Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Mechanistic studies of reactive oxygen species mediated electrochemical radical reactions of alkyl iodides†
Tsz-Kan
Ma
*,
Diyuan
Li
 and
Jonathan D.
Wilden
and
Jonathan D.
Wilden
 *
*
Department of Chemistry, University College London, 20 Gordon Street, London, WC1H 0AJ, UK. E-mail: j.wilden@ucl.ac.uk
First published on 27th July 2021
Abstract
Mechanistic studies of a reactive oxygen species mediated electrochemical radical reaction of alkyl iodides are described. Hydroxyl radicals and ozone are identified to be the active species involved in the formation of alkyl radicals under mildly reducing potential (−1.0 V vs. Ag QRE) in buffered acidic conditions (pH 3.6).
Carbon-centered radicals are important synthetic intermediates in organic synthesis. To date, the generation of alkyl radicals heavily relies on the homolytic cleavage of labile carbon–halogen (C–X) bonds. In particular, n-tributyltin hydride and azobisisobutyronitrile (AIBN) mediated radical chain reaction of alkyl halides is the dominant approach to produce predictable C-centered radicals chemoselectively under mild conditions.1 Although organotin reagents have been proven to be extremely versatile to construct carbon–carbon (C–C) bonds, the substantial toxicity of organotin compounds has limited their large-scale applications. To circumvent this issue, alternative tin-free radical chain reaction initiators such as organoboranes and thiols have been employed but their efficiency and scope are often limited.2,3
Recent advances in photocatalysed processes provides alternative approaches to generate C-centered radicals, representing a significant step to move away from using any toxic organotin reagents.4 On the other hand, synthetic organic electrochemistry has also gained popularity as an alternative method to manipulate organic compounds via direct addition or removal of electrons to generate reactive radical species.5 However, direct electrochemical activation of alkyl halides via reduction has limited synthetic utility due to the requirement of highly reducing potentials.
Recently, our group has developed a highly efficient methodology to activate alkyl iodides with the use of air and electricity as the promotors (Scheme 1).6 The classic Giese reaction could be carried out electrochemically under mildly reducing conditions (−1.0 V vs. Ag QRE) in semi-aqueous media with inexpensive graphite electrodes. Molecular oxygen is selectively activated to mediate the radical reactions of alkyl iodides. Herein, we report results of our studies into the mechanism of this intriguing and novel reaction sequence.
We first sought to determine the active initiator derived from molecular oxygen which starts the reaction sequence. According to our previous studies, the presence of molecular oxygen and water is essential for the reaction to occur.6 We originally postulated that hydroxyl radicals were involved in the activation of the iodide, however, subsequent experiments have suggested that an alternative mediator must also be involved. Accordingly, we wondered if the agent was ozone. To test our hypothesis that ozone could be the oxidant to activate the alkyl iodide, a sample of neat isopropyl iodide was subjected to a constant stream of ozone and it quickly turned brown. After that, this sample was exposed to a reducing potential (constant potential, 1.0 V vs. Ag wire quasi-reference electrode, graphite rod working electrode, divided cell) in an acidic acetonitrile-water solution (pH 2) containing phenyl vinyl sulfone under an inert atmosphere saturated with argon (Scheme 2). Gratifyingly, the ozonised alkyl iodide was fully consumed and reacted with phenyl vinyl sulfone to give the corresponding alkyl sulfone (74%). It is postulated that ozonation of isopropyl iodide produced an unstable trioxide intermediate, which decomposes into the corresponding alkyl radical, IO˙ radical and molecular oxygen to initiate the radical reaction.7,8 The proposed trioxide intermediate is analogous to the reaction between aqueous ozone with halide anions as well as alkanes.7–10 The necessity of molecular oxygen and water in the reaction vessel could be explained by the requirement of ozone production electro-catalytically. The formation of ozone relies on the reaction between adsorbed hydroxyl free radicals and molecular oxygen at the anode (eqn (3a)–(3d)) as direct oxidation of water to ozone requires a much higher potential (E° = 1.51 V) and is unlikely to occur under the operating potential (Scheme 3).11 To further validate this hypothesis, excess ethyl vinyl ether was added to the anodic chamber to quench the ozone generated from the anode where another sample of isopropyl iodide and phenyl vinyl sulfone were subjected to the standard conditions in the cathodic chamber. Indeed, addition of ethyl vinyl ether has completely terminated the reaction and the substrates are fully recovered. In addition, subjecting anethole to the reaction condition at the anodic chamber resulted in the formation of the corresponding 4-methoxybenzaldehyde (2%) and 1-(4-methoxyphenyl)propane-1,2-diol (30%).
