 Open Access Article
Open Access ArticleChiral porous CN-bridged coordination polymer mimicking MOF-74 and showing magnetization photoswitching†
Michał
Magott
 * and
Dawid
Pinkowicz
* and
Dawid
Pinkowicz
 *
*
Faculty of Chemistry, Jagiellonian University, Gronostajowa 2, 30-387 Kraków, Poland. E-mail: michal.magott@uj.edu.pl; dawid.pinkowicz@uj.edu.pl
First published on 9th September 2021
Abstract
A chiral porous cyanide-bridged framework {[MnII(L)]2[WIV(CN)8]·10H2O}n (1; L = 2,6-bis[1-(2-(N-methylamino)ethylimino)ethyl]-pyridine) showing a strong structural similarity to MOF-74 has been prepared and characterised. The crystallised water molecules can be easily removed below 60 °C, leading to a distinct crystal colour change and the activation of its photomagnetic properties – constituting the so called photomagnetic sponge behaviour of this system. The complete dehydration of 1 proceeds through a single-crystal-to-single-crystal transformation and the resulting anhydrous framework {[MnII(L)]2[WIV(CN)8]}n (1anh) was studied using single-crystal X-ray diffraction.
Metal–organic frameworks (MOFs), coordination polymers characterised by metal nodes connected by organic linkers, are regarded as the new generation of “smart” sorption materials. Terephthalate-based MOFs seem to attract most of the attention,1 with MOF-74 being the prominent example of a material characterised by a remarkable stability of the coordination network and unique sorption properties.2 The modular characteristics of its skeleton, constructed from divalent metal cations and 2,5-dihydroxybenzene-1,4-dicarboxylate, were utilized to tailor specific functionalities, such as the sorption of carbon dioxide,3–5 conductivity6–8 or gas separation.9,10 One of the approaches to further improve the properties of this framework is based on its decoration with a specifically designed mixture of active metal sites.11,12
Herein, we demonstrate a cyanide-bridged coordination polymer {[MnII(L)]2[WIV(CN)8]·10H2O}n (1; L = 2,6-bis[1-(2-(N-methylamino)ethylimino)ethyl]-pyridine), see Fig. S1 in the ESI†) mimicking the topology of MOF-74 despite a completely different preparation strategy involving the use of an octacyanometallate anion as a linker. Octacyanometallates in combination with cationic transition metal complexes are known to form functional bimetallic three-dimensional (3-D) coordination polymers (CPs) by design.13 Chirality of the CPs is usually achieved by the intentional incorporation of chiral ligands at the self-assembly stage14–16 and the crystallisation of chiral CPs from achiral building blocks is unexpected and rare.17,18 In the particular case of 1 the combination of [WIV(CN)8]4− with achiral [MnII(L)]2+ serendipitously leads to a unique chiral CP that not only mimics MOF-74's topology and porosity, but also shows single-crystal-to-single-crystal (SCSC) transformation induced by water sorption, different photomagnetic behaviour depending on the hydration level and spontaneous resolution of the enantiomeric forms by crystallisation.
