Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Glassy behaviour of mechanically amorphised ZIF-62 isomorphs†
Michael F.
Thorne
 a,
Adam F.
Sapnik
a,
Adam F.
Sapnik
 a,
Lauren N.
McHugh
a,
Lauren N.
McHugh
 a,
Alice M.
Bumstead
a,
Alice M.
Bumstead
 a,
Celia
Castillo-Blas
a,
Celia
Castillo-Blas
 a,
Dean S.
Keeble
a,
Dean S.
Keeble
 b,
Maria
Diaz Lopez
b,
Phillip A.
Chater
b,
Maria
Diaz Lopez
b,
Phillip A.
Chater
 b,
David A.
Keen
b,
David A.
Keen
 c and
Thomas D.
Bennett
c and
Thomas D.
Bennett
 *a
*a
aDepartment of Materials Science and Metallurgy, University of Cambridge, 27 Charles Babbage Road, Cambridge, Cambridgeshire CB3 0FS, UK. E-mail: tdb35@cam.ac.uk
bDiamond Light Source Ltd, Diamond House, Harwell Campus, Didcot, Oxfordshire OX11 0DE, UK
cISIS Facility, Rutherford Appleton Laboratory, Harwell Campus, Didcot, Oxfordshire OX11 0QX, UK
First published on 16th August 2021
Abstract
Zeolitic imidazolate frameworks (ZIFs) can be melt-quenched to form glasses. Here, we present an alternative route to glassy ZIFs via mechanically induced amorphisation. This approach allows various glassy ZIFs to be produced in under 30 minutes at room temperature, without the need for melt-quenching.
Metal–organic frameworks (MOFs) are hybrid materials consisting of inorganic nodes connected through organic linkers.1 Major applications proposed for MOFs include gas storage, separation,2 and heterogeneous catalysis.3
Several members of a sub-category of MOFs, zeolitic imidazolate frameworks (ZIFs), have been shown to undergo melting, and form liquids of identical composition to their crystalline parent materials.4 ZIF-62 [M(Im)2−x(bIm)x] where x ≥ 0.05, M = Zn2+ or Co2+, Im = [C3H3N2]− and bIm = [C7H5N2]−, exhibits a melting event at ca. 430 °C to form a ZIF liquid.5 Quenching of this liquid yields a glass (agZIF-62, ag = melt-quenched glass), with a continuous random network topology akin to that of amorphous SiO2.5–9
A mechanochemical (solid-state milling) approach to crystalline ZIF-62 has recently been reported.10 This, unlike conventional solvothermal synthesis, allows for direct stoichiometric control over both ligand and metal ratios.10 This facilitates control over melting points (Tm) and subsequent glass transition temperatures (Tg), i.e. second order phase transitions, in which an amorphous material transforms to a liquid-like state upon heating.
The formation of ZIF glasses by melt-quenching is limited by the necessity for appropriate crystalline framework densities and thermally stable linkers.11 The production of glassy materials via mechanical force has been demonstrated for metallic alloy glasses,12 organic pharmaceuticals,13 and two dimensional coordination polymers.14 For example, the two dimensional coordination polymer system [M(1,2,4-triazole)2(H2PO4)2] (where M = Cd2+, Cr2+, or Mn2+) can be vitrified by either melt-quenching or by the application of mechanical force.15
Mechanical amorphisation of ZIFs has been studied for archetypal materials such as ZIF-4 [Zn(Im)2], and ZIF-8 [Zn(mIm)2] mIm = [C4H5N2]−, although a detailed investigation into the thermal response of mechanically amorphised systems has yet to be performed.16,17 Here, we investigate the mechanochemical amorphisation of ZIF-62 and its subsequent glass like behaviour.
