Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Tandem catalysts for the selective hydrogenation of butadiene with hydrogen generated from the decomposition of formic acid†
D. H.
Carrales-Alvarado
a,
A. B.
Dongil
 *a,
A.
Guerrero-Ruiz
*a,
A.
Guerrero-Ruiz
 *bc and
I.
Rodríguez-Ramos
*bc and
I.
Rodríguez-Ramos
 ac
ac
aInstituto de Catálisis y Petroleoquímica, CSIC, c/Marie Curie No. 2, Cantoblanco, Madrid 28049, Spain. E-mail: a.dongil@csic.es; Fax: +34915854760
bDpto. Química Inorgánica y Técnica, Facultad de Ciencias UNED, Senda del Rey 9, Madrid 28040, Spain. E-mail: aguerrero@iccia.uned.es
cUA UNED-ICP(CSIC), Grupo de Diseño y Aplicación de Catalizadores Heterogéneos, Madrid, Spain. E-mail: a.dongil@csic.es; aguerrero@iccia.uned.es; Fax: +34915854760
First published on 10th May 2021
Abstract
We report for the first time the selective hydrogenation of 1,3-butadiene to butene using formic acid as the hydrogen source with 1 wt% Pd/carbon in a continuous flow reactor. The catalytic results show that the selectivity is even higher when formic acid is used compared to gas hydrogen.
The chemical industry needs about 40 million tons of hydrogen per year for chemical transformations. Of them, refineries are the second largest consumer with 25%. As is well-known, biomass upgrading is even more hydrogen demanding than upgrading fossil-based molecules, since biomass holds a larger amount of oxygen which needs to be reduced for valorisation. However, nowadays around 96% of hydrogen is obtained from fossil derived compounds.1 So, taking all this into account if we want to change to more sustainable chemical production, it is mandatory to find alternative hydrogen sources. In this context, the use of biomass derived molecules emerges as an excellent and well-suited solution to be used in future biorefinery schemes. Among those molecules, formic acid (FA) is highly interesting since it is produced in large amounts as a subproduct of cellulose hydrogenolysis, and its decomposition to H2 takes place under mild conditions.2,3
One of the limitations of using FA is that the dehydrogenation reaction to produce CO and H2O also takes place at low temperatures, ca. 150 °C.2 This reaction reduces the H2 availability and the as-produced CO can poison the catalyst, so an optimal design of the catalytic system must be done. In this regard, some Pd optimised systems for liquid-phase FA decomposition have been recently reported.4
Recently we have developed a Pd-based catalyst supported on carbon nanofibers, which is promising for conjugated double bond olefins and alkyne hydrogenations.5 For this catalyst, we demonstrated that Pd4S nanoparticles are exceptionally active, stable and selective for gas phase hydrogenation, even under more challenging industrial conditions of moderate pressure.
We selected this reaction as the starting point to study tandem reactions with formic acid, since the partial hydrogenation of alkadienes is one of the most important and challenging processes in the chemical industry.
To date the selective hydrogenation of C–C bonds in dienes using FA directly as the hydrogen source has not been reported. And there are no studiesthat address the critical question of the role of the secondary by-products formed in the formic acid decomposition reaction, in particular H2O and CO.
In this study we have simultaneously performed the decomposition of FA and the hydrogenation of 1,3-butadiene (HBD), FA being the hydrogen supplier. The catalysts used are based on Pd supported on carbon nanofibers and commercial high surface area graphites. Two commercial carbon nanofibers, Pyrograph (PR24-HHT and PR24-PS), were used as supports. The difference between them is the final treatment temperature. While PR24-HHT was subjected to temperatures over 3000 °C, PR24-PS was subjected to temperatures of 700 °C. This indicates that the former holds a more graphitic surface with less reactive sites, implying larger nanoparticles. The catalytic materials were prepared by incipient wetness impregnation, with a 1 wt% Pd loading, and reduced as detailed in the ESI.† As for these catalysts we can manage the selectivity to butenes, and we aimed to verify the catalytic properties to hydrogenate butadiene when the hydrogen reactant is produced in the same catalytic bed by FA decomposition.