Compound 1 crystallises in the form of dark yellow needle crystals from a water–acetonitrile solution comprising [MnII(L)]Cl2 and K4[WIV(CN)8]·2H2O (see the Experimental section of the ESI† for details). Its 3-D coordination framework in the trigonal crystal system shows a strong resemblance to MOF-74 (Fig. 1a and b). Crystals of 1 consist of –MnII(L)-(μ-NC)-WIV(CN)6-(μ-CN)– helical rods running along the c direction, with the octacyanotungstate(IV) moieties connecting the neighbouring rods. Contrary to MOF-74 crystallising in a centrosymmetric R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) space group, 1 undergoes a spontaneous resolution into a racemic mixture of enantiopure crystals in two enantiomorphic space groups: P3121 and P3221. The homochirality of the single crystals of 1 is related to the aforementioned 31 or 32 helical rods within the crystal (Fig. S2, ESI†), similar to the chiral [Mn3(HCOO)4(D-Cam)]n MOF.19 We hypothesize that chirality is transferred from one helix to another by flexible [W(CN)8] linkers, which are directly incorporated into both helical rods, in contrast to the rigid organic linkers simply connecting the well-separated inorganic SBUs of opposite handedness in the structure of MOF-74.20 The chirality of a single cyanide-bridged helix may originate from the conformation of the N-CH3 groups of the ligand, similar to the [MnII(L)(H2O)]2[MoIII(CN)7]·6H2O molecules, which crystallise in the chiral P21 space group.21 Moreover, the ligand itself attains a slightly helical conformation of donor atoms, which may be associated with the twist of the helices forming the coordination skeleton (Fig. S2, ESI†). In the crystal structure of 1 there are two types of water molecules: four molecules per Mn2W formula unit form direct hydrogen bonds with the nitrogen atoms of the terminal cyanide ligands and the remaining six molecules occupy the interior of the pores (Fig. 2). The crystallised water molecules in the structure of 1 can be completely removed below 60 °C, as demonstrated by the thermogravimetric analysis (TGA) under a dry nitrogen atmosphere (Fig. S3, ESI†). The anhydrous coordination framework is stable up to 250 °C, which is uncommon for this class of compounds.22 A clear step-like feature at 270 °C marks the decomposition temperature of anhydrous {[MnII(L)]2[WIV(CN)8]}n (1anh). Interestingly, the dehydrated crystals of 1 do not show any signs of deterioration (no cracking or turning opaque) and preserve their chirality. Nevertheless, the single-crystal X-ray diffraction pattern for anhydrous {[MnII(L)]2[WIV(CN)8]}n (1anh) is characterised by a relatively high mosaicity, hence, it is difficult to obtain a good-quality structural model for large crystals of 1anh using a commercial diffractometer. The determination of the crystal structure was possible for a very small crystal of 1anh using synchrotron radiation at 30 K (Diamond Light Source, United Kingdom). Comparison of the structural models of 1 and 1anh (Tables S1, S2 and Fig. S4, S5, ESI†) indicates that the dehydration process only slightly affects the cyanide-bridged framework and the conformation of the organic ligands. The unit cell volume at room temperature shrinks from 4005.8(2) Å3 for 1 to 3786.3(7) Å3 for 1anh upon dehydration (data based on the Pawley analysis of the experimental PXRD patterns; for details see Fig. S6 and S7 in the ESI†). Interestingly, the a period related to the opening of the channels shrinks by 2.1% as compared to the 1.4% decrease of the c period upon dehydration. This results in the stability of the material, which loses and reabsorbs water reversibly in at least three dehydration–rehydration cycles as demonstrated by the powder X-ray diffraction studies (PXRD) (Fig. S8, ESI†). The most pronounced difference between the PXRD for 1 and 1anh is an approximately 50% line broadening for the latter (Fig. S9, ESI†), which most probably results from the crystal lattice distortion induced by water removal. This is supported by the narrowing of the diffraction peaks upon rehydration, which also confirms that there is no permanent damage associated with the H2O removal.
