Wearable triboelectric nanogenerators for heart rate monitoring†
Sophia
Shen‡
,
Xiao
Xiao‡
,
Xiao
Xiao
and
Jun
Chen
 *
*
Department of Bioengineering, University of California, Los Angeles, Los Angeles, CA 90095, USA. E-mail: jun.chen@ucla.edu
First published on 11th May 2021
Abstract
Cardiovascular diseases are currently the leading cause of death globally and are projected to remain the leading cause in 2040, making heart rate an important physiological indicator that should be regularly monitored. Current heart rate monitoring techniques, including photoplethysmography and electrocardiography, are inconvenient for continuous biomonitoring in a wearable way. With a collection of compelling features, such as light weight and high sensitivity, triboelectric nanogenerators (TENGs) have become an emerging and cost-effective biotechnology for long-term and continuous heart rate monitoring in a wearable manner. In this review, we systematically discuss the biomechanics of heart beat, working mechanisms of TENGs, and exemplary applications of TENGs for self-powered heart rate monitoring. Finally, we conclude with a discussion of the potential for technology development in the future.
Introduction
As an important part of vital biological parameters, heart rate reveals one's physical status, including sleep state, infections, medications, the state of the heart, and blood-related issues (such as anaemia).1 Using heart rate data, heart rate variability (HRV) can be determined, which is a measure of how well the heart responds to stress, with a high HRV corresponding to a healthy heart.2 From 2018 to 2020, the top two leading causes of death in China and US, namely, ischaemic heart disease and stroke, were of cardiovascular origin.3,4 To further emphasize the impact of heart-related diseases, the forecasted top two leading causes of death in 2040 were still ischaemic heart disease and stroke.5 To monitor the heart status and ensure body health, heart rate is an important indicator that has to be monitored constantly, which can be achieved through wearable pulse monitors. Furthermore, regular medical care to monitor the heart may not be available to everybody, as only 56.9% of the world's population today can walk to a healthcare facility within 60 minutes.6 This lack of widespread accessibility to regular check-ups makes technology such as wearable heart rate sensors extremely important to maintain health.7Most modern heart rate sensors use one of two technologies, photoplethysmography (PPG), which utilizes light to detect blood flow through arteries,8 and electrocardiography (ECG), which utilizes the body's own electrical signals to measure heart rate.9,10 However, there are limitations with both PPG and ECG that make them unideal as a method for regularly monitoring heart rate. Though PPG is convenient, since it is light-based, factors such as ambient light, skin colour, and distance from the artery can vastly affect the accuracy of the results.11 On the other hand, ECG is very accurate because it uses multiple electrodes to directly measure the electrical activity of the heart, but it is not very convenient to use due to the multiple electrodes needed.12,13 In addition to these limitations, neither PPG nor ECG is self-powered, which means that any portable versions cannot be continuously worn as they need time to charge.
To combat all the limitations that come with the mainstream wearable heart rate monitors, triboelectric nanogenerators (TENGs)14 are a rapidly expanding technology that can serve as a self-powered device.15–19 By converting mechanical energy into power, TENGs can reach voltage outputs of more than 1000 V, and have high signal-to-noise ratios and pressure sensitivity that allows for accuracy in heart rate measurements.20,21 Moreover, they are light-weight due to their small structure and can be made using a variety of materials,22 such as synthetic polymers,23 silicone rubber,24 silk,25 and cotton.26 Additionally, as they are relatively small, TENGs are a suitable wearable device for regular use.16,21,27 Besides, harvesting energy from human movement to drive other devices by TENGs is also fast emerging.28–30 In fact, many wearable TENG-based heart rate monitors have already been developed, each with their own unique design.31,32
In this paper, we will first examine the mechanics behind heart rate and give an introduction to the working principle of TENGs. Next, we will review both various thin-film and textile-based self-powered wearable TENGs for heart rate monitoring and the use of TENGs as a power source. Finally, we will end with a summary of the future prospects of wearable TENG-based heart rate sensors and why wearable TENGs have the potential to be an extremely popular method of pulse monitoring.
Biomechanics of heart beat
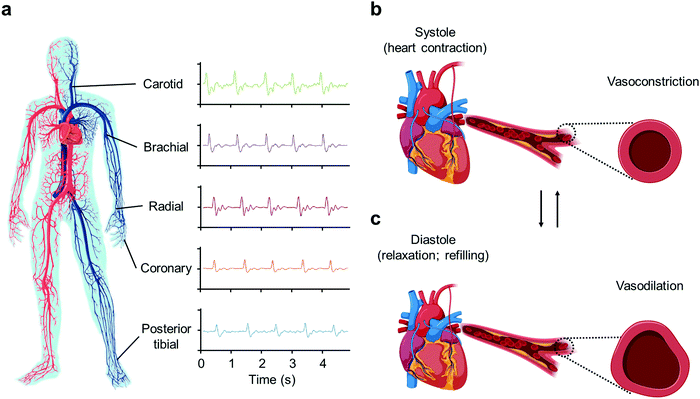
Heart rate can be measured in various locations on the human body where arteries are located, as illustrated in Fig. 1a. For example, when measuring heart rate from the wrist area such as in devices like a Fitbit or an Apple Watch, it is the pulse from the radial artery that is being measured.33 When the heart beats, it sends electrical signals that allow the heart to undergo two phases—systole and diastole, shown in Fig. 1b and c, respectively.34,35 During systole, the heart contracts and causes vasoconstriction to occur, which pumps blood throughout the body. Then, during diastole, the heart relaxes and allows blood to flow back to it, causing vasodilation. This blood flow is what is used to measure heart rate in PPG, while the electrical signals sent throughout the body are monitored in ECG.Additionally, heart rate can also be measured in another manner, as vasoconstriction and vasodilation not only cause blood to flow but also alter arterial pressure. Vasoconstriction causes arterial vessels to tighten and decreases their radius in order to push blood away from the heart, and as a result, increases arterial pressure. In contrast, vasodilation causes arterial vessels to loosen and increases their radius, which causes a decrease in arterial pressure.36 These changes in arterial pressure can be measured and plotted on a graph, as depicted in Fig. 2a.