We adapted the previously reported mechanosynthesis of ZIF-62 to access a series of materials with increasing bIm![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Im ratio.10,18 The maximum reported bIm:Im ratio in prior mechanochemical syntheses of ZIF-62 was [Zn(Im)1.75(bIm)0.25],10 whereas that for solution phase synthesis was [Zn(Im)1.65(bIm)0.35].5,19 Here, crystalline [Zn(Im)1.70(bIm)0.30] was successfully formed after 30 minutes of grinding. However, further increasing the bIm content by 0.05 resulted in an amorphous product, with the formula am[Zn(Im)1.65(bIm)0.35] (am = mechanically amorphised), being produced after 30 minutes of grinding (Fig. 1a and Fig. S1, ESI†).
Im ratio.10,18 The maximum reported bIm:Im ratio in prior mechanochemical syntheses of ZIF-62 was [Zn(Im)1.75(bIm)0.25],10 whereas that for solution phase synthesis was [Zn(Im)1.65(bIm)0.35].5,19 Here, crystalline [Zn(Im)1.70(bIm)0.30] was successfully formed after 30 minutes of grinding. However, further increasing the bIm content by 0.05 resulted in an amorphous product, with the formula am[Zn(Im)1.65(bIm)0.35] (am = mechanically amorphised), being produced after 30 minutes of grinding (Fig. 1a and Fig. S1, ESI†).
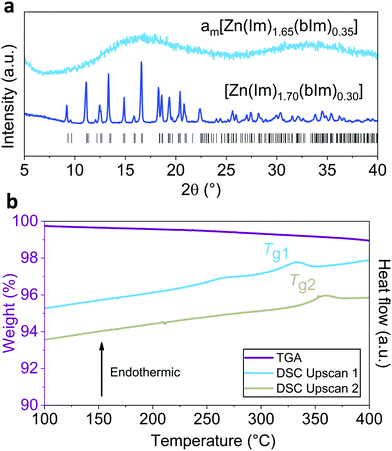 | ||
| Fig. 1 (a) Powder X-ray diffraction (PXRD) of am[Zn(Im)1.65(bIm)0.35], [Zn(Im)1.70(bIm)0.30] with calculated Bragg positions for ZIF-62. (b) TGA-DSC scans showing thermal response of am[Zn(Im)1.65(bIm)0.35] during heating upscan 1 and 2. Full TGA and DSC scans are available, including a DSC upscan of unevacuated am[Zn(Im)1.65(bIm)0.35] to identify solvent loss events (Fig. S2 and S3, ESI†). | ||
Thermogravimetric analysis (TGA), differential scanning calorimetry (DSC), optical microscopy, and thermomechanical analysis (TMA) were conducted on am[Zn(Im)1.65(bIm)0.35] (Fig. 1) (Fig. S2–S5, ESI†). Interestingly, upon a first heating upscan in the DSC, am[Zn(Im)1.65(bIm)0.35] exhibited a glass transition (Tg1 = 318 °C). This Tg1 is evidence of glassy behaviour and highlights the different thermal response of this material when compared to crystalline ZIF-62. Crystalline ZIF-62 displays a Tm on a first heating scan, at significantly higher temperatures than Tg1 seen here.10 To further investigate the glassy behaviour of am[Zn(Im)1.65(bIm)0.35], the softening temperature (Ts) was determined from TMA. This confirmed a brittle to soft transition at Tg1, with the Ts for am[Zn(Im)1.65(bIm)0.35] being 322 °C (Fig. S4, ESI†). This amZIF-62 sample has a completely different thermal history to melt-quenched ZIF glasses seen previously,10 or for the melt-quenched ag[Zn(Im)1.70(bIm)0.30] reported here (Fig. S6–S10, ESI†).5,10Tg values for melt-quenched systems are usually reported from the second DSC heating scan, as the first heating scan is required to melt the crystalline material.10
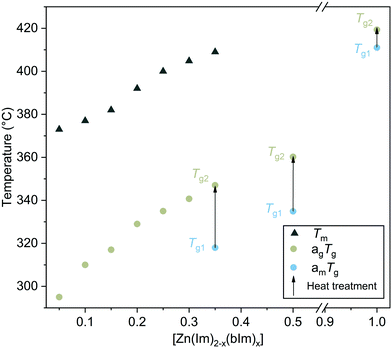
Given the dependence of Tg on thermal history,20 a second heat treatment to 450 °C was performed on am[Zn(Im)1.65(bIm)0.35] to give the material equivalent thermal history to melt-quenched systems.10 This allowed for a more consistent comparison between am[Zn(Im)1.65(bIm)0.35] and melt-quenched glasses. After this second heat treatment, a second glass transition temperature (Tg2) was observed (Fig. 1b). This Tg2 = 347 °C continues the trend as seen for melt-quenched ag[Zn(Im)2−x(bIm)x] systems previously reported experimentally (Fig. 2),19 and predicted from topological constraint theory.21,22 Consequently, after initial heat treatment, am[Zn(Im)1.65(bIm)0.35] may now be considered equivalent to a melt-quenched glass (Fig. 2). From this point onwards, am[Zn(Im)1.65(bIm)0.35] after initial heat treatment will therefore be denoted as ag[Zn(Im)1.65(bIm)0.35], for consistency.