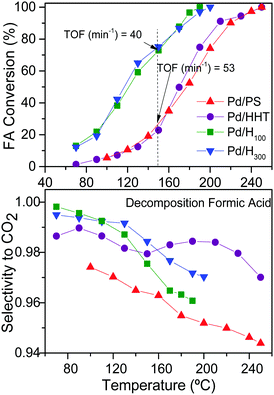
Measurements of the catalyst activity both for vapor phase FA decomposition and for HBD were carried out in a fixed-bed flow reactor and the details are given in the ESI.† The first step of this study was done to assess the temperature that could offer at least a H2/BD ratio of 2 and the highest selectivity to CO2 for all the tested catalysts. Hence, Fig. 1 shows the curves of conversion of FA and selectivity to CO2vs. reaction temperatures. Experimental data indicated that Pd/H100 and Pd/H300 have similar behaviour, reaching 100% conversion at 190 °C. The conversion profile shifted to higher temperatures for Pd/HHT and Pd/PS, so 100% conversion was obtained at 250 °C. On the other hand, the selectivity to CO2 was high for all the catalysts and in the range of 94–97% with minor differences within the experimental error. These selectivity values resulted in CO concentrations of 0.04–0.20 mL min−1. However, when analysing the activity per surface mol (TOF) estimated using the CO uptake from chemisorption analyses, the values, included in Fig. 1, indicate that Pd loaded onto nanofibers is slightly more active than that on the commercial graphites.
The reaction mechanism of FA decomposition has been described previously by a formate mediated path, which can adsorb either mono or bidentate.6 Tentatively, the bidentate formate species would be more favoured on the largest Pd particles observed on the nanofibers HHT, in Fig. 2 and Table 1, and this could explain the higher activity of these catalysts. The adsorption through both carbonyl and hydroxyl groups in a bidentate mode promotes the formation of HCOO which is the precursor of CO and could also explain the lower selectivity of the nanofibers compared to the graphites. As for the catalyst Pd/PS, despite the microscopy showing a high proportion of small nanoparticles, it also displays larger particles (see Fig. 2c) that allow the identification of the Pd(111) phases by XRD, as shown in Fig S2a and b (ESI†). This probably favours a similar catalytic behaviour on FA decomposition to that observed on Pd/HHT.
| Catalyst | S BET (m2 g−1) | CO-uptake (μmol g−1) | PdTEM (nm) |
|---|---|---|---|
| a Surface area of the reduced catalyst. | |||
| Pd/HHT | 44 | 2.3 | 3.2 |
| Pd/PS | 41 | 2.3 | 2.4 |
| Pd/H100 | 69 | 10.1 | 2.2 |
| Pd/H300 | 272 | 10.7 | 2.2 |
According to the results obtained through the light-off curves, the conversion and selectivity obtained at 200 °C should be adequate to obtain a H2/BD ratio over 2 in every catalyst, i.e. 2.4 for Pd/H100 and Pd/H300 and 2.1 for Pd/HHT and Pd/PS. These ratios would be suitable to perform the HBD tests avoiding apparent selectivity due to limiting hydrogen reactant conditions.
Hence, we evaluated the catalysts in FA decomposition at 200 °C for 600 minutes and the obtained H2 flow (mL min−1) is included in Fig. S3a and b (ESI†). Under these conditions, the H2 flow obtained reached a quite stable value of 3.3 mL min−1 for Pd/H100, 3.4 mL min−1 for Pd/H300, 2.4 mL min−1 for Pd/PS and 2.1 mL min−1 for Pd/HHT. However, these values resulted in lower H2/BD ratios than those expected by a previous experiment at this temperature, which for Pd/HHT is indeed below 2, i.e. 1.5, as shown in Fig. 3c. This results from the slight deactivation of the catalysts under steady state operation. This minor deactivation of the catalysts could be explained by CO poisoning which is produced through the formic acid dehydration reaction, and would remain irreversibly adsorbed on the Pd surface.