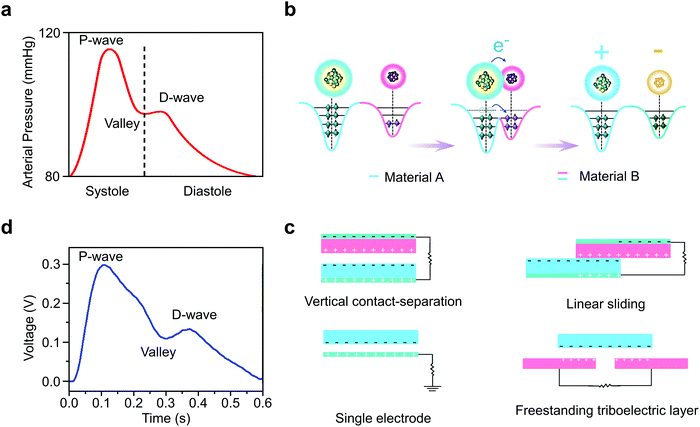 | ||
| Fig. 2 Arterial pressure induced electricity generation via wearable TENGs for heart rate monitoring. (a and d) Comparison of pressure within large arteries during a cardiac cycle with the voltage signals recorded during a respiration rate monitoring test, showing the high reliability of TENG heart rate monitoring.46 (b) The atomic-scale electron-cloud potential-well model of electricity generation via the conversion of biomechanical motions into electricity.39 (c) The device-scale illustration of electricity generation. | ||
Mechanism of TENGs
TENGs are a rapidly developing technology that uses the triboelectric effect along with physical movement to generate electricity.37,38 As shown in Fig. 2b, the triboelectric effect requires the use of two materials with different electron affinity. When in close proximity with each other, electron transfer can occur between the materials. After separation, the two surfaces remain charged, creating a voltage difference between the two materials.39 This cycle then repeats when the two are brought together again.40Using this basic principle, as demonstrated in Fig. 2c, there are currently four working modes of TENGs that are used: vertical contact-separation, linear sliding, single-electrode, and freestanding triboelectric layer.41 The vertical contact-separation mode functions through repeated contact and separation between two oppositely charged materials.40 The linear sliding mode uses the displacement between the two materials to create a flow of electrons, creating an alternating current.42 The single-electrode mode does not require connection of the two materials; rather, one material is attached to the load and ground, while the other material is free to slide as in the linear sliding mode, drawing electrons to and from the ground in order to create current.43,44 Lastly, the freestanding triboelectric layer requires the use of a pair of symmetric electrodes, which generates an alternating current between due to electrostatic induction.45
These four working modes of TENGs can be used to measure arterial pulse waves by utilizing the aforementioned pressure changes associated with vasoconstriction and vasodilation. As seen in Fig. 2d, the resulting voltage plot generated from a wearable TENG heart rate sensor is very similar to the arterial pressure wave shown previously in Fig. 2a, containing the characteristic P-wave, D-wave, and valley.46 This demonstrates the feasibility and accuracy of using wearable TENGs as heart rate sensors.
Self-powered HR sensors
Thin-film based wearable TENGs
The first wearable TENGs developed for heart rate monitoring were thin-film based and were used by attaching the TENG directly to the skin. In 2014, a membrane-based triboelectric sensor (M-TES) was developed for use in both surveillance and healthcare monitoring (Fig. 3a).47 Relying on air pressure changes to induce triboelectric charges, the M-TES utilizes a latex membrane layered on top of a layer of fluorinated ethylene propylene (FEP) and two layers of copper electrodes separated by acrylic. Pressure changes from a through air-conducting channel in the centre cause the membrane to expand and contract, resulting in potential differences between the two electrodes. The M-TES reports an average sensitivity of 0.04 V kPa−1 (and higher sensitivity with smaller size) and stability even after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif)
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles, accurately measuring a heart rate of 72 beats per minute (Fig. 3a).
000 cycles, accurately measuring a heart rate of 72 beats per minute (Fig. 3a).
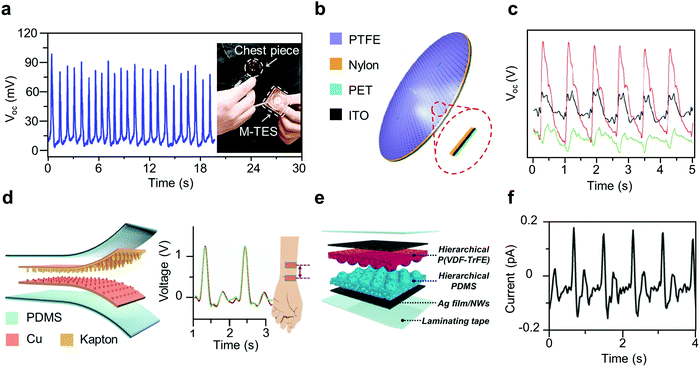 | ||
| Fig. 3 Thin-film based TENGs as self-powered sensors for heart rate monitoring. (a) Output voltage signals of the M-TES as a heartbeat monitor, inset: setup of the M-TES as a heartbeat monitor.47 (b) Schematic illustrations of the BMS. (c) The pulse waves acquired using a BMS from three different sites of a participant: carotid artery, left wrist, and the chest.48 (d) Schematic structure and radial artery pulse signal output generated by the SUPS.33 (e) Schematic illustration of humid-insensitive TESs worn on the wrist. (f) Radial artery pulse waves detected by ultra-flexible TESs attached on the wrist.49 | ||
In 2015, Yang et al. reported an eardrum-inspired bionic membrane sensor (BMS) that can be used for health monitoring (Fig. 3b).48 The BMS is an oval, multi-layered structure, which contains an indium tin oxide (ITO) electrode and a nylon electrification layer supported by a thin layer of poly-ethylene terephthalate (PET), all attached to an outwardly tented layer of polytetrafluoroethylene (PTFE). The BMS acts as a single-electrode mode TENG, generating electricity upon compression of the PTFE layer during vasoconstriction and vasodilation of the arteries. With a pressure sensitivity of 51 mV Pa−1 and consistent performance over 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles, the BMS can accurately monitor heart rate when attached to the carotid artery, chest, and wrist (Fig. 3c).