The different Tg values for am[Zn(Im)1.65(bIm)0.35], before and after heat treatment, indicate structural changes may have occurred during heating. A possible property of the material which may change upon heat treatment is its skeletal density. The He pycnometric densities of am[Zn(Im)1.65(bIm)0.35] and ag[Zn(Im)1.65(bIm)0.35] (i.e. before and after heat treatment) are similar, with values of 1.584 (±0.002) g cm−3 and 1.576 (±0.003) g cm−3 respectively (Fig. S11, ESI†). This suggests the increase in Tg is not due to a significant change in skeletal density.
To investigate whether pore volume differences can be observed between am[Zn(Im)1.65(bIm)0.35] and ag[Zn(Im)1.65(bIm)0.35] CO2 gas sorption experiments were performed (Fig. S12, ESI†). This is important as the free space available in the pores of the materials may affect the steric freedom of the linkers, and therefore influence Tg.23 Both materials showed almost identical isotherms, with a maximum CO2 uptake of 23.6 cm3 g−1 in both cases. Further to this, the pore widths were determined to be 3.6 Å in both cases, and maximum pore volume of 0.043 cm3 g−1 was found for am[Zn(Im)1.65(bIm)0.35] and ag[Zn(Im)1.65(bIm)0.35]. These similarities in pore size and volume rule out changes in pore space causing changes in Tg. Additionally, the CO2 isotherms do not show a large difference in hysteresis, and as no mass loss from potential Zn–CO2/Zn–H2O sites is seen in TGA, it is unlikely that a substantial number of defects caused by severed Zn–N bonds are present in am[Zn(Im)1.65(bIm)0.35] (Fig. S2, ESI†).
The temperature difference between Tg1 and Tg2 is akin to the difference between Tm in mechanochemically and solvothermally produced crystalline ZIF-62 systems with identical chemical composition. For example, the Tm offset of mechanochemically produced crystalline ZIF-62 is lower than solvothermally produced ZIF-62 in various different reports, yet the resultant Tgs are identical.5,10,24 This is assumed to be a particle size effect seen when performing DSC at a constant heating rate, on the same material with different particle sizes.
Here, scanning electron microscopy (SEM) revealed the production of nanoscale am[Zn(Im)1.65(bIm)0.35] particles directly after mechanosynthesis. This was followed by their coalescence to form connected globular particles larger than 10 μm, after heat treatment to form ag[Zn(Im)1.65(bIm)0.35] (Fig. S13, ESI†). Therefore, the difference in Tg1 and Tg2 is likely a kinetic effect seen in DSC caused by particle agglomeration, rather than a thermodynamic lowering of Tg caused by structural changes in the amorphous materials. To further investigate the change from Tg1 to Tg2, with particle agglomeration, a set of DSC experiments on am[Zn(Im)1.65(bIm)0.35] were performed (Fig. S14 and S15, ESI†). These results show Tg2 is only dependant on maximum temperature during heating, and therefore support the hypothesis that particle agglomeration affects Tg2.