Although this lower H2/BD ratio was also used for BD hydrogenation for comparison, for the purpose of this research we tested both Pd/PS and Pd/HHT at 250 °C for 600 minutes. At this temperature, the H2/BD ratio was ca. 2.1 and 2.3 for Pd/HHT and Pd/PS respectively, so these conditions can be used to evaluate BD selective hydrogenation to butenes.
Then, once the best conditions were selected, we evaluated the hydrogenation of BD using FA and H2 from the line. We observed that for every catalyst, under the studied conditions, the conversion of FA in the presence and absence of BD was similar within experimental error. These results are in contrast to those reported in the hydrogenation of ethylene and propylene where authors observed a higher conversion of olefins in the presence of formic acid.7 This was explained by the faster consumption of hydrogen in the presence of the olefin, which would decrease the concentration of hydrogen on the surface allowing more formic acid molecules to react.
The average selectivity to butenes along with the H2/BD ratios is shown in Fig. 3c. The experiments with the H2 line were performed using a H2/BD ratio of 3. The reliability of the experiments was assessed by testing the catalyst Pd/H100 using a 2.5 H2/BD ratio, indicating that the selectivity to butenes is not affected within these ratios.
The results in Fig. 3c clearly demonstrate that not only it is possible to use formic acid as the hydrogen source, but also that the selectivity to butenes is enhanced significantly compared to the selectivity when using hydrogen from the line. The selectivity to butenes using H2 from the line at 200 °C followed the trend: Pd/HHT > Pd/PS > Pd/H300 > Pd/H100. However, the highest value was quite modest, ca. 25%, and the latter catalyst barely displayed any selectivity to butenes. Moreover, the selectivity profile in Fig. S4b (ESI†) showed that it decreases during the first 60 minutes on stream by 39% and 28% for Pd/PS and Pd/HHT respectively at 200 °C, and then the values remain quite constant. This behaviour might be due to modifications on the active phase due to carbon covering. Coke deposition on low coordinated sites seems to enhance the diffusion rate of subsurface hydrogen, favouring the hydrogenation of alkenes.8,9
With regard to the effect of the temperature in the experiments using line H2, interestingly the selectivity to butenes with the catalyst Pd/HHT increases at 250 °C compared to 200 °C (30 vs. 25%), this being much more produced for Pd/PS (75 vs. 8%). Also, the selectivity of Pd/PS at 250 °C increases during the first 180 min from 52 to 75%.
On the one hand, the particle size of Pd can influence the selectivity since it is possible that smaller particles, which are more positively charged, favour the BD and further butene adsorption through their electron rich double bonds, leading to the complete hydrogenation to the alkane.10 Thus, the small particle size of both Pd/H100 and Pd/H300 could lead to a stronger adsorption of reactants on the surface of Pd/H100 and Pd/H300, responsible for their lower selectivity to butenes. Moreover, Pd hydride species could be more active in the hydrogenation to the alkane since more H atoms are available to react. The Pd hydride formation can occur during the reduction step of the catalysts or during the reaction even at room temperature, and their presence was indeed verified by the appearance of negative peaks during the H2-TPR analyses in Fig. S5 (ESI†) indicative of H2 evolution from the hydride.11 While Pd hydride is noticeable for all the tested catalysts, and no apparent H2 consumption is observed during the TPR experiment, the temperature at which it appears is higher for Pd/H300 than Pd/H100, which means that more stable hydride species are formed on Pd/H300. It could then be expected that the Pd hydride of Pd/H100 can provide more readily available H atoms for the hydrogenation of butenes, which can explain the lower selectivity of Pd/H100 compared to Pd/H300. Unfortunately, the small Pd size on Pd/H100 and Pd/H300 does not allow the identification of any Pd-hydride diffraction in XRD in Fig. S1 (ESI†) and whose position could be used to compare the extent of hydrogen adsorption on the catalysts.