space group, 1 undergoes a spontaneous resolution into a racemic mixture of enantiopure crystals in two enantiomorphic space groups: P3121 and P3221. The homochirality of the single crystals of 1 is related to the aforementioned 31 or 32 helical rods within the crystal (Fig. S2, ESI†), similar to the chiral [Mn3(HCOO)4(D-Cam)]n MOF.19 We hypothesize that chirality is transferred from one helix to another by flexible [W(CN)8] linkers, which are directly incorporated into both helical rods, in contrast to the rigid organic linkers simply connecting the well-separated inorganic SBUs of opposite handedness in the structure of MOF-74.20 The chirality of a single cyanide-bridged helix may originate from the conformation of the N-CH3 groups of the ligand, similar to the [MnII(L)(H2O)]2[MoIII(CN)7]·6H2O molecules, which crystallise in the chiral P21 space group.21 Moreover, the ligand itself attains a slightly helical conformation of donor atoms, which may be associated with the twist of the helices forming the coordination skeleton (Fig. S2, ESI†). In the crystal structure of 1 there are two types of water molecules: four molecules per Mn2W formula unit form direct hydrogen bonds with the nitrogen atoms of the terminal cyanide ligands and the remaining six molecules occupy the interior of the pores (Fig. 2). The crystallised water molecules in the structure of 1 can be completely removed below 60 °C, as demonstrated by the thermogravimetric analysis (TGA) under a dry nitrogen atmosphere (Fig. S3, ESI†). The anhydrous coordination framework is stable up to 250 °C, which is uncommon for this class of compounds.22 A clear step-like feature at 270 °C marks the decomposition temperature of anhydrous {[MnII(L)]2[WIV(CN)8]}n (1anh). Interestingly, the dehydrated crystals of 1 do not show any signs of deterioration (no cracking or turning opaque) and preserve their chirality. Nevertheless, the single-crystal X-ray diffraction pattern for anhydrous {[MnII(L)]2[WIV(CN)8]}n (1anh) is characterised by a relatively high mosaicity, hence, it is difficult to obtain a good-quality structural model for large crystals of 1anh using a commercial diffractometer. The determination of the crystal structure was possible for a very small crystal of 1anh using synchrotron radiation at 30 K (Diamond Light Source, United Kingdom). Comparison of the structural models of 1 and 1anh (Tables S1, S2 and Fig. S4, S5, ESI†) indicates that the dehydration process only slightly affects the cyanide-bridged framework and the conformation of the organic ligands. The unit cell volume at room temperature shrinks from 4005.8(2) Å3 for 1 to 3786.3(7) Å3 for 1anh upon dehydration (data based on the Pawley analysis of the experimental PXRD patterns; for details see Fig. S6 and S7 in the ESI†). Interestingly, the a period related to the opening of the channels shrinks by 2.1% as compared to the 1.4% decrease of the c period upon dehydration. This results in the stability of the material, which loses and reabsorbs water reversibly in at least three dehydration–rehydration cycles as demonstrated by the powder X-ray diffraction studies (PXRD) (Fig. S8, ESI†). The most pronounced difference between the PXRD for 1 and 1anh is an approximately 50% line broadening for the latter (Fig. S9, ESI†), which most probably results from the crystal lattice distortion induced by water removal. This is supported by the narrowing of the diffraction peaks upon rehydration, which also confirms that there is no permanent damage associated with the H2O removal.
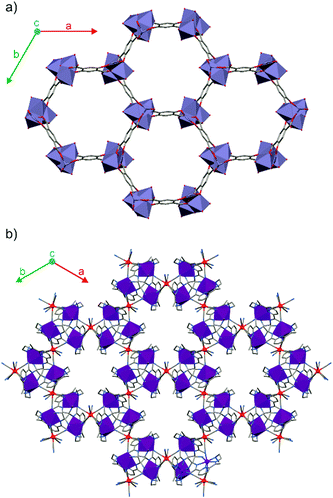 | ||
| Fig. 1 Fragments of the crystal structure demonstrating the coordination framework of MOF-74 (a) and 1 (P3121 enantiomer) (b) as seen along the crystallographic c axis. | ||
The desorption/adsorption of water was also studied using IR spectroscopy. Fig. S10 (ESI†) shows the spectra recorded in three consecutive dehydration/rehydration cycles. Each dehydration leaves the skeletal vibrations in the fingerprint region unaffected and very similar to the precursor [MnII(L)]Cl2·H2O (Fig. S11, ESI†), but leads to the disappearance of the broad O–H bands in the 3600–3000 cm−1 region – in line with the complete desorption of H2O. Dehydration also causes a slight shift of the cyanide stretching vibration bands from 2106 cm−1 for 1 to 2097 cm−1 for 1anh. Each rehydration results in the complete recovery of the initial IR spectrum: the O–H bands reappear and the C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N bands shift back to 2106 cm−1. This confirms the reversibility of the water sorption properties in 1.