ZIF-62 is a compositional series [Zn(Im)2−x(bIm)x], with the lower limit of x = 0.05.5,10 However, the upper limit of x has never been identified.25 To further investigate mechanically induced amorphisation, and determine an upper limit of x for amZIF-62, the bIm content was increased in the mechanosynthesis. This yielded am[Zn(Im)1.5(bIm)0.5] and am[Zn(Im)1.0(bIm)1.0], with attempts to yield x > 1.0 resulting in partial ZIF-7 synthesis (Fig. S16–S18, ESI†). These high bIm content amZIF-62 systems also yielded a Tg1 and subsequent higher Tg2 values after heat treatment to form their equivalent agZIF-62 systems. These Tg2 values also agree with an increase of Tg with bIm content (Fig. 2 and Fig. S19–S24, ESI†). The Tg1 values for am[Zn(Im)1.50(bIm)0.50] and am[Zn(Im)1.0(bIm)1.0] were 335 °C and 411 °C respectively. The Tg2 values were of the respective agZIFs were 360 °C and 419 °C. The Tg2 value for ag[Zn(Im)1.0(bIm)1.0] represents by far the highest Tg value ever measured for a ZIF glass, driven by its high bIm content.23
To understand the production of amZIFs, an ex situ kinetics study on am[Zn(Im)1.65(bIm)0.35] formation was performed (Fig. S25 and S26, ESI†). This showed initial production of crystalline [Zn(Im)1.65(bIm)0.35]. The [Zn(Im)1.65(bIm)0.35] remained crystalline until mechanically induced amorphisation after 22 minutes, with no further change in the diffraction pattern after 30 minutes.
We note that crystalline ZIFs with the cag topology, such as ZIF-62 and ZIF-4, have 4 and 8-membered rings of ZnN4 tetrahedra.26 In ZIF-4 there are no bIm linkers, however, when bIm linkers are included as in ZIF-62, the bulky six membered aromatic rings of bIm may exclude DMF from the pore space of the ZIF (Fig. S27, ESI†). When the bIm content is increased, such as in the case of [Zn(Im)1.65(bIm)0.35], a larger amount of bIm linkers will occupy the free space within the pores. We hypothesise that increasing the bIm content of ZIF-62 is correlated with a reduction in DMF content within the ZIF structure. This was confirmed by TGA measurements, which showed a lower mass loss due to solvent evaporation from ZIF-62 samples with a higher bIm content (Fig. S28, ESI†). The lower level of DMF per mol of Zn in [Zn(Im)1.65(bIm)0.35], with respect to [Zn(Im)1.75(bIm)0.25] (19.74 g mol−1vs. 26.51 g mol−1), may reduce the ability of the material to withstand mechanical collapse. This has previously been found for solvated and fully evacuated ZIFs.27 Further to this, DSC was performed on crystalline [Zn(Im)1.65(bIm)0.35] after 15 minutes of mechanosynthesis (Fig. S29, ESI†). This showed a Tm of 409 °C, (Fig. 2) and Tg of 346 °C. The enthalpy of fusion increases with bIm content in ZIF-62 systems, therefore the enthalpy of fusion for crystalline [Zn(Im)1.65(bIm)0.35] was expected to be higher than [Zn(Im)1.70(bIm)0.30].24 However here, the enthalpy of fusion for crystalline [Zn(Im)1.65(bIm)0.35] was far lower than [Zn(Im)1.70(bIm)0.30] (7.57 J g−1vs. 12.57 J g−1) indicating a lower level of crystallinity in [Zn(Im)1.65(bIm)0.35]. Therefore, we hypothesise that the amorphisation of [Zn(Im)1.65(bIm)0.35] is due to lower DMF content in the pores, and also lower crystallinity with respect to other mechanosynthetic ZIF-62 systems.
