Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Distinctly different reactivity of bis(silylenyl)-versus phosphanyl-silylenyl-substituted o-dicarborane towards O2, N2O and CO2†
Yun
Xiong
 a,
Shenglai
Yao
a,
Shenglai
Yao
 a,
Ales
Ruzicka
a,
Ales
Ruzicka
 b and
Matthias
Driess
b and
Matthias
Driess
 *a
*a
aMetalorganics and Inorganic Materials, Department of Chemistry, Technische Universität Berlin, Straße des 17, Juni 135, Sekr. C2, Berlin 10623, Germany. E-mail: matthias.driess@tu-berlin.de; Web: http://www.driess.tu-berlin.de Fax: +49-30-314-29732
bDepartment of General and Inorganic Chemistry, Faculty of Chemical Technology, University of Pardubice, Studentska 573, 532 10 Pardubice, Czech Republic
First published on 18th May 2021
Abstract
In stark contrast to the reactivity of the bis-silylenyl dicarborane CB–Si2 (1) [CB = ortho-C,C′-C2B10H10, Si = PhC(tBuN)2Si] towards O2, N2O, and CO2, yielding the same dioxygenation product CB–Si2O2 (2) with a four-membered 1,3,2,4-disiladioxetane ring, the activation of the latter small molecules with the phosphanyl-silylenyl-functionalised CB–SiP (3) {P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) P[N(tBu)CH2]2} affords with O2 the CB–Si(
P[N(tBu)CH2]2} affords with O2 the CB–Si(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)P(
O)P(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) silanone-phosphine oxide (4), with N2O the CB–Si(
O) silanone-phosphine oxide (4), with N2O the CB–Si(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)P silanone-phosphine (5), and with CO2 the CB–Si(O2C
O)P silanone-phosphine (5), and with CO2 the CB–Si(O2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)P silicon carbonate-phosphine (6) and CB–C(
O)P silicon carbonate-phosphine (6) and CB–C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)OSiOP ester (7), respectively.
O)OSiOP ester (7), respectively.
For many decades, the activation of ubiquitous small molecules such as H2, N2, O2, CO, CO2, N2O, etc. has been considered to be a domain of transition-metal chemistry and metalloenzymes, including their low molecular-weight functional models. However, recent advances in low-valent non- and semi-metal chemistry have provided a plethora of impressive evidence with transition-metal-like reactivity of main-group elements in small molecule activation.1–6 This is in part due to the availability of reactive lone pair electrons and relatively low-lying empty acceptor orbitals of main-group elements in unusual low oxidation states, which can mimic the electronic situation of transition-metal centres and even mediate chemical bond cleavage of unactivated small molecules. Striking examples comprise the activation of dihydrogen by acyclic silylenes with a highly reactive silicon(II) centre7–10 and the fixation of dinitrogen by a borylene as a carbene analogue.11,12 In recent years, we have been interested in the chemistry of chelating N-heterocyclic silylenes (NHSis) that feature two adjacent divalent silicon atoms as highly reactive sites.13–15 Such bis-NHSis not only can serve as steering ligands for coordination of main-group elements in unusual low oxidation states and transition metals for catalysis,13–15 they can also mediate cooperative small molecule activation through sufficiently close proximity of the two silicon(II) centres.16,17 For instance, the bis-NHSis with a Si⋯Si distances between 2.6 and 4.5 Å are capable reacting with CO at ambient