N bands shift back to 2106 cm−1. This confirms the reversibility of the water sorption properties in 1.
The porosity of powdered 1anh was experimentally confirmed by the type I nitrogen adsorption isotherm at 77 K (Fig. S12, ESI†). Its total surface area SBET = 270.1(5) m2 g−1 is very similar to the 264 m2 g−1 reported for the chiral Zn-MOF-74-L-Pro, but significantly smaller than the 1168 m2 g−1 observed for the archetypical Zn-MOF-74.20 The t-plot analysis of N2 sorption yields a micropore volume Vmicro = 0.0749(8) cm3 g−1, which corresponds to 10.6% of the unit cell volume. This value is smaller than the 17% volume of voids determined for 1anh using Mercury 2020.3.0 (contact surface; probe radius 1.8 Å, grid spacing 0.5 Å).23 However, the contact surface by definition accounts for the volume in the crystal that is accessible to the single probe molecule moving inside the pore, whereas in the nitrogen adsorption measurement some space may be blocked by already adsorbed molecules. This would explain the smaller experimental microporosity than that derived from the crystal structure.
Despite the fact that the removal of H2O from 1 only weakly influences the entire framework, it leads to pronounced changes in its physical properties. The crystals change colour from yellow to orange-red upon dehydration (Fig. 3 inset), which is reflected in the change of the UV-vis spectra (red solid line for 1anhvs. orange solid line for 1 in Fig. 3). Such changes cannot be attributed to the octacyanotungstate(IV) moiety, which does not show intense absorption bands above 500 nm (Fig. S13, ESI†).24 On the other hand, the absorption band observed for 1anh stretches up to 600 nm, which is much further than in the case of the MnII(L)Cl2·H2O precursor (yellow dashed line in Fig. 3). Such a decrease in the energy of the d–d transition is unexpected upon substitution of chloride with a strong field ligand – cyanide, especially as there are no systematic changes in the MnII–N bond lengths between the precursor [MnII(L)Cl2]·1.78EtOH·0.22H2O,25 the pristine 1 and 1anh (Table S2, ESI†). Therefore we attribute this broad and relatively intense absorption to the metal-to-ligand charge transfer band (MLCT). The energy of the CT transition often depends on the solvent,26,27 which explains the colour change upon dehydration, despite negligible changes in the coordination environment of MnII.
The presence of the MnII nodes (S = 5/2) makes 1 and 1anh paramagnetic. These paramagnetic centres are relatively well separated, and both phases show no signs of long range magnetic ordering down to 2 K (detailed description of their ground state magnetic properties can be found in Fig. S14 and S15 in the ESI†). However, octacyanometallates based on molybdenum(IV) or tungsten(IV) have been demonstrated to show photomagnetic effects associated with the transition from the diamagnetic S = 0 to the paramagnetic S = 1 state upon blue light irradiation.28–31 In our previous work we have demonstrated that in {[MnII(imH)]2[WIV(CN)8]}n this can lead to photo-induced magnetic ordering with the critical temperature as high as 93 K and the emergence of the magnetic hysteresis above the boiling point of liquid nitrogen.32 We have also demonstrated that this photomagnetic effect is completely quenched after water adsorption, constituting the first “photomagnetic sponge” example. Taking into account the reversible water sorption in 1, we decided to investigate its photomagnetic behaviour. Similar to the previously reported hydrated {[MnII(imH)(H2O)2]2[WIV(CN)8]·4H2O}n, 1 is completely unaffected by the 450 nm light irradiation at 10 K (P = 6–10 mW cm−2; 15 h), as demonstrated in Fig. S16 (ESI†). Hence the field-cooled magnetization