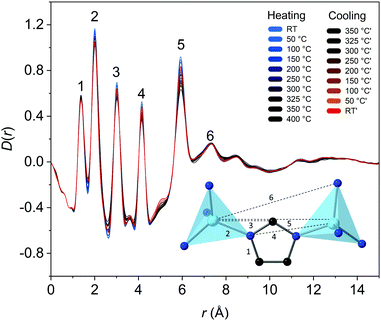
To investigate the local and long range structure in am[Zn(Im)1.65(bIm)0.35] upon heat treatment to form ag[Zn(Im)1.65(bIm)0.35], variable temperature pair distribution function (VT PDF) data was collected (Fig. 3 and Fig. S30–S36, ESI†), as well as calculations to aid in the assignment of the peaks (Fig. S34–S36, ESI†). To assist in the refinement of the structure against the experimental D(r), modifications were made to a previously published ZIF-62 CIF (see methods).10 The calculated D(r) showed a good agreement with experimental data for [Zn(Im)1.65(bIm)0.35] at room temperature (Fig. S34, ESI†), and the exported structure showed a chemically sensible unit cell (Fig. S35, ESI†). From this, the weighted partial PDF correlations from individual atom–atom pairs, gij(r), can be obtained.28 From these correlations, D(r) peak assignments can be made (Fig. S36, ESI†).
Upon heat treatment to 400 °C, there was no evidence of crystallisation, with no evidence of long-range order observed at any temperature in the D(r) (Fig. 3), or sharp Bragg peaks in the S(Q) (Fig. S33, ESI†). The VT PDF highlights how similar the amZIF and agZIF are on a structural level, as no major changes before and after heat treatment can be seen in the D(r) or S(Q).
To further illustrate mechanically induced amorphisation in ZIFs, a similar process of increasing large linker content for the glass-forming ZIF-UC-5 compositional series, [Zn(Im)2−x(ClbIm)x] (where ClbIm = [C7H4ClN2]−) was performed (Fig. S37–S40, ESI†).10,29 Interestingly, the switch from forming crystalline ZIFs to producing amZIFs occurred at x = 0.30 as opposed to x = 0.35 in the case ZIF-62 (Fig. S41, ESI†). The sterically larger ClbIm in ZIF-UC-5 may exclude a greater amount of solvent from the pores of the ZIF-UC-5 relative to bIm in ZIF-62.30 This may be the reason for the production of am[Zn(Im)1.70(ClbIm)0.30] when the equivalent ZIF-62 system remains crystalline after 30 minutes of grinding. Further to this, am[Zn(Im)1.65(ClbIm)0.35] was formed after 30 minutes of mechanosynthesis.
An ex situ kinetics study of am[Zn(Im)1.65(ClbIm)0.35] formation revealed a similar trend to am[Zn(Im)1.65(bIm)0.35] (Fig. S41, ESI†). As for am[Zn(Im)1.65(bIm)0.35], am[Zn(Im)1.65(ClbIm)0.35] was amorphous after 30 minutes of grinding after going through an intermediate cag topology crystalline state.
Thermal analysis confirmed the glassy behaviour of both amZIF-UC-5 samples (Fig. S42–S47, ESI†). Tg1s of 306 °C and 303 °C for am[Zn(Im)1.65(ClbIm)0.35] and am[Zn(Im)1.70(ClbIm)0.30] were found respectively. Heat treatment of both samples at 450 °C however yielded Tg2s of 324 °C and 321 °C, i.e. the expected Tg values for melt-quenched glasses with these compositions, based on the trend in Tg values as a function of the linker ratio (Fig. S46, ESI†). TMA of am[Zn(Im)1.65(ClbIm)0.35] indicated a Ts of 332 °C, further confirming the glassy behaviour of amZIF-UC-5 (Fig. S47, ESI†). As with amZIF-62, PDF analysis showed the retention of the Zn–Im–Zn unit upon amorphisation and loss of long-range order, in both amZIF-UC-5 samples (Fig. S48–S51, ESI†).