On the other hand, the selectivity results obtained with Pd/PS and Pd/HHT can be tentatively ascribed to the effect of particle size and the presence of sulphur species on Pd/PS. At 200 °C using line H2, the selectivity to butenes is higher on the catalyst Pd/HHT in agreement with their larger average Pd particle size and related to electronic effects, as explained above. For the Pd/PS catalyst, possibly two opposite indiscernible effects should be considered: the presence of a large proportion of small particles which tend to diminish the selectivity, and the sulphur species which could promote the selectivity to butenes. In our previous work we showed that the sulphur species are less prone to adsorb hydrogen and/or butadiene. The role of this S–Pd species is explained by changes in the availability of surface and subsurface hydrogen.5,12 However, upon increasing the reaction temperature up to 250 °C, the selectivity trend changes and now Pd/PS are much more selective than Pd/HHT to butenes. A control experiment with Pd/H300 using the H2 line at 250 °C indicated that for this catalyst the selectivity did not vary compared to that obtained at 200 °C. Since the particle size of the spent catalyst Pd/PS (Fig. S1a, ESI†) does not vary either at 200 °C or at 250 °C, another phenomenon should explain this difference.
The higher selectivity obtained at 250 °C for both Pd/PS and Pd/HHT could be related to the favoured fast recombination of H atoms from the hydride at higher temperatures, which would result in the reduced availability of hydrogen to hydrogenate the butenes. In contrast, the palladium hydride formed on Pd/H300 is more stable, likely hampering the recombination of H atoms, hence not affecting selectivity.
Finally, we evaluated the target process and the selectivity to butenes using formic acid as the hydrogen source at 200 or 250 °C was measured. The higher selectivity at 200 °C with Pd/PS and Pd/HHT is well-explained by the lower H2/BD ratio, and only the selectivity at 250 °C will be considered in the discussion. The selectivity followed the trend Pd/PS (99%) > Pd/HHT (73%) > Pd/H100 (44%) > Pd/H300 (5%). In Fig. S3 (ESI†) the progress of production of H2 and H2 not consumed in the conversion of BD is shown. These profiles indicate that the difference in H2 produced and consumed agrees well with the theoretical BD fed and consumed in the HBD reaction.
Besides the measured concentration of H2 in the outlet, the significantly different selectivity reached with Pd/H100 and Pd/H300 despite using a similar H2/BD ratio during the experiments with FA confirms that other parameters are responsible for directing the selectivity to partial hydrogenation products. There are several factors that could explain the higher selectivity obtained with formic acid. Firstly, CO is produced during formic acid decomposition (dehydration of FA), in small quantities of ca. 0.2–0.08 mL min−1 and decreases as follows: H300 > H100 > HHT > PS. Since CO is known to be a poisonous molecule that could chemisorb on Pd, as a tentative hypothesis the improved selectivity to butenes when using FA as the source of hydrogen could be linked with the presence of CO as a by-product. In fact, recent studies of the promoter effect of CO on selectivity to butenes have been reported.13 Another option is related to the adsorption of reactants in a different position, e.g. tilted vs. parallel. The role of hydride diffusion could also be considered. But since this phenomenon has a lower energetic barrier compared to FA decomposition, the effect should indeed be the opposite to that observed.7
Since the reaction temperature also influences the selectivity, the hydrogenation with formic acid can only be compared at the same temperature. Overall, the fact that with Pd/H100 and Pd/H300 the selectivity enhancement is more pronounced when the reaction is performed using formic acid (from 0–5% to 45–75%) can indicate that smaller particles are more influenced during formic acid decomposition to be more selective to butenes. This is better explained if CO poisoning of the most active sites of the catalysts occurs. Also, this explanation agrees with the estimated amount of CO produced from FA dehydration which is higher for Pd/H100 and Pd/H300. In contrast, although Pd/PS and Pd/HHT also increase the selectivity when FA is used at 250 °C, the relative increase is lower, i.e. from 30–75% to 75–100%.