curve recorded after illumination closely resembles the measurement for the non-irradiated sample (Fig. S17, ESI†). On the other hand, 1anh shows an almost five-fold increase of the magnetization after 48 hours of 450 nm irradiation at 10 K (Fig. S16, ESI†). Moreover, the photo-induced state shows bifurcation of the field-cooled and zero field-cooled magnetization curves at Tc = 48 K (Fig. 4). This remarkably high photo-induced magnetic ordering temperature is bested only by the aforementioned {[MnII(imH)]2[MIV(CN)8]}n family of photomagnets (M = Mo with Tc = 72 K and M = W with Tc = 93 K, respectively)32,33 and matches that of the {[CoII(4-Mepy)(pym)]2[CoII(H2O)2][WV(CN)8]2·4H2O}n system34 which is characterised by the same magnetic ordering temperature of 48 K. Due to the long-range magnetic ordering, 1anh in its photo-induced state shows distinct magnetic hysteresis at 2 K (Fig. S18 and S19, ESI†), characterised by the coercive field of Hc = 550 Oe, remanence Mr = 0.6 Nβ and saturation magnetisation Ms = 8.8 Nβ. In the case of 1anh the transition to the photo-induced state is not completely reversible – after 2 hours at 300 K a clear χT maximum of 15.6 cm3 K mol−1 is still observed at 6.8 K (Fig. S20, ESI†) suggesting that the relaxation of the photo-induced high-spin W(IV) is not complete at room temperature. In an attempt to characterise the structure of the photo-induced state and understand the photoswitching mechanism, we have performed photo-crystallography experiments on 1anh using synchrotron radiation (Diamond Light Source, beamline I19) and prepared the elusive heptacyanotungstate(IV) complex anion35 by photo-irradiation of octacyanotungstate(IV) (new photochemical route). Details of these experiments and the crystal structure of the photoproduct can be found in the Photomagnetic irreversibility section of the ESI.†
 | ||
| Fig. 4 Field-cooled (full circles) and zero field-cooled (open circles) magnetization curves for 1anh before irradiation (black) and after a 450 nm light irradiation (red). | ||
In summary, we have demonstrated a multifunctional CN-bridged CP that topologically mimics MOF-74. Its multifunctionality is associated with three key features: porosity, chirality and photomagnetism, which are expected to be strongly intertwined e.g. the guest-induced single-crystal-to-single-crystal transformation results in the “photomagnetic sponge behaviour” accompanied by a colour change. Further studies of the sorption properties, including possible chiral separation36,37 and magneto-chiral effects are underway.38,39
This work was financed by the Polish Ministry of Science and Higher Education within the Diamond Grant project (0192/DIA/2017/46) and the National Science Centre Poland within the Opus project 2020/37/B/ST5/02735. The authors acknowledge the Diamond Light Source beamtime (proposal no. CY24501-1) and the assistance of the I19 staff.
Conflicts of interest
There are no conflicts to declare.Notes and references
- J. Winarta, B. Shan, S. M. McIntyre, L. Ye, C. Wang, J. Liu and B. Mu, Cryst. Growth Des., 2020, 20, 1347–1362 CrossRef CAS
.
- N. L. Rosi, J. Kim, M. Eddaoudi, B. L. Chen, M. O'Keeffe and O. M. Yaghi, J. Am. Chem. Soc., 2005, 127, 1504–1518 CrossRef CAS PubMed
.
- A. R. Millward and O. M. Yaghi, J. Am. Chem. Soc., 2005, 127, 17998–17999 CrossRef CAS PubMed
.
- D.-A. Yang, H.-Y. Cho, J. Kim, S.-T. Yang and W.-S. Ahn, Energy Environ. Sci., 2012, 5, 6465–6473 RSC
.
- T. M. McDonald, W. R. Lee, J. A. Mason, B. M. Wiers, C. S. Hong and J. R. Long, J. Am. Chem. Soc., 2012, 134, 7056–7065 CrossRef CAS PubMed
.
- W. J. Phang, W. R. Lee, K. Yoo, D. W. Ryu, B. Kim and C. S. Hong, Angew. Chem., Int. Ed., 2014, 53, 8383–8387 CrossRef CAS PubMed
.