In conclusion, we demonstrate formation of amorphous ZIF-62 and ZIF-UC-5 directly by mechanochemical reaction. These amZIFs exhibit Tgs upon heat treatment, even though they have not been melt-quenched. This presents an alternate route to glassy ZIFs which circumvents the requirement to melt-quench a crystalline material to form a glass. These amZIFs have Tg values lower than expected, however heat treating them to agglomerate particles, results in Tg values consistent with agZIFs. Further to this the upper limit of bIm content in amZIF-62 was determined. By formation of am[Zn(Im)1.0(bIm)1.0], the highest bIm content ZIF glass to date, we have extended the agZIF-62 chemical space up to a composition of [Zn(Im)2−x(bIm)x] where 0.05 ≤ x ≤ 1 and produced the ZIF glass with the highest Tg identified so far. This mechanochemical amorphisation method to form glassy ZIFs demonstrates a route to a wider variety of glass-forming systems not seen previously and removes the requirement for melt-quenching crystalline materials.
Conflicts of interest
There are no conflicts to declare.References
- H.-C. Zhou, J. R. Long and O. M. Yaghi, Chem. Rev., 2012, 112, 673–674 CrossRef CAS PubMed.
- B. Li, H. M. Wen, W. Zhou and B. Chen, J. Phys. Chem. Lett., 2014, 5, 3468–3479 CrossRef CAS PubMed.
- H. X. Zhang, M. Liu, T. Wen and J. Zhang, Coord. Chem. Rev., 2016, 307, 255–266 CrossRef CAS.
- T. D. Bennett, J. C. Tan, Y. Yue, E. Baxter, C. Ducati, N. J. Terrill, H. H. M. Yeung, Z. Zhou, W. Chen, S. Henke, A. K. Cheetham and G. N. Greaves, Nat. Commun., 2015, 6, 1–7 Search PubMed.
- L. Frentzel-Beyme, M. Kloß, P. Kolodzeiski, R. Pallach and S. Henke, J. Am. Chem. Soc., 2019, 141, 12362–12371 CrossRef CAS PubMed.
- T. To, S. S. Sørensen, M. Stepniewska, A. Qiao, L. R. Jensen, M. Bauchy, Y. Yue and M. M. Smedskjaer, Nat. Commun., 2020, 11, 1–9 Search PubMed.
- C. Zhou, M. Stepniewska, L. Longley, C. W. Ashling, P. A. Chater, D. A. Keen, T. D. Bennett and Y. Yue, Phys. Chem. Chem. Phys., 2018, 20, 18291–18296 RSC.
- A. Qiao, H. Tao, M. P. Carson, S. W. Aldrich, L. M. Thirion, T. D. Bennett, J. C. Mauro and Y. Yue, Opt. Lett., 2019, 44, 1623 CrossRef CAS PubMed.
- M. A. Ali, J. Ren, T. Zhao, X. Liu, Y. Hua, Y. Yue and J. Qiu, ACS Omega, 2019, 4, 12081–12087 CrossRef CAS PubMed.
- M. F. Thorne, M. L. R. Gómez, A. M. Bumstead, S. Li and T. D. Bennett, Green Chem., 2020, 22, 2505–2512 RSC.
- R. Gaillac, P. Pullumbi and F. X. Coudert, J. Phys. Chem. C, 2018, 122, 6730–6736 CrossRef CAS.
- L. C. Zhang, Z. Q. Shen and J. Xu, Mater. Sci. Eng., A, 2005, 394, 204–209 CrossRef.
- N. S. Trasi and S. R. Byrn, AAPS PharmSciTech, 2012, 13, 772–784 CrossRef CAS PubMed.
- W. Chen, S. Horike, D. Umeyama, N. Ogiwara, T. Itakura, C. Tassel, Y. Goto, H. Kageyama and S. Kitagawa, Angew. Chem., Int. Ed., 2016, 55, 5195–5200 CrossRef CAS PubMed.
