Abstract
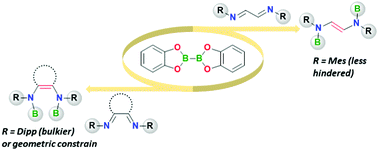
Diazabutadiene derivatives have been identified as a distinct class of reagents, capable of cleaving B–B bonds of diboron(4). The cleavage is accompanied by the formation of a new C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond and the product geometry is highly dependent on the substituents on the DAB units. Preliminary mechanistic investigations suggest a concerted mechanism and the absence of any radical intermediates.
C bond and the product geometry is highly dependent on the substituents on the DAB units. Preliminary mechanistic investigations suggest a concerted mechanism and the absence of any radical intermediates.
- This article is part of the themed collection: 2021 Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...