Organic guest molecule induced ultrafast breathing of an epitaxially grown metal–organic framework on a self-assembled monolayer†
Abstract
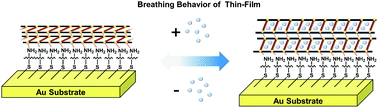
We report epitaxially grown new two-dimensional metal–organic framework (MOF) thin films on a self-assembled monolayer (SAM). We fabricated these epitaxial thin-films using stepwise layer-by-layer seeding followed by solvothermal treatment. The MOF thin films exhibit ultrafast structural flexibility (through breathing) compared to their bulk samples upon uptake of organic guest molecules.
- This article is part of the themed collections: 2021 Emerging Investigators and Celebrating the 75th Anniversary of the Korean Chemical Society (KCS)


 Please wait while we load your content...
Please wait while we load your content...