- W. Chen, S. Horike, D. Umeyama, N. Ogiwara, T. Itakura, C. Tassel, Y. Goto, H. Kageyama and S. Kitagawa, Angew. Chem., Int. Ed., 2016, 55, 5195–5200 CrossRef CAS PubMed.
- T. D. Bennett, S. Cao, J. C. Tan, D. A. Keen, E. G. Bithell, P. J. Beldon, T. Friscic and A. K. Cheetham, J. Am. Chem. Soc., 2011, 133, 14546–14549 CrossRef CAS PubMed.
- S. Cao, T. D. Bennett, D. A. Keen, A. L. Goodwin and A. K. Cheetham, Chem. Commun., 2012, 48, 7805–7807 RSC.
- A. M. Bumstead, M. F. Thorne and T. D. Bennett, Faraday Discuss., 2021, 225, 210–225 RSC.
- L. Frentzel-Beyme, M. Kloß, R. Pallach, S. Salamon, H. Moldenhauer, J. Landers, H. Wende, J. Debus and S. Henke, J. Mater. Chem. A, 2019, 7, 985–990 RSC.
- Y. Zhang, R. D. Adams and L. F. M. Da Silva, J. Adhes., 2014, 90, 327–345 CrossRef CAS.
- Y. Yang, C. J. Wilkinson, K. H. Lee, K. Doss, T. D. Bennett, Y. K. Shin, A. C. T. Van Duin and J. C. Mauro, J. Phys. Chem. Lett., 2018, 9, 6985–6990 CrossRef CAS PubMed.
- M. L. Ríos Gómez, G. I. Lampronti, Y. Yang, J. C. Mauro and T. D. Bennett, Dalton Trans., 2020, 49, 850–857 RSC.
- A. M. Bumstead, M. L. Ríos Gómez, M. F. Thorne, A. F. Sapnik, L. Longley, J. M. Tuffnell, D. S. Keeble, D. A. Keen and T. D. Bennett, CrystEngComm, 2020, 22, 3627–3637 RSC.
- A. Qiao, T. D. Bennett, H. Tao, A. Krajnc, C. M. Doherty, A. W. Thornton, J. C. Mauro, G. N. Greaves and Y. Yue, Sci. Adv., 2018, 4, 1–8 CrossRef PubMed.
- V. Nozari, C. Calahoo, L. Longley, T. D. Bennett and L. Wondraczek, J. Chem. Phys., 2020, 153, 204501 CrossRef CAS PubMed.
- R. N. Widmer, G. I. Lampronti, S. Chibani, C. W. Wilson, S. Anzellini, S. Farsang, A. K. Kleppe, N. P. M. Casati, S. G. Macleod, S. A. T. Redfern, F. X. Coudert and T. D. Bennett, J. Am. Chem. Soc., 2019, 141, 9330–9337 CrossRef PubMed.
- E. F. Baxter, T. D. Bennett, A. B. Cairns, N. J. Brownbill, A. L. Goodwin, D. A. Keen, P. A. Chater, F. Blanc and A. K. Cheetham, Dalton Trans., 2016, 45, 4258–4268 RSC.
- D. A. Keen and T. D. Bennett, Phys. Chem. Chem. Phys., 2018, 20, 7857–7861 RSC.
- J. Hou, M. L. Ríos Gómez, A. Krajnc, A. McCaul, S. Li, A. M. Bumstead, A. F. Sapnik, Z. Deng, R. Lin, P. A. Chater, D. S. Keeble, D. A. Keen, D. Appadoo, B. Chan, V. Chen, G. Mali and T. D. Bennett, J. Am. Chem. Soc., 2020, 142, 3880–3890 CrossRef CAS PubMed.
- J. Yang, Y. B. Zhang, Q. Liu, C. A. Trickett, E. Gutiérrez-Puebla, M. Á. Monge, H. Cong, A. Aldossary, H. Deng and O. M. Yaghi, J. Am. Chem. Soc., 2017, 139, 6448–6455 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1cc03469c |
| This journal is © The Royal Society of Chemistry 2021 |