000 cycles, the BMS can accurately monitor heart rate when attached to the carotid artery, chest, and wrist (Fig. 3c).
The self-powered ultrasensitive pressure sensor (SUPS), developed in 2017, is based on the vertical contact-separation mode (Fig. 3d).33 The SUPS is made of a nanostructured Kapton (n-Kapton) film as one triboelectric layer with an ultrathin layer of Cu as its electrode, while a nanostructured Cu film developed using n-Kapton serves as the both the other triboelectric layer and its electrode. This design results in a sensor with a high signal-to-noise ratio of 45 dB and a long-term performance of 10 million cycles, ideal for regional artery PWV measurement. Even when pressed on the radial artery at two locations 4.5 cm apart, it results in an accurate measurement (Fig. 3d).
Similar to the SUPS, the TES functions through the vertical contact-separation mode and is a skin-inspired triboelectric sensor developed in 2018. Similar to human skin, the TES has gradient stiffness along with interlocked and microridged polymers, with a stiff layer of poly(vinylidenefluoride-co-trifluoroethylene) (P(VDF-TrFE)) and a soft layer of poly(dimethylsiloxane) (PDMS), as shown in Fig. 3e.49 This microridge structure also creates a variation in the gap distance and contact area that removes the need for bulky spacers. In addition, it is packaged with laminating tape to create a humidity-proof heart rate sensor. The TES is extremely flexible and has a pressure sensitivity of 0.55 V kPa−1, which allows for reliable measurement of even the weak radial artery pulse waves (Fig. 3f).
TENGs can also be created through heating, such as the expandable microsphere-based TENG introduced in 2019.46 This TENG consists of two layers of FEP films with Cu electrodes, where one layer has an additional coating of a PDMS and microsphere mixture that is heated to allow for microsphere expansion. The deformation of microspheres in response to pressure is what drives this TENG, as increasing pressure results in larger contact area due to deformation. With a maximum pressure sensitivity of 150 mV Pa−1 and no performance degradation after 5000 cycles, it is able to accurately measure heart rate from both chest and wrist areas.
Thin-film wearable TENGs as heart rate sensors are a reliable, small, and low-cost alternative to the heart rate sensors widely available today. However, as wearable heart rate monitors are devices that are designed to be worn long-term, comfort is an aspect of the design that needs to be considered. Owing to their different materials and fabrication processes, thin-film based TENGs are not as wearable as the textiles in clothing. To bridge this gap, a possible alternative is to create textile-based wearable TENGs.
Textile-based wearable TENGs
Owing to their superior comfort during long-term use, textile-based wearable TENGs as heart rate sensors are a quickly expanding field.50 As early as 2017, a highly stretchable energy-harvesting textile (SEHT) was developed to monitor heart rate (Fig. 4a).51 The SEHT is a single-thread based TENG, created with a multi-twisted stainless-steel conducting thread acting as an electrode with a soft silicone rubber coating as the triboelectric layer. In order for a single-thread based TENG to be possible, it is designed to be worn against the skin, which acts as the other electrode. The SEHT is then sown in a serpentine shape onto both sides of a portion of an elastic textile, generating electricity upon deformation due to skin contact when worn. Attaching a single thread to the wrist generates a wrist pulse, demonstrating its use as a heart rate monitor (Fig. 4b).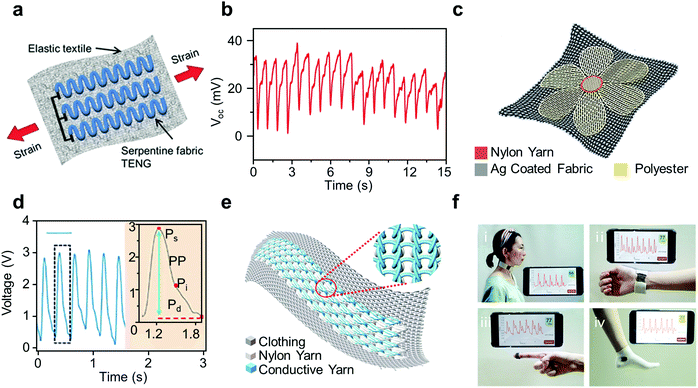 | ||
| Fig. 4 Textile based TENGs as self-powered sensors for heart rate monitoring. (a) Schematic illustration of the SEHT. (b) Measurement result of pulse signals from a 32-year-old female using SEHT.51 (c) Schematic illustration of the as-fabricated TS. (d) The acquired pulse wave signals of a 22-year-old woman using TS.54 (e) Schematic illustration of the combination of TATSA and clothes. (f) Measurement of the pulse using TATSA at the (e1) neck, (e2) wrist, (e3) fingertip, and (e4) ankle.57 | ||
In addition to single-thread based textile TENGs which are sown onto other materials, textile-based TENGs can also include TENGs where the entire textile is a TENG. For example, the weaving constructed self-powered pressure sensor (WCSPS) is a multilayer-structured TENG created in 2018 with interlaced PTFE strips above a layer of PET and ITO, which serve as an electrification layer and electrode, respectively, all under a protective layer of PDMS.52 Due to the interlaced PTFE strips, the WCSPS shows a pressure sensitivity of 45 mV Pa−1. With high pressure sensitivity and no performance degradation after 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles, the WCSPS can consistently and reliably measure heart rate at various locations, such as the fingertip, wrist, ear, and ankle.
