionics, 1993, 63–65, 148 CrossRef CAS.
- H. Rieck and R. Hoppe, Z. Anorg. Allg. Chem., 1973, 400, 311 CrossRef CAS.
- M. Y. Chern and F. J. DiSalvo, J. Solid State Chem., 1990, 533, 528 CrossRef.
- H. Yamane and F. J. DiSalvo, J. Solid State Chem., 1995, 119, 375 CrossRef CAS.
- J. K. Bendyna, P. Höhn, Y. Prots and R. Kniep, Z. Kristallogr. - New Cryst. Struct., 2007, 222, 484 Search PubMed.
- G. R. Kowach, H. Y. Lin and F. J. Disalvo, J. Solid State Chem., 1998, 9, 1–9 CrossRef.
- R. Niewa and F. J. DiSalvo, J. Alloys Compd., 1998, 279, 153–160 CrossRef CAS.
- P. Höhn and R. Kniep, Z. Naturforsch., 1992, 47b, 477–481 CrossRef.
- J. K. Bendyna, P. Höhn and R. Kniep, Z. Kristallogr. - New Cryst. Struct., 2007, 222, 165 CAS.
- Y. Laurent, J. Lang and M. T. Le Bihan, Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1968, 24, 494 CrossRef CAS.
- N. E. Brese and M. O’Keeffe, Acta Crystallogr., Sect. B: Struct. Sci., 1991, 47, 192 CrossRef.
- N. E. Brese and M. O’Keeffe, Crystal chemistry of inorganic nitrides in Complexes, Clusters and Crystal Chemistry, Springer-Verlag, Berlin/Heidelberg, 2006, p. 307 Search PubMed.
- D. H. Gregory, J. Chem. Soc., Dalton Trans., 1999, 259 RSC.
- A. Ovchinnikov, M. Bobnar, Y. Prots, W. Schnelle, P. Höhn and Y. Grin, Crystals, 2018, 8, 235 CrossRef.
- M. E. Fisher, Am. J. Phys., 1964, 32, 343 CrossRef.
- J. Etourneau, J. Portier and F. Ménil, J. Alloys Compd., 1992, 188, 1 CrossRef CAS.
- D. Li, K. Lee, B. Y. Wang, M. Osada, S. Crossley, H. R. Lee, Y. Cui, Y. Hikita and H. Y. Hwang, Nature, 2019, 572, 624 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2096979. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d1cc04001d |
| This journal is © The Royal Society of Chemistry 2021 |