- L. Sun, C. H. Hendon, M. A. Minier, A. Walsh and M. Dincă, J. Am. Chem. Soc., 2015, 137, 6164–6167 CrossRef CAS PubMed
.
- M. K. Sarango-Ramírez, D.-W. Lim, D. I. Kolokolov, A. E. Khudozhitkov, A. G. Stepanov and H. Kitagawa, J. Am. Chem. Soc., 2020, 142, 6861–6865 CrossRef PubMed
.
- D. Britt, H. Furukawa, B. Wang, T. G. Glover and O. M. Yaghi, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 20637–20640 CrossRef CAS PubMed
.
- E. D. Bloch, L. J. Murray, W. L. Queen, S. Chavan, S. N. Maximoff, J. P. Bigi, R. Krishna, V. K. Peterson, F. Grandjean, G. J. Long, B. Smit, S. Bordiga, C. M. Brown and J. R. Long, J. Am. Chem. Soc., 2011, 133, 14814–14822 CrossRef CAS PubMed
.
- L. J. Wang, H. Deng, H. Furukawa, F. Gándara, K. E. Cordova, D. Peri and O. M. Yaghi, Inorg. Chem., 2014, 53, 5881–5883 CrossRef CAS PubMed
.
- Z. Ji, T. Li and O. M. Yaghi, Science, 2020, 369, 674–680 CrossRef CAS PubMed
.
- S. Chorazy, J. J. Zakrzewski, M. Magott, T. Korzeniak, B. Nowicka, D. Pinkowicz, R. Podgajny and B. Sieklucka, Chem. Soc. Rev., 2020, 49, 5945–6001 RSC
.
- K. Inoue, H. Imai, P. S. Ghalsasi, K. Kikuchi, M. Ohba, H. Ōkawa and J. V. Yakhmi, Angew. Chem., Int. Ed., 2001, 40, 4242–4245 CrossRef CAS PubMed
.
- J. Milon, M.-C. Daniel, A. Kaiba, P. Guionneau, S. Brandès and J.-P. Sutter, J. Am. Chem. Soc., 2007, 129, 13872–13878 CrossRef CAS PubMed
.
- S. Chorazy, R. Podgajny, W. Nitek, T. Fic, E. Görlich, M. Rams and B. Sieklucka, Chem. Commun., 2013, 49, 6731–6733 RSC
.
- D. Pinkowicz, R. Podgajny, W. Nitek, M. Rams, A. M. Majcher, T. Nuida, S.-I. Ohkoshi and B. Sieklucka, Chem. Mater., 2011, 23, 21–31 CrossRef CAS
.
- S.-I. Ohkoshi, S. Takano, K. Imoto, M. Yoshikiyo, A. Namai and H. Tokoro, Nat. Photonics, 2014, 8, 65–71 CrossRef CAS
.
- J. Zhang, S. Chen, H. Valle, M. Wong, C. Austria, M. Cruz and X. Bu, J. Am. Chem. Soc., 2007, 129, 14168–14169 CrossRef CAS PubMed
.
- A. Gheorghe, B. Strudwick, D. M. Dawson, S. E. Ashbrook, S. Woutersen, D. Dubbeldam and S. Tanase, Chem. – Eur. J., 2020, 26, 13957–13965 CrossRef CAS PubMed
.
- K. Qian, X.-C. Huang, C. Zhou, X.-Z. You, X.-Y. Wang and K. R. Dunbar, J. Am. Chem. Soc., 2013, 135, 13302–13305 CrossRef CAS PubMed
.
- K. Nakabayashi, S. Chorazy, Y. Miyamoto, T. Fujimoto, K. Yazawa, D. Takahashi, B. Sieklucka and S.-I. Ohkoshi, CrystEngComm, 2016, 18, 9236–9242 RSC
.
- C. F. Macrae, I. Sovago, S. J. Cottrell, P. T. A. Galek, P. McCabe, E. Pidcock, M. Platings, G. P. Shields, J. S. Stevens, M. Towler and P. A. Wood, J. Appl. Crystallogr., 2020, 53, 226–235 CrossRef CAS PubMed
.