000 cycles, the WCSPS can consistently and reliably measure heart rate at various locations, such as the fingertip, wrist, ear, and ankle.
Many clothes made today involve the use of sewing machines, so it is reasonable to suggest that perhaps a textile-based TENG could be fabricated using a sewing machine as well. In fact, in 2018, polyvinylidene fluoride (PVDF) fibres with a sewing machine were used to make a textile-based TENG with programmable patterns.53 Combined with a supportive PET thread, the PVDF was sewn onto a fabric consisting of a conductive electrode fabric sandwiched between two layers of supportive fabric. When the PVDF threads come in contact with nylon fabric on an aluminium electrode from pressure changes such as that from carotid arterial pulse, the resulting voltage waveform can be used to track heart rate in real time. Additionally, the use of a sewing machine allows for custom PVDF-based embroidery on clothing, increasing its aesthetic appeal.
The aesthetic appeal of textile-based TENGs is highly important as they will be worn, but sometimes custom patterns are not feasible. As a result, TENGs can be designed with aesthetics in mind, such as the textile-based sensor (TS) developed in 2019 (Fig. 4c).54 The TS consists of a flower-shaped textile made up of 3-ply-twisted polyester–metal hybrid fibres embossed on a silver-coated fabric. The TS has a sensitivity of 3.88 V kPa−1 and maintains its durability and reliability after 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles, making it capable of monitoring heart rate when sown onto wristbands, headbands, and clothes (Fig. 4d).
000 cycles, making it capable of monitoring heart rate when sown onto wristbands, headbands, and clothes (Fig. 4d).
Another method to create custom designs using textiles is through weaving with yarn. In 2020, a triboelectric sensing textile was created by weaving together core–shell yarns.55 The weft yarn contained a stainless-steel core wrapped by either a nylon or PTFE filament, while the warp yarn was comprised of a pure nylon or PTFE filament. Electricity is generated upon contact between the yarns upon deformation. With a pressure sensitivity of 1.33 V kPa−1 and 0.32 V kPa−1 in the pressure ranges of 1.95–3.13 kPa and 3.20–4.61 kPa and consistent performance after 4200 cycles, this sensing textile can consistently measure heart rate from the carotid artery.56 When weaving yarns together, it is important to consider not only the materials used but also the pattern of weaving (Fig. 4e). The triboelectric all-textile sensor array (TATSA) was introduced in 2020 and involves the knitting of a conductive yarn and a nylon yarn together into a full cardigan stitch.57 This results in a higher contact area for the triboelectric effect, leading to a pressure sensitivity of 7.84 mV Pa−1 and stability after more than 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles. When sewn into fabric and attached to various parts of the body, the heart rate can be measured, as seen in Fig. 4f.
000 cycles. When sewn into fabric and attached to various parts of the body, the heart rate can be measured, as seen in Fig. 4f.
The use of machines in the design of textile-based TENGs is limited to creating custom patterns alone. The sensor textile, reported in 2020, utilizes a facile electrospinning technique to create a sensor made with a layer of PVDF/Ag nanowire nanofibrous membrane (NFM) and a layer of ethyl cellulose NFM between two layers of conductive fabric.58 The rough structure of the nanofibers allows for the sensor textile to have a pressure sensitivity of 1.67 V kPa−1 in the pressure range of 0–3 kPa and 0.20 V kPa−1 in the range of 3–32 kPa, with stability after 7200 cycles. As a result, when placed on the neck, the sensor textile can measure the real-time heart rate of the carotid artery.
All of the heart rate sensors mentioned previously require a form of direct measurement from the artery; however, sometimes this may not be feasible. The single layered ultra-soft smart textile, presented in 2020, is a non-invasive method of monitoring physiological symptoms during sleep.24 Similar to the SEHT, the textile consists of a single thread of fibre woven into a serpentine shape. The fibre was formed by twisting polyester yarns around a stainless steel rod, which was then inserted into a hollow silicone fibre. Using the commercial fabric as the substrate, the single-layered ultra-soft smart textile shows a pressure sensitivity of 10.79 mV Pa−1. With the assistance of a bandpass filter, the pulse waveform can be clearly obtained for heart rate measurement. By incorporating a smaller portion of the textile directly into clothing, it can be worn as a wearable heart rate monitor.
Textile-based wearable TENGs are a comfortable, reliable, and fashionable means for long-term heart rate monitoring. However, many studies do not focus much on verifying the results of the pulse measurement with verified forms of heart rate measurement, such as the use of ECG. With further validation of these results, textile-based wearable TENGs could become a device available for commercial and professional use.