The electrochemical radical reaction of alkyl iodide could also be extended to intramolecular reductive cyclisation (Table 1). When alkyl iodide 1 was subjected to a mild reducing potential (constant potential, 1.0 V vs. Ag wire quasi-reference electrode, graphite rod working electrode, divided cell) in an acidified MeCN–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, pH 1) solution, it was reductively cyclised into the corresponding pyrrolidine 2 (31%) with N-allyltosylamide 3 (23%) as a by-product (Table 1, entry 1). N-Allyltosylamide 3 is likely to be produced via β-elimination from an anionic intermediate, terminated by the abstraction of the hydrogen atom from the solvent.12 The effect of pH was also examined by adjusting the pH of the solvent with various buffer solutions. It was found that optimal conversion occurred (Table 1, entry 2) at pH 3.6 and no reaction occurred at pH 7.
1, pH 1) solution, it was reductively cyclised into the corresponding pyrrolidine 2 (31%) with N-allyltosylamide 3 (23%) as a by-product (Table 1, entry 1). N-Allyltosylamide 3 is likely to be produced via β-elimination from an anionic intermediate, terminated by the abstraction of the hydrogen atom from the solvent.12 The effect of pH was also examined by adjusting the pH of the solvent with various buffer solutions. It was found that optimal conversion occurred (Table 1, entry 2) at pH 3.6 and no reaction occurred at pH 7.
| Entry | Solvent | pH | Time, h | 2,a % | 3,a % |
|---|---|---|---|---|---|
| a Isolated yield. b pH adjusted with HCl. c pH adjusted with acetate buffer. d pH adjusted with phosphate buffer. e Alkyl iodide 1 was fully recovered. | |||||
| 1 | MeCN–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
1b | 24 | 31 | 23 |
| 2 | MeCN–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
3.6c | 6 | 40 | 37 |
| 3 | MeCN–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
5.7c | 24 | Trace | 33 |
| 4 | MeCN–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
7d | 24 | N/Re | N/Re |
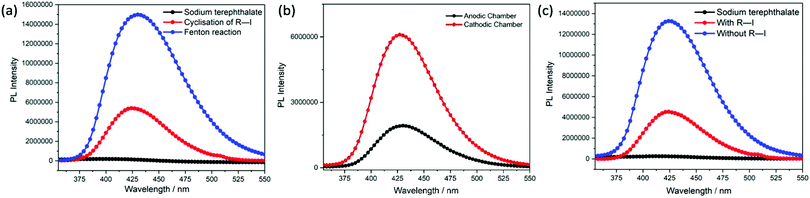
To further investigate the role of reactive oxygen species in the reaction, sodium terephthalate was used as the molecular fluorescence probe for the detection of hydroxyl radicals.13 Sodium terephthalate is a non-fluorescent compound that would react with hydroxyl radicals to form a fluorescent aromatic hydroxylated product, namely sodium 2-hydroxyterephthalate, that is known to show a characteristic emission at 430 nm.14 Therefore, sodium terephthalate was introduced to the reaction mixture under different conditions to detect the presence of hydroxyl radicals.
First, a classic Fenton reaction was carried out in the presence of this molecular probe to validate this approach.15 An aliquot of the reaction mixture was extracted, and the fluorescence emission was recorded. By comparing that emission spectrum (Fig. 1a, blue curve) with the emission spectrum of sodium terephthalate (Fig. 1a, black curve), hydroxyl radicals were detected, giving rise to the intense fluorescence emission at 430 nm. By applying this technique to the cathodic chamber of the reaction vessel where radical cyclisation of alkyl iodide 1 occurred, the same emission was observed (Fig. 1a, red curve), confirming the presence of hydroxyl radicals in the reaction mixture.