- M. F. A. Hendrickx, V. S. Mironov, L. F. Chibotaru and A. Ceulemans, Inorg. Chem., 2004, 43, 3142–3150 CrossRef CAS PubMed
.
- A. Dees, A. Zahl, R. Puchta, N. J. R. van Eikema Hommes, F. W. Heinemann and I. Ivanović-Burmazović, Inorg. Chem., 2007, 46, 2459–2470 CrossRef CAS PubMed
.
- V. W.-W. Yam, K. H.-Y. Chan, K. M.-C. Wong and N. Zhu, Chem. – Eur. J., 2005, 11, 4535–4543 CrossRef PubMed
.
- P. Li, J. M. Maier, J. Hwang, M. D. Smith, J. A. Krause, B. T. Mullis, S. M. S. Strickland and K. D. Shimizu, Chem. Commun., 2015, 51, 14809–14812 RSC
.
- N. Bridonneau, J. Long, J. L. Cantin, J. von Bardeleben, S. Pillet, E. E. Bendeif, D. Aravena, E. Ruiz and V. Marvaud, Chem. Commun., 2015, 51, 8229–8232 RSC
.
- M. Magott, O. Stefańczyk, B. Sieklucka and D. Pinkowicz, Angew. Chem., Int. Ed., 2017, 56, 13283–13287 CrossRef CAS PubMed
.
- X. Qi, S. Pillet, C. de Graaf, M. Magott, E.-E. Bendeif, P. Guionneau, M. Rouzières, V. Marvaud, O. Stefańczyk, D. Pinkowicz and C. Mathonière, Angew. Chem., Int. Ed., 2020, 59, 3117–3121 CrossRef CAS PubMed
.
- X. Qi, P. Guionneau, E. Lafon, S. Perot, B. Kauffmann and C. Mathonière, Magnetochemistry, 2021, 7, 97 CrossRef
.
- M. Magott, M. Reczyński, B. Gaweł, B. Sieklucka and D. Pinkowicz, J. Am. Chem. Soc., 2018, 140, 15876–15882 CrossRef CAS PubMed
.
- M. Magott, B. Gaweł, M. Sarewicz, M. Reczyński, K. Ogorzały, W. Makowski and D. Pinkowicz, Chem. Sci., 2021, 12, 9176–9188 RSC
.
- N. Ozaki, H. Tokoro, Y. Hamada, A. Namai, T. Matsuda, S. Kaneko and S.-I. Ohkoshi, Adv. Funct. Mater., 2012, 22, 2089–2093 CrossRef CAS
.
- F. J. Birk, D. Pinkowicz and K. R. Dunbar, Angew. Chem., Int. Ed., 2016, 55, 11368–11371 CrossRef CAS PubMed
.
- F. Luo, C. Yan, L. Dang, R. Krishna, W. Zhou, H. Wu, X. Dong, Y. Han, T.-L. Hu, M. O’Keeffe, L. Wang, M. Luo, R.-B. Lin and B. Chen, J. Am. Chem. Soc., 2016, 138, 5678–5684 CrossRef CAS PubMed
.
- B. Van de Voorde, B. Bueken, J. Denayer and D. De Vos, Chem. Soc. Rev., 2014, 43, 5766–5788 RSC
.
- M. Atzori, G. L. J. A. Rikken and C. Train, Chem. – Eur. J., 2020, 26, 9784–9791 CrossRef CAS PubMed
.
- M. Atzori, I. Breslavetz, K. Paillot, K. Inoue, G. L. J. A. Rikken and C. Train, J. Am. Chem. Soc., 2019, 141, 20022–20025 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, thermogravimetric analysis, powder X-ray diffraction patterns, magnetic measurements, UV-vis, and IR spectra and CIF files. CCDC 2094734 (1 right-handed), 2094735 (1 left-handed), 2094736 (1anh right-handed). For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d1cc03870b |
| This journal is © The Royal Society of Chemistry 2021 |