Sustainable power sources for HR sensors
The lack of research on the accuracy of wearable TENGs that directly monitor heart rate means that they are an alternative to modern heart rate monitors that may take a while before they can be commercially available. However, long-term monitoring is more effective the earlier it begins, so it is ideal to first have a device that can simply be adapted to commercially available pulse sensors. As a result, the use of a TENG as the power source for wearable heart rate sensors allows for a device to have both the accuracy of a commercial heart rate sensor and the self-powered, low-cost qualities of TENGs. Developed in 2017, the downy-structure-based TENG (D-TENG) is a TENG that can serve as a power source for heart rate sensors.59 The D-TENG, whose structure is shown in Fig. 5a, is created with grids that are composed of PTFE and copper thin films that are able to slide past each other. It is able to harvest vibrational energy from activities such as walking through the freestanding triboelectric layer mode upon sliding of the PTFE and copper thin films. As the D-TENG is responsible for powering the heart rate sensor, it is important that it produces the highest power possible. This can be done by varying the number of grid numbers and testing the output power under consistent mechanical excitation, which in this case was 10 Hz. As shown in Fig. 5b, the use of four grids led to the maximum power output and was thus chosen for the D-TENG. Upon integration of the D-TENG with a body sensor network (BSN) that includes a heart rate sensor, an accurate pulse waveform can be generated that contains the distinguishing markers of an arterial pulse waveform (Fig. 5c). The voltage waveform contains all the characteristic peaks of an arterial pulse waveform, such as Ps, the systolic peak that is part of the P-wave, and Pi, the point of inflection.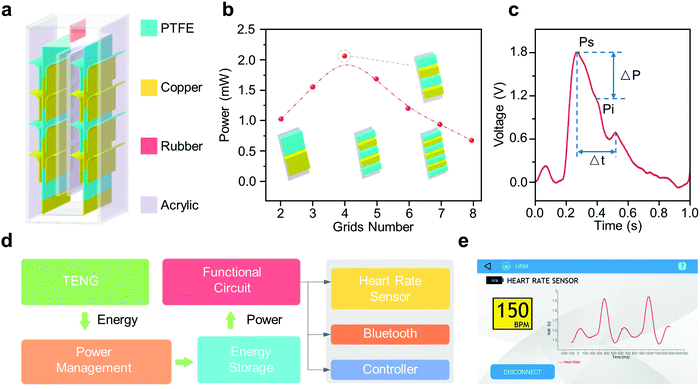 | ||
| Fig. 5 TENGs as sustainable power sources for heart rate monitoring. (a) Schematic illustration of the device structure of the D-TENG. (b) Output power of the D-TENG with different grid numbers under a consistent mechanical excitation of 10 Hz. (c) Heart-rate signal acquired by the BSN with an integrated D-TENG. (d) System diagram of the complete power-supplying system. (e) Enlarged view to show the whole software interface of the real-time acquired heart-rate signals.59 | ||
What differentiates the D-TENG from the previously discussed wearable TENGs is its integration in a power management circuit to supply power to the commercial heart rate sensor (Fig. 5d). The circuit serves to improve both the storage efficiency and the compatibility between electrical outputs of the D-TENG and commercial electronics. In this way, the heart rate sensor, Bluetooth module, and controller can function as if powered by a battery, able to work together to display the pulse waveform on a cell phone via Bluetooth (Fig. 5e). The D-TENG and BSN is a viable method to combine both TENGs and pre-existing heart rate monitors for an accurate and self-powered heart rate sensor. The further development of similar devices serves as a viable solution to the immediate and increasing need for regular heart rate monitoring.
Concluding remarks
Wearable TENGs made for heart rate monitoring are a cheaper and more convenient method of long-term pulse monitoring for cardiovascular diseases. They are able to combine various elements essential for a modern-day portable healthcare system (Fig. 6), such as Internet of Things (IoT), integrated chips (ICs), artificial intelligence (AI), disease monitoring, and point-of-care (POC). However, there are still many aspects of these elements that need to be perfected before they can be used commercially.The IoT was a term first created in 2006 and has since seen a huge increase in popularity,60 and describes the system of interconnected devices in our daily lives and their ability to connect with each other and transfer data among each other.61 For example, the TATSA is able to transmit the measured heart rate through Bluetooth to ones’ smartphone, allowing the user to save their pulse information and track daily changes.57 Future development of TENG-based self-powered bioelectronics could further integrate the IoT.62–65 This includes features such as securely and directly sending heart rate information to your physician using the internet.
The integration of IoT into these sensors may be limited by the physical hardware through its IC. As a wearable device, it should be made as small as possible so as to not obstruct our day-to-day life.66 Although the TENGs themselves are very compact, they still need to transmit their data to an IC that can process and transmit the data, and this IC reduces the compactness of the TENG. With the emerging field of nanoelectronics that can create smaller devices that consume less power, integration of nanocircuits with wearable TENGs is sure to create a truly nonintrusive device for long-term pulse monitoring.
Without the proper IC, devices such as the BSM would not be possible. In the BSM, the D-TENG could not be directly connected to the commercial electronics to power them. It was through the use of an IC that allowed the BSM to bridge this gap and create a functioning heart rate sensor. However, there are still many such design issues with wearable TENGs. For example, their accuracy may vary when there is additional signal noise, such as through walking or other movements while wearing the device, which could result in false pulse waveforms. Future improvements in the IC aspect of a TENG could lead to reduction of signal noise.
To further improve user experience, the use of AI or machine learning is a viable solution that, similar to IoT, has seen an increase in popularity recently.67–70 For example, when the body sweats, the slipperiness caused by this can lead to inaccuracies during heart rate measurement since there is no longer a precise fit of the TENG. However, a machine learning algorithm that can determine the amount of slipperiness and adjust the resulting output waveform can be implemented to reduce the impact that sweating has on the pulse waveform. With the use of AI and machine learning, future TENGs could give an accurate pulse waveform in any situation.
A vital aspect of healthcare monitoring is the ability to detect when there are abnormalities that could potentially point to diseases. Currently, the wearable TENGs that are designed for heart rate monitoring are able to assist in disease monitoring through the measurement of pulse waves, but are unable to determine their significance by themselves. As a result, a future development of wearable TENG pulse sensors could be to compare daily heart rate data and automatically notify the user if an abnormal heart rate persists for a long period of time, which can be indicative of a serious cardiovascular disease.
POC is a healthcare term that refers to when a healthcare service is administered.71 By gathering the most relevant information at the POC, it allows physicians to efficiently find the best treatment for a patient. Wearable TENGs for pulse monitoring can contribute to the POC system by providing more, and thus more accurate and representative, HRV readings for a physician to examine. In the future, development of wearable TENGs could allow for the automatic detection and treatment of common, treatable cardiovascular diseases such as administration of antiarrhythmic drugs.