The most interesting finding of the present work is the significantly higher selectivity to butenes obtained when using formic acid as the hydrogen source. These results are relevant since they show that it is possible to directly hydrogenate C–C double bonds using formic acid as the source of hydrogen in the same catalytic bed, feeding simultaneously both reactants. Interestingly, selectivity to butenes is much higher than that obtained with the same catalyst using hydrogen gas as a reactant. In short, this tandem reaction gives place to an active and very selective catalyst for the partial HBD into butenes. This is an example of reutilization of a natural by-product that can additionally serve as a selectivity promoter for the partial hydrogenation of butadiene into butenes.
This work was supported by the Spanish Agencia Estatal de Investigación (AEI) and EU (FEDER) (projects CTQ2017-89443-C3-1-R and CTQ2017-89443-C3-3-R). DHCA acknowledges financial support from the Consejo Nacional de Ciencia y Tecnología, CONACyT, México [grant numbers 2019-000029-01EXTV-00057].
Conflicts of interest
There are no conflicts to declare.Notes and references
- https://hydrogeneurope.eu/hydrogen-industry. .
- F. Valentini, V. Kozell, C. Petrucci, A. Marrocchi, Y. Gu, D. Gelman and L. Vaccaro, Energy Environ. Sci., 2019, 12, 2646 RSC.
- K. Mori, Y. Futamura, S. Masuda, H. Kobayashi and H. Yamashita, Nat. Commun., 2019, 10, 4094–4103 CrossRef PubMed.
- N. Yan, et al. , Angew. Chem., Int. Ed., 2020, 59, 20183–20191 CrossRef PubMed.
- A. Y. Liu, Y. Li, J. A. Anderson, J. Feng, A. Guerrero-Ruiz, I. Rodríguez-Ramos, A. J. McCue and D. Li, J. Catal., 2020, 383, 51–59 CrossRef.
- R. Schimmenti, R. Cortese, D. Duca and M. Mavrikakis, ChemCatChem, 2010, 9, 1610 CrossRef.
- D. A. Bulushev and J. H. Ross, Catal. Today, 2011, 12, 42 CrossRef.
- W. Ludwig, A. Savara, R. J. Madix, S. Schauermann and H.-J. Freund, J. Phys. Chem. C, 2012, 116, 3539 CrossRef CAS.
- M. Wilde, K. Fukutani, W. Ludwig, B. Brandt, J.-H. Fischer, S. Schauermann and H.-J. Freund, Angew. Chem., Int. Ed., 2008, 47, 9289 CrossRef CAS PubMed.
- A. Cooper, B. Bachiller-Baeza, J. A. Anderson, I. Rodríguez-Ramos and A. Guerrero-Ruiz, Catal. Sci. Technol., 2014, 4, 1446 RSC.
- S. F. Parker, H. C. Walker, S. K. Callear, E. Grünewald, T. Petzold, D. Wolf, K. Möbus, J. Adam, S. D. Wieland, M. Jiménez-Ruiz and P. W. Albers, Chem. Sci., 2019, 10, 480 RSC.
- M. V. Morales, A. Guerrero-Ruiz, E. Castillejos, E. Asedegbega-Nieto and I. Rodríguez-Ramos, Carbon, 2020, 157, 120–129 CrossRef CAS.
- J. F. Yang, B. Hu, W. Xia, B. Peng, J. Shen and M. Muhler, J. Catal., 2018, 365, 55 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Details of materials and methods, characterization of materials and reaction results. See DOI: 10.1039/d1cc01954f |
| This journal is © The Royal Society of Chemistry 2021 |