With this encouraging result in hand, the focus was switched to examine the origin of hydroxyl radicals in the reaction vessel (Fig. 1b). Sodium terephthalate was added to a solution of acetonitrile–water (pH 3.6) with sodium chloride as the electrolyte and exposed to a mild reducing potential (constant potential, 1.0 V vs. Ag wire quasi-reference electrode, graphite rod working electrode, divided cell). It is observed that hydroxyl radicals are present at both the anodic and cathodic chambers, but they are more concentrated at the cathodic chambers relatively. The relative low concentration of hydroxyl radicals at the anodic chamber is consistent with the fact that only small amount of adhered hydroxyl radicals are generated at the anode to facilitate the electro-catalytic ozone generation process.16 The formation of hydroxyl radicals at the cathodic chamber is possible via various one-electron redox reactions (eqn (4a)–(4g)) (Scheme 4) under the operating potential.17–19 The operative reactions generate oxygenic radicals which would lead to the formation of hydrogen peroxide and homolysed to form hydroxyl radicals. This would also indicate that a buffered acidic solvent system would be essential for the steady formation of hydroxyl radicals at the cathodic chamber to facilitate the radical reactions of alkyl iodides. Moreover, a low pH environment would also inhibit the auto-decomposition of hydrogen peroxide, another reactive oxygen species that could homolyse into hydroxyl radicals. At higher pH, the decomposition of hydrogen peroxide into molecular oxygen and water would dominate, limiting the supply of hydroxyl radicals and, therefore, lead to no reactivity.20
Lastly, the effect of alkyl iodide on the consumption of hydroxyl radicals was examined. Control experiments have shown that the relative concentration of hydroxyl radicals decreases when alkyl iodide 1 is present (Fig. 1c) and indicated that hydroxyl radicals are involved directly in facilitating the radical cyclisation of alkyl iodide 1.
Following with the results of these experiments, we propose the following mechanism for the generation of alkyl radicals (Scheme 5). Two productive reaction pathways are responsible for the oxidation of alkyl iodide. The reaction is initiated by the generation of ozone at the anode via an electro-catalytic process and the resulting ozone is reacted with the alkyl iodide in the cathodic chamber, by slow diffusion in the closed divided cell via the pressure equalizer channel as well as the semiporous sintered glass divider. Reaction between ozone and alkyl iodide generated an unstable trioxide intermediate that subsequently fragment into the corresponding alkyl radical, IO˙ radical and molecular oxygen. The generated IO˙ radicals lead to the accumulation of hypoiodous acid that is known to react with superoxide radicals to yield highly reactive hydroxyl radicals to oxidise more alkyl iodides into the corresponding unstable I(II) species that fragment to yield hypoiodous acid and alkyl radicals.21 As a result, alkyl iodides could be activated by both productive pathways. Furthermore, additional redox reactions of molecular oxygen and hydrogen peroxide would also supply more hydroxyl radicals to facilitate the radical reaction of alkyl iodides.
The reactivity of alkyl iodide 1 could be rationalised as follows (Scheme 6): Once the primary radical is formed, there are two competing reaction pathways, which lead to the formation of pyrrolidine and N-allyltosylamide. When the primary alkyl radical is reduced by the electrode, the resulting anion would then undergo rapid elimination to produce ethene and an anionic intermediate, which could be protonated to give N-allyltosylamide. Another pathway involves the reaction of the primary radical with the allyl group via an intramolecular pathway to give a cyclised primary radical intermediate, which can then be reduced by the electrode to the corresponding anion to be protonated and form the pyrrolidine.
In conclusion, these experimental results indicate that under the standard electrochemical conditions, alkyl iodide are first ozonolysed to yield an unstable intermediate that fragments into the corresponding alkyl and IO radicals as well as molecular oxygen. This activates a superoxide radicals and hypoiodous acid mediated ‘redox relay’ reaction sequence to oxidise more alkyl iodides into the corresponding unstable I(II) species to produce more alkyl radicals. The use of sodium terephthalate as a fluorescence molecular probe provided insight to the role of hydroxyl radicals in this reaction sequence. Further studies on other radical reactions with this electrochemical approach are ongoing in our laboratory.
We wish to thank the Leverhulme Trust (RPG-2019-183) for generous financial support of our programme. The authors also gratefully acknowledge Dr D. MacMillan and Dr K. Karu for mass spectrometry and Dr A. Aliev for NMR support.
Conflicts of interest
There are no conflicts to declare.Notes and references
- G. J. Rowlands, Annu. Rep. Prog. Chem., Sect. B: Org. Chem., 2011, 107, 19–33 RSC.
- V. Darmency and P. Renaud, Radicals in Synthesis I, Springer-Verlag, Berlin/Heidelberg, 2006, vol. 263, pp. 71–106 Search PubMed.
- A. Studer and S. Amrein, Synthesis, 2002, 835–849 CrossRef CAS.
- S. Crespi and M. Fagnoni, Chem. Rev., 2020, 120, 9790–9833 CrossRef CAS.
- (a) M. Yan, Y. Kawamata and P. S. Baran, Chem. Rev., 2017, 117, 13230–13319 CrossRef CAS PubMed; (b) D. Pollok and S. R. Waldvogel, Chem. Sci., 2020, 11, 12386–12400 RSC.
- D. Li, T.-K. Ma, R. J. Scott and J. D. Wilden, Chem. Sci., 2020, 11, 5333–5338 RSC.
- Y. Sakamoto, A. Yabushita, M. Kawasaki and S. Enami, J. Phys. Chem. A, 2009, 113, 7707–7713 CrossRef CAS PubMed.
- T. M. Hellman and G. A. Hamilton, J. Am. Chem. Soc., 1974, 96, 1530–1535 CrossRef CAS.
- S. W. Hunt, M. Roeselová, W. Wang, L. M. Wingen, E. M. Knipping, D. J. Tobias, D. Dabdub and B. J. Finlayson-Pitts, J. Phys. Chem. A, 2004, 108, 11559–11572 CrossRef CAS.
- Q. Liu, L. M. Schurter, C. E. Muller, S. Aloisio, J. S. Francisco and D. W. Margerum, Inorg. Chem., 2001, 40, 4436–4442 CrossRef CAS PubMed.
- Y.-H. Wang and Q.-Y. Chen, Int. J. Electrochem., 2013, 2013, 1–7 Search PubMed.
- A. Hosomi, M. Hojo, J. Yoshizawa, Y. Funahashi, R. Okada, S. Nakamura and J. Tateiwa, Heterocycles, 1998, 49, 85 CrossRef.
- A. Gomes, E. Fernandes and J. L. F. C. Lima, J. Biochem. Biophys. Methods, 2005, 65, 45–80 CrossRef CAS PubMed.
- B. Tang, L. Zhang and Y. Geng, Talanta, 2005, 65, 769–775 CrossRef CAS PubMed.
- H. J. H. Fenton, J. Chem. Soc., Trans., 1894, 65, 899–910 RSC.
- Y.-H. Wang and Q.-Y. Chen, Int. J. Electrochem., 2013, 2013, 1–7 Search PubMed.
- J.-M. Noël, A. Latus, C. Lagrost, E. Volanschi and P. Hapiot, J. Am. Chem. Soc., 2012, 134, 2835–2841 CrossRef PubMed.
- M. H. Shao, P. Liu and R. R. Adzic, J. Am. Chem. Soc., 2006, 128, 7408–7409 CrossRef CAS.
- S. C. Perry, D. Pangotra, L. Vieira, L. I. Csepei, V. Sieber, L. Wang, C. Ponce de León and F. C. Walsh, Nat. Rev. Chem., 2019, 3, 442–458 CrossRef CAS.
- L. Szpyrkowicz, C. Juzzolino and S. N. Kaul, Water Res., 2001, 35, 2129–2136 CrossRef CAS.
- L. P. Candeias, K. B. Patel, M. R. L. Stratford and P. Wardman, FEBS Lett., 1993, 333, 151–153 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1cc03019a |
| This journal is © The Royal Society of Chemistry 2021 |