In conclusion, TENGs are a rapidly developing technology, as evidenced by the rapid increase in papers related to TENGs (Fig. 6), that has a wide variety of applications. Wearable TENGs for heart rate monitoring are a developing part of the Internet of medical things that, with collaboration between engineers, physicians, and the general public, shows much promise for the future.
Author contributions
J. C. initiated and supervised the review writing. All authors contributed to literature research and manuscript writing.Conflicts of interest
The authors declare no competing interests.Acknowledgements
This is an invited article to Prof. Jun Chen for the 2021 ChemComm Emerging Investigators Award. The authors acknowledge the Henry Samueli School of Engineering & Applied Science and the Department of Bioengineering at the University of California, Los Angeles for the startup support. J. C. also acknowledges the 2020 Okawa Foundation Research Grant. X. X. thanks Ms Kedi Xie for providing the stellar schematic diagram.References
- G. Q. Zhang and W. Zhang, Ageing Res. Rev., 2009, 8, 52–60 CrossRef PubMed.
- J. Fatisson, V. Oswald and F. Lalonde, Heart Int., 2016, 11, e32–e40 Search PubMed.
- L.-Y. Ma, W.-W. Chen, R.-L. Gao, L.-S. Liu, M.-L. Zhu, Y.-J. Wang, Z.-S. Wu, H.-J. Li, D.-F. Gu, Y.-J. Yang, Z. Zheng and S.-S. Hu, J. Geriatr. Cardiol., 2020, 17, 1–8 Search PubMed.
- S. S. Virani, A. Alonso, E. J. Benjamin, M. S. Bittencourt, C. W. Callaway, A. P. Carson, A. M. Chamberlain, A. R. Chang, S. Cheng, F. N. Delling, L. Djousse, M. S. V. Elkind, J. F. Ferguson, M. Fornage, S. S. Khan, B. M. Kissela, K. L. Knutson, T. W. Kwan, D. T. Lackland, T. T. Lewis, J. H. Lichtman, C. T. Longenecker, M. S. Loop, P. L. Lutsey, S. S. Martin, K. Matsushita, A. E. Moran, M. E. Mussolino, A. M. Perak, W. D. Rosamond, G. A. Roth, U. K. A. Sampson, G. M. Satou, E. B. Schroeder, S. H. Shah, C. M. Shay, N. L. Spartano, A. Stokes, D. L. Tirschwell, L. B. VanWagner and C. W. Tsao, Circulation, 2020, 141, e139–e596 Search PubMed.
- K. J. Foreman, N. Marquez, A. Dolgert, K. Fukutaki, N. Fullman, M. McGaughey, M. A. Pletcher, A. E. Smith, K. Tang, C.-W. Yuan, J. C. Brown, J. Friedman, J. He, K. R. Heuton, M. Holmberg, D. J. Patel, P. Reidy, A. Carter, K. Cercy, A. Chapin, D. Douwes-Schultz, T. Frank, F. Goettsch, P. Y. Liu, V. Nandakumar, M. B. Reitsma, V. Reuter, N. Sadat, R. J. D. Sorensen, V. Srinivasan, R. L. Updike, H. York, A. D. Lopez, R. Lozano, S. S. Lim, A. H. Mokdad, S. E. Vollset and C. J. L. Murray, Lancet, 2018, 392, 2052–2090 CrossRef.
- D. J. Weiss, A. Nelson, C. A. Vargas-Ruiz, K. Gligorić, S. Bavadekar, E. Gabrilovich, A. Bertozzi-Villa, J. Rozier, H. S. Gibson, T. Shekel, C. Kamath, A. Lieber, K. Schulman, Y. Shao, V. Qarkaxhija, A. K. Nandi, S. H. Keddie, S. Rumisha, P. Amratia, R. Arambepola, E. G. Chestnutt, J. J. Millar, T. L. Symons, E. Cameron, K. E. Battle, S. Bhatt and P. W. Gething, Nat. Med., 2020, 26, 1835–1838 CrossRef CAS PubMed.
- Q. Zheng, Q. Tang, Z. L. Wang and Z. Li, Nat. Rev. Cardiol., 2021, 18, 7–21 CrossRef PubMed.
- A. A. Alian and K. H. Shelley, in Monitoring Technologies in Acute Care Environments: A Comprehensive Guide to Patient Monitoring Technology, ed. J. M. Ehrenfeld and M. Cannesson, Springer New York, New York, NY, 2014, pp. 165–178 DOI:10.1007/978-1-4614-8557-5_19.
- M. P. Witvliet, E. P. M. Karregat, J. C. L. Himmelreich, J. S. S. G. de Jong, W. A. M. Lucassen and R. E. Harskamp, J. Electrocardiol., 2021, 66, 33–37 CrossRef PubMed.
- M. Ajmal and F. Marcus, Am. J. Med., 2021, 134, 430–434 CrossRef PubMed.
- C. El-Hajj and P. A. Kyriacou, Biomed. Signal Process Control, 2020, 58, 101870 CrossRef.
- H. Ruppel, M. Funk, H. P. Kennedy, C. P. Bonafide, S.-F. Wung and R. Whittemore, Heart Lung, 2018, 47, 502–508 CrossRef PubMed.
- B. R. Chaitman and D. D. Miller, J. Nucl. Cardiol., 1995, 2, 267–270 CrossRef CAS PubMed.
- J. Lama, A. Yau, G. Chen, A. Sivakumar, X. Zhao and J. Chen, J. Mater. Chem. A, 2021 10.1039/D1TA02518J.
- L. Jin, X. Xiao, W. Deng, A. Nashalian, D. He, V. Raveendran, C. Yan, H. Su, X. Chu, T. Yang, W. Li, W. Yang and J. Chen, Nano Lett., 2020, 20, 6404–6411 CrossRef CAS PubMed.
