Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Organometallic small molecule kinase inhibitors – direct incorporation of Re and 99mTc into Opaganib®†
Raphael
Lengacher
 a,
Youchao
Wang
b,
Henrik
Braband
a,
Olivier
Blacque
a,
Youchao
Wang
b,
Henrik
Braband
a,
Olivier
Blacque
 a,
Gilles
Gasser
a,
Gilles
Gasser
 b and
Roger
Alberto
b and
Roger
Alberto
 *a
*a
aUniversity of Zurich, Department of Chemistry, Zurich, Switzerland. E-mail: ariel@chem.uzh.ch
bChimie ParisTech, PSL University, CNRS, Institute of Chemistry for Life and Health Sciences, Laboratory for Inorganic Chemical Biology, Paris F-75005, France
First published on 20th November 2021
Abstract
[(η5-Cp)ReI(CO)3] was incorporated into the kinase inhibitor Opaganib®. The resulting bioorganometallic complex showed a similar anti-cancer activity to Opaganib® against PC-3 cancer cells. The IC50 value for the kinase SK2 is 30x higher than that of Opaganib®. The 99mTc homologue was synthesized, completing a matched-pair for molecular theranostics.
Small molecule kinase inhibitors (SMKIs) have emerged as highly relevant compounds in the clinical treatment of cancer and central nervous system (CNS) diseases.1 This is evidenced by no less than 28 SMKIs being approved by the FDA between 2001 and 2015.2,3
Incorporating a radioactive label into SMKIs allows for the tracking of the drug's distribution inside the body, enabling cancer diagnostics by positron emission tomography (PET) or single photon emission computed tomography (SPECT) imaging, depending on the isotope employed. Furthermore, an accurate quantification of kinase expression in tumour lesions in vivo accelerates drug discovery.4 A large number of radiolabelled SMKIs have been reported so far. Most of these are labelled with the PET radionuclides 18F and 11C since the non-radioactive isotopologues contain these elements in the lead structure.5 The monitoring of tyrosine kinase inhibition requires prolonged times for allowing sufficient accumulation of the radiolabelled inhibitor, thus, radioisotopes with a longer half-life time are favourable. A number of 99mTc labelled SMKIs have been prepared, in which a chelator for coordinating Tc is appended to a SMKI.6–10 In this “pendent approach”, although the radiolabelled 99mTc complex is spatially separated from the SMKI pharmacophore, the inclusion of an appended 99mTc radiolabelled complex adversely affects pharmacokinetics and target receptor binding. Therefore, such 99mTc-radiolabelled tracers cannot be used to image SMKI distribution and site-specific accumulation in vivo. Alternatively, in the integrated approach, the radiolabelled metal complex moiety comprises an integral chemical component of the pharmacophore, and its inclusion is important for receptor binding.11,12 The less the overall structure of the inhibitor changes, the less its pharmacology is affected. Examples are artificial amino acid mimics or integrated ferrocifen,13–15 which pioneered this field.
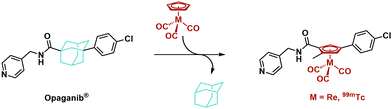
99mTc cytectrenes ([(η5-Cp)99mTcI(CO)3] complexes) have been employed as imaging agents mainly along the pendent but rarely the integrated approach.20 There are some previous examples for the integrated approach, e.g. the [(η5-C5H4-R)Re(CO)3] derivative of tamoxifen, a selective estrogen-receptor modulator by Jaouen and coworkers.16 Highly substituted rhenocene as ligands for the estrogen receptor have been presented by Katzenellenbogen and coworkers.17 The non-steriodal antiandrogen flutamide by Benny et al. is another example18 or Luyts macrocyclic peptides with integrated or pendent chelators.21,22 Some representative examples are given in Scheme 1. Not all of these examples describe both, the rhenium and the 99mTc compounds, but they align with the concept of an integrated approach. The cytectrene moiety usually replaces a phenyl ring. With metal complex-derivatives of protein kinase inhibitors, Meggers and co-workers showed that higher affinities and selectivities as compared to the organic lead structures could be obtained.19,23Scheme 1d shows staurosporine and a derived ruthenium complex. The affinity of the latter for S6K1 was substantially higher, confirming that the metal complex is essential for inhibition. We herein report the synthesis of two organometallic analogues of the SMKI Opaganib. Opaganib® comprises a bulky adamantyl group (Fig. 1). We have designed our organometallic derivatives to retain both the pyridyl and chlorophenyl groups, while the cytectrene replaces the central adamantyl. In this way, the two terminal groups are separated from each other by three carbon atoms, as in the lead Opaganib® (Fig. 1).
 | ||
| Scheme 1 Examples for the integrated approach; tamoxifen and the [(η5-C5H5)Re(CO)3] analogue (a),16 a de novo estrogen receptor ligand (b),17 a flutamide in which the complex participates in binding (c)18 and a staurosporine kinase inhibitor (d).19 | ||
Opaganib®, also known as ABC294640, is an orally available, competitive and highly selective sphingosine kinase-2 (SK2) inhibitor (Ki = 9 μM).24–26 Opaganib® accumulates in tumours and induces apoptosis.26 This accumulation makes Opaganib® an interesting lead for the development of radio imaging agents. The adamantyl moiety was replaced by a [(η5-Cp)MI(CO)3] (M = Re, 99mTc) complex (Fig. 1), which showed very similar IC50 values towards PC-3 cells, exemplifying the validity of the integrated approach.
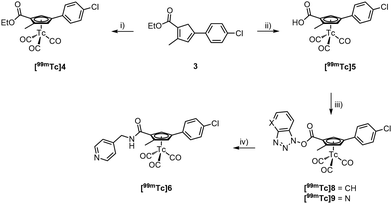
The formation of the core cyclopentadiene (Cp) synthesis followed a report of Hatanaka et al.27 We applied this procedure to the synthesis of various Cp-based building blocks over the recent years,15,28,29 however, always with alkylated but not with arylated cyclopentadienes. By reacting 2-bromo-4-chloroacetophenone (1) with the Wittig salt 227 under basic conditions, Cp 3 was obtained in 22% yield. We emphasize that this procedure is applicable to other bromo-acetyl aromatics of which numerous precursors for pharmaceuticals are commercially available; this method can thus principally be extended to other pharmaceuticals. Upon reaction of [Re2(CO)10] with 3 at high temperature, the Re complex 4 was obtained in 72% yield (see ESI†). The ester in compound 4 was hydrolysed to its carboxylate 5 with aqueous NaOH (1 M) at high temperatures in 58% yield. Subsequent amide bond formation with 4-methylaminepyridine gave the [(η5-Cp)ReI(CO)3] incorporating Opaganib® analogue 6 in good yield (67%, Scheme 2).
Single crystals were obtained for compounds 3, 4, and 6. Crystallographic details for 3 and 4 can be found in the ESI.† Single crystals of 6 (Fig. 2) were obtained by slow evaporation of a H2O/MeCN solution after being spiked with a small amount of NH4PF6. The distance between the Cp centroid and Re1 was calculated to be 1.958 Å. Re–CO bond distances are all within the same range with 1.907(4) Å (Re1–C1), 1.912(4) Å (Re1–C2), and 1.923(3) Å (Re1–C3) and Re–C–O bond angles of 175.2(3)° (Re1–C1–O1), 175.7(3)° (Re1–C2–O2), and 178.97(3)° (Re1–C3–O3). The structural properties are in line with similar complexes known in the literature (Scheme 2).15,30–36
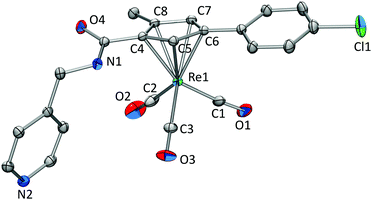 | ||
| Fig. 2 Displacement ellipsoid representation of 6. Hydrogens are omitted for clarity. Thermal ellipsoids represent 35% probability. | ||
We attempted to prepare the pure cyclopentadiene analogue of Opaganib® for activity comparison with the lead and for a direct labelling with the fac-[99mTc(CO)3]+ core. However, this was unsuccessful as 3 could not be hydrolysed. Most likely, the cyclopentadiene is deprotonated under these alkaline conditions. The resulting negative charge is delocalized, including the ester group, which leads to a fulvene-like anion. In this form, the ester carbon is inaccessible for nucleophilic attack. Approaches for acidic hydrolysis were equally unsuccessful. Thus, for preparing the homologue [99mTc]6, a post-labelling functionalization procedure had to be employed.37 Post-labelling modifications are less desired for routine application since they rely on multi-step radiosynthesis, However, for conceptual assessments it is still an option.20 Accordingly, 3 was reacted with [99mTc(H2O)3(CO)3]+ (7) in an EtOH/H2O solution. Fulvene formation is inhibited in [99mTc]4 after the formation of the cytectrenes and the ester group hydrolyses easily depending on the labelling conditions, [99mTc]4 or [99mTc]5 form in high radiochemical purity (90% RCP, resp. 80% RCP) in one step (Scheme 3).
Along peptide bond formation chemistry, [99mTc]5 was activated with DCC and HOBt or HOAt in a CH2Cl2/MeCN mixture at r.t., forming the activated ester [99mTc]8/[99mTc]9 within 30 min. Its formation was monitored by radio-HPLC. Addition of excess 4-aminomethylpyridine for 30 min at r.t. afforded [99mTc]6 with 99% RCP after HPLC purification (Fig. 3). The lipophilicity of both compounds 6 (Rt = 21.82 min) and Opaganib® (Rt = 22.90 min) is remarkably similar although, 6 is slightly more hydrophilic.
The cytotoxicity of 6 and Opaganib® towards PC3 cancer cell line (human caucasian prostate adenocarcinoma cells) and non-cancerous RPE1 cell line (retinal pigment epithelium cells) was investigated using a well-known fluorometric cell viability assay (Resazurin).38 Cisplatin was used as a control since it is a well-known metal-based anticancer drug. As shown in Table 1, Opaganib® displayed a moderate toxicity on both PC3 and RPE1 cell lines, with EC50 values of ca. 37 μM. As hypothesized, the EC50 values for 6 are similar to the ones of Opaganib® on both cell lines, demonstrating that 6 induces similar cytotoxicity to Opaganib® (Fig. 4). To furthermore confirm that 6 competitively binds to SK2, IC50 values of Opaganib and 6 towards SK2 were determined. Whereas a Ki value for Opaganib is available (vide supra), no IC50 has been reported. We found that Opaganib has an IC50 towards SK2 of 15.8 ± 3.0 μM and 6 a surprisingly low one of 0.4 ± 0.05 μM (ESI†), ca. 30x lower than Opaganib®. This is in agreement with other findings in which bioorganometallic analogues of pharmaceuticals displayed higher selectivities and affinities for receptors.39–41
In conclusion, cold Re and radioactive 99mTc were both successfully incorporated into the lead structure of the SMKI Opaganib®, representing the first examples of organometallic SMKI analogues. Furthermore, [99mTc]6 represents a 99mTc radiolabelled SMKI following a truly integrated approach. 6 possess similar activity to Opaganib® versus PC-3 cancer cells, corroborating the validity of the concept presented in this article. Compound 6 exhibits a 30x higher affinity for SK2 compared with Opaganib, exemplifying that the integration of a metal complex into a pharmaceutical does not necessarily lead to loss of activity. The post-labelling modification procedure for the 99mTc homologue of 6 was established but is not ideal for direct application. Preparation of cyclopentadienes carrying two different functionalities is not routine. The presented synthetic concept still represents a general procedure towards other, multi-functional cytectrene complexes with rhenium and 99mTc. The search for alternative routes towards other cyclopentadiene analogues is currently ongoing.
This work was financially supported by an ERC Consolidator Grant PhotoMedMet to G. G. (GA 681679) and has received support under the program Investissements d’Avenir launched by the French Government and implemented by the ANR with the reference ANR-10-IDEX-0001-02 PSL (G. G.). Y. W. thanks the China Scholarship Council for financial support. The authors recognize financial support and the contribution of the analytical services of the chemistry department at the University of Zurich.
Conflicts of interest
The authors declare no conflicts of interest.Notes and references
- P. Wu, T. E. Nielsen and M. H. Clausen, Drug Discovery Today, 2016, 21, 5–10 CrossRef CAS PubMed
.
- P. Wu, T. E. Nielsen and M. H. Clausen, Trends Pharmacol. Sci., 2015, 36, 422–439 CrossRef CAS PubMed
.
- Z. O’Brien and M. Fallah Moghaddam, Expert Opin. Drug Metab. Toxicol., 2013, 9, 1597–1612 CrossRef PubMed
.
- J. K. Willmann, N. van Bruggen, L. M. Dinkelborg and S. S. Gambhir, Nat. Rev. Drug Discovery, 2008, 7, 591–607 CrossRef CAS PubMed
.
- J. W. Hicks, H. F. VanBrocklin, A. A. Wilson, S. Houle and N. Vasdev, Molecules, 2010, 15, 8260–8278 CrossRef CAS PubMed
.
- C. Fernandes, C. Oliveira, L. Gano, A. Bourkoula, I. Pirmettis and I. Santos, Bioorg. Med. Chem., 2007, 15, 3974–3980 CrossRef CAS PubMed
.
- J. K. Lim, K. Negash, S. M. Hanrahan and H. F. VanBrocklin, J. Labelled Compd. Radiopharm., 2000, 43, 1183–1191 CrossRef CAS
.
- M. Shaul, G. Abourbeh, O. Jacobson, Y. Rozen, D. Laky, A. Levitzki and E. Mishani, Bioorg. Med. Chem., 2004, 12, 3421–3429 CrossRef CAS PubMed
.
- F. Mattner Quart, J. Nucl. Med., 2001, 45, S6 Search PubMed
.
- C. Fernandes, I. C. Santos, I. Santos, H. J. Pietzsch, J. U. Kunstler, W. Kraus, A. Rey, N. Margaritis, A. Bourkoula, A. Chiotellis, M. Paravatou-Pestsotas and I. Pirmettis, Dalton Trans., 2008, 3215–3225 RSC
.
- M. Bartholoma, J. Valliant, K. P. Maresca, J. Babich and J. Zubieta, Chem. Commun., 2009, 493–512 RSC
.
- T. Kniess, M. Laube, F. Wust and J. Pietzsch, Dalton Trans., 2017, 46, 14435–14451 RSC
.
- G. Jaouen, A. Vessieres and S. Top, Chem. Soc. Rev., 2015, 44, 8802–8817 RSC
.
- S. Gaire, B. R. Schrage and C. J. Ziegler, Inorg. Chem., 2021, 60(14), 10105–10108 CrossRef CAS PubMed
.
- R. Lengacher, J. Csucker, D. Hernandez-Valdes, B. Spingler, H. Braband and R. Alberto, Bioconjugate Chem., 2021, 32, 1393–1398 CrossRef CAS PubMed
.
- S. Top, A. Vessieres, P. Pigeon, M. N. Rager, M. Huche, E. Salomon, C. Cabestaing, J. Vaissermann and G. Jaouen, ChemBioChem, 2004, 5, 1104–1113 CrossRef CAS PubMed
.
- E. S. Mull, V. J. Sattigeri, A. L. Rodriguez and J. A. Katzenellenbogen, Bioorg. Med. Chem., 2002, 10, 1381–1398 CrossRef CAS PubMed
.
- H. He, J. E. Morely, E. Silva-Lopez, B. Bottenus, M. Montajano, G. A. Fugate, B. Twamley and P. D. Benny, Bioconjugate Chem., 2009, 20, 78–86 CrossRef CAS PubMed
.
- J. Qin, R. Rajaratnam, L. Feng, J. Salami, J. S. Barber-Rotenberg, J. Domsic, P. Reyes-Uribe, H. Liu, W. Dang, S. L. Berger, J. Villanueva, E. Meggers and R. Marmorstein, J. Med. Chem., 2015, 58, 305–314 CrossRef CAS PubMed
.
- R. Lengacher and R. Alberto, Coord. Chem. Rev., 2021, 437, 213869 CrossRef CAS
.
- J. L. Hickey, E. J. Simpson, J. Hou and L. G. Luyt, Chem. – Eur. J., 2015, 21, 568–578 CrossRef CAS PubMed
.
- D. Rosita, M. A. DeWit and L. G. Luyt, J. Med. Chem., 2009, 52, 2196–2203 CrossRef CAS PubMed
.
- E. Meggers, Curr. Opin. Chem. Biol., 2007, 11, 287–292 CrossRef CAS PubMed
.
- C. D. Britten, E. Garrett-Mayer, S. H. Chin, K. Shirai, B. Ogretmen, T. A. Bentz, A. Brisendine, K. Anderton, S. L. Cusack, L. W. Maines, Y. Zhuang, C. D. Smith and M. B. Thomas, Clin. Cancer Res., 2017, 23, 4642–4650 CrossRef CAS PubMed
.
- P. Gao, Y. K. Peterson, R. A. Smith and C. D. Smith, PLoS One, 2012, 7, e44543 CrossRef CAS PubMed
.
- K. J. French, Y. Zhuang, L. W. Maines, P. Gao, W. Wang, V. Beljanski, J. J. Upson, C. L. Green, S. N. Keller and C. D. Smith, J. Pharmacol. Exp. Ther., 2010, 333, 129 CrossRef CAS PubMed
.
- M. Hatanaka, Y. Himeda and I. Ueda, J. Chem. Soc., Perkin Trans. 1, 1993, 2269–2274 RSC
.
- R. Lengacher, H. Braband, J. Csucker and R. Alberto, Eur. J. Inorg. Chem., 2021, 1611–1614 CrossRef CAS
.
- A. Frei, B. Spingler and R. Alberto, Chem. – Eur. J., 2018, 24, 10156–10164 CrossRef CAS PubMed
.
- Z. Li, M. Cui, J. Dai, X. Wang, P. Yu, Y. Yang, J. Jia, H. Fu, M. Ono, H. Jia, H. Saji and B. Liu, J. Med. Chem., 2013, 56, 471–482 CrossRef CAS PubMed
.
- J. Jia, M. Cui, J. Dai and B. Liu, Dalton Trans., 2015, 44, 6406–6415 RSC
.
- J. Jia, K. Zhou, J. Dai, B. Liu and M. Cui, Eur. J. Med. Chem., 2016, 124, 763–772 CrossRef CAS PubMed
.
- X. Li, S. Chen, Z. Liu, Z. Zhao and J. Lu, J. Organomet. Chem., 2018, 871, 28–35 CrossRef CAS
.
- S. Masi, S. Top, L. Boubekeur, G. Jaouen, S. Mundwiler, B. Spingler and R. Alberto, Eur. J. Inorg. Chem., 2004, 2013–2017, DOI:10.1002/ejic.200300731
.
- D. Li, Y. Chen, X. Wang, W. Deuther-Conrad, X. Chen, B. Jia, C. Dong, J. Steinbach, P. Brust, B. Liu and H. Jia, J. Med. Chem., 2016, 59, 934–946 CrossRef CAS PubMed
.
- H. W. Peindy N’Dongo, P. D. Raposinho, C. Fernandes, I. Santos, D. Can, P. Schmutz, B. Spingler and R. Alberto, Nucl. Med. Biol., 2010, 37, 255–264 CrossRef PubMed
.
- T. W. Spradau, W. B. Edwards, C. J. Anderson, M. J. Welch and J. A. Katzenellenbogen, Nucl. Med. Biol., 1999, 26, 1–7 CrossRef CAS PubMed
.
- S. A. Ahmed, R. M. Gogal and J. E. Walsh, J. Immunol. Methods, 1994, 170, 211–224 CrossRef CAS PubMed
.
- D. Can, B. Spingler, P. Schmutz, F. Mendes, P. Raposinho, C. Fernandes, F. Carta, A. Innocenti, I. Santos, C. T. Supuran and R. Alberto, Angew. Chem., Int. Ed., 2012, 51, 3354–3357 CrossRef CAS PubMed
.
- E. Meggers, Chem. Commun., 2009, 1001–1010, 10.1039/b813568a
.
- F. W. Monnard, T. Heinisch, E. S. Nogueira, T. Schirmer and T. R. Ward, Chem. Commun., 2011, 47, 8238–8240 RSC
.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2094782–2094784. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d1cc03678e |
| This journal is © The Royal Society of Chemistry 2021 |