- S. Zhang, M. Bick, X. Xiao, G. Chen, A. Nashalian and J. Chen, Matter, 2021, 4, 845–887 CrossRef.
- J. Chen and Z. L. Wang, Joule, 2017, 1, 480–521 CrossRef CAS.
- Z. Wu, W. Ding, Y. Dai, K. Dong, C. Wu, L. Zhang, Z. Lin, J. Cheng and Z. L. Wang, ACS Nano, 2018, 12, 5726–5733 CrossRef CAS PubMed.
- N. N. Zhang, C. Y. Tao, X. Fan and J. Chen, J. Mater. Res., 2017, 32, 1628–1646 CrossRef CAS.
- Y. Fang, X. Zhao, T. Tat, X. Xiao, G. Chen, J. Xu and J. Chen, Matter, 2021, 4, 1102–1105 CrossRef.
- T. Tat, A. Libanori, C. Au, A. Yau and J. Chen, Biosens. Bioelectron., 2021, 171, 112714 CrossRef CAS PubMed.
- Y. Zhou, W. Deng, J. Xu and J. Chen, Cell Rep. Phys. Sci., 2020, 1, 100142 CrossRef.
- Y. Su, J. Wang, B. Wang, T. Yang, B. Yang, G. Xie, Y. Zhou, S. Zhang, H. Tai, Z. Cai, G. Chen, Y. Jiang, L. Q. Chen and J. Chen, ACS Nano, 2020, 14, 6067–6075 CrossRef CAS PubMed.
- Z. Zhou, S. Padgett, Z. Cai, G. Conta, Y. Wu, Q. He, S. Zhang, C. Sun, J. Liu, E. Fan, K. Meng, Z. Lin, C. Uy, J. Yang and J. Chen, Biosens. Bioelectron., 2020, 155, 112064 CrossRef CAS PubMed.
- Q. Niu, L. Huang, S. Lv, H. Shao, S. Fan and Y. Zhang, Nano Energy, 2020, 74, 104837 CrossRef CAS.
- M. Zhu, Q. Shi, T. He, Z. Yi, Y. Ma, B. Yang, T. Chen and C. Lee, ACS Nano, 2019, 13, 1940–1952 CAS.
- Z. M. Lin, Z. Y. Wu, B. B. Zhang, Y. C. Wang, H. Y. Guo, G. L. Liu, C. Y. Chen, Y. L. Chen, J. Yang and Z. L. Wang, Adv. Mater. Technol., 2019, 4, 1800360 CrossRef.
- C. Yan, Y. Y. Gao, S. L. Zhao, S. L. Zhang, Y. H. Zhou, W. L. Deng, Z. W. Li, G. Jiang, L. Jin, G. Tian, T. Yang, X. Chu, D. Xiong, Z. X. Wang, Y. Z. Li, W. Q. Yang and J. Chen, Nano Energy, 2020, 67, 104235 CrossRef CAS.
- Z. M. Lin, J. Chen and J. Yang, J. Nanomater., 2016, 2016, 5651613 Search PubMed.
- K. Dong, J. Deng, W. Ding, A. C. Wang, P. Wang, C. Cheng, Y.-C. Wang, L. Jin, B. Gu, B. Sun and Z. L. Wang, Adv. Energy Mater., 2018, 8, 1801114 CrossRef.
- X. Peng, K. Dong, C. Ye, Y. Jiang, S. Zhai, R. Cheng, D. Liu, X. Gao, J. Wang and Z. L. Wang, Sci. Adv., 2020, 6, eaba9624 CrossRef CAS PubMed.
- F. Yi, Z. Zhang, Z. Kang, Q. Liao and Y. Zhang, Adv. Funct. Mater., 2019, 29, 1808849 CrossRef CAS.
- H. Ouyang, J. Tian, G. Sun, Y. Zou, Z. Liu, H. Li, L. Zhao, B. Shi, Y. Fan, Y. Fan, Z. L. Wang and Z. Li, Adv. Mater., 2017, 29, 1703456 CrossRef PubMed.
- Y.-J. Deng, J. Dong, J.-R. Zhou, D. Chen and J. Chen, Clin. Imaging, 2021, 69, 120–125 CrossRef PubMed.
- Z. Alizadeh Sani, A. Shalbaf, H. Behnam and R. Shalbaf, J. Digit. Imaging, 2015, 28, 91–98 CrossRef PubMed.
- A. Radtke, K. Popov, A. M. Bronstein and M. A. Gresty, Lancet, 2000, 356, 736–737 CrossRef CAS.
- J. Chen, Y. Huang, N. N. Zhang, H. Y. Zou, R. Y. Liu, C. Y. Tao, X. Fan and Z. L. Wang, Nat. Energy, 2016, 1, 16138 CrossRef CAS.
- Z. L. Wang, J. Chen and L. Lin, Energy Environ. Sci., 2015, 8, 2250–2282 RSC.
- X. Xiao, G. Chen, A. Libanori and J. Chen, Trends Chem., 2021, 3, 279–290 CrossRef CAS.
- X. Zhao, H. Askari and J. Chen, Joule, 2021 DOI:10.1016/j.joule.2021.03.013.
- Y. Zi and Z. L. Wang, APL Mater., 2017, 5, 074103 CrossRef.
- S. Wang, L. Lin, Y. Xie, Q. Jing, S. Niu and Z. L. Wang, Nano Lett., 2013, 13, 2226–2233 CrossRef CAS PubMed.
- Y. Yang, H. Zhang, J. Chen, Q. Jing, Y. S. Zhou, X. Wen and Z. L. Wang, ACS Nano, 2013, 7, 7342–7351 CrossRef CAS PubMed.
- Y. Yang, Y. S. Zhou, H. Zhang, Y. Liu, S. Lee and Z. L. Wang, Adv. Mater., 2013, 25, 6594–6601 CrossRef CAS PubMed.
- S. Wang, Y. Xie, S. Niu, L. Lin and Z. L. Wang, Adv. Mater., 2014, 26, 2818–2824 CrossRef CAS PubMed.
- Z. Liu, Z. Zhao, X. Zeng, X. Fu and Y. Hu, Nano Energy, 2019, 59, 295–301 CrossRef CAS.
- P. Bai, G. Zhu, Q. Jing, J. Yang, J. Chen, Y. Su, J. Ma, G. Zhang and Z. L. Wang, Adv. Funct. Mater., 2014, 24, 5807–5813 CrossRef CAS.
- J. Yang, J. Chen, Y. Su, Q. Jing, Z. Li, F. Yi, X. Wen, Z. Wang and Z. L. Wang, Adv. Mater., 2015, 27, 1316–1326 CrossRef CAS PubMed.
- M. Ha, S. Lim, S. Cho, Y. Lee, S. Na, C. Baig and H. Ko, ACS Nano, 2018, 12, 3964–3974 CrossRef CAS PubMed.
- G. Chen, Y. Li, M. Bick and J. Chen, Chem. Rev., 2020, 120, 3668–3720 CrossRef CAS PubMed.
- Y.-C. Lai, J. Deng, S. L. Zhang, S. Niu, H. Guo and Z. L. Wang, Adv. Funct. Mater., 2017, 27, 1604462 CrossRef.
- K. Meng, J. Chen, X. Li, Y. Wu, W. Fan, Z. Zhou, Q. He, X. Wang, X. Fan, Y. Zhang, J. Yang and Z. L. Wang, Adv. Funct. Mater., 2019, 29, 1806388 Search PubMed.
- Y.-E. Shin, J.-E. Lee, Y. Park, S.-H. Hwang, H. G. Chae and H. Ko, J. Mater. Chem. A, 2018, 6, 22879–22888 RSC.
- K. Meng, S. Zhao, Y. Zhou, Y. Wu, S. Zhang, Q. He, X. Wang, Z. Zhou, W. Fan, X. Tan, J. Yang and J. Chen, Matter, 2020, 2, 896–907 CrossRef.
- K. R. S. D. Gunawardhana, N. D. Wanasekara and R. D. I. G. Dharmasena, iScience, 2020, 23, 101360 CrossRef CAS PubMed.
- M. Lou, I. Abdalla, M. Zhu, X. Wei, J. Yu, Z. Li and B. Ding, ACS Appl. Mater. Interfaces, 2020, 12, 19965–19973 CrossRef CAS PubMed.
- W. Fan, Q. He, K. Meng, X. Tan, Z. Zhou, G. Zhang, J. Yang and Z. L. Wang, Sci. Adv., 2020, 6, eaay2840 CrossRef CAS PubMed.
- M. Lou, I. Abdalla, M. Zhu, J. Yu, Z. Li and B. Ding, ACS Appl. Mater. Interfaces, 2020, 12, 1597–1605 CrossRef CAS PubMed.
- Z. Lin, J. Chen, X. Li, Z. Zhou, K. Meng, W. Wei, J. Yang and Z. L. Wang, ACS Nano, 2017, 11, 8830–8837 CrossRef CAS PubMed.
- M. Dachyar, T. Y. M. Zagloel and L. R. Saragih, Heliyon, 2019, 5, e02264 CrossRef CAS PubMed.
- N. Fantana, T. Riedel, J. Schlick, S. Ferber, J. Hupp, S. Miles, F. Michahelles and S. Svensson, Internet of Things: Converging Technologies for Smart Environments and Integrated Ecosystems, 2013, pp. 153–204 Search PubMed.
- S.-N. Lai, C.-K. Chang, C.-S. Yang, C.-W. Su, C.-M. Leu, Y.-H. Chu, P.-W. Sha and J. M. Wu, Nano Energy, 2019, 60, 715–723 CrossRef CAS.
- J. M. Wu, C. C. Lee and Y. H. Lin, Nano Energy, 2015, 14, 102–110 CrossRef CAS.
- Y. Zou, J. Liao, H. Ouyang, D. Jiang, C. Zhao, Z. Li, X. Qu, Z. Liu, Y. Fan, B. Shi, L. Zheng and Z. Li, Appl. Mater. Today, 2020, 20, 100699 CrossRef.
- H. Ouyang, Z. Liu, N. Li, B. Shi, Y. Zou, F. Xie, Y. Ma, Z. Li, H. Li, Q. Zheng, X. Qu, Y. Fan, Z. L. Wang, H. Zhang and Z. Li, Nat. Commun., 2019, 10, 1821 CrossRef PubMed.
- Y. Lin, Y. Fang, J. Yue and B. Tian, Trends Chem., 2020, 2, 519–534 CrossRef CAS.
- D. Ben-Israel, W. B. Jacobs, S. Casha, S. Lang, W. H. A. Ryu, M. de Lotbiniere-Bassett and D. W. Cadotte, Artif. Intell. Med., 2020, 103, 101785 CrossRef PubMed.
- G. Q. Zhao, J. Yang, J. Chen, G. Zhu, Z. D. Jiang, X. Y. Liu, G. X. Niu, Z. L. Wang and B. Zhang, Adv. Mater. Technol., 2019, 4, 1800167 CrossRef.
- A. Haque, A. Milstein and F.-F. Li, Nature, 2020, 585, 193–202 CrossRef CAS PubMed.
- Z. Zhou, K. Chen, X. Li, S. Zhang, Y. Wu, Y. Zhou, K. Meng, C. Sun, Q. He, W. Fan, E. Fan, Z. Lin, X. Tan, W. Deng, J. Yang and J. Chen, Nat. Electron., 2020, 3, 571–578 CrossRef.
- M. Ebell, J. Am. Board Fam. Pract., 1999, 12, 225–235 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1cc02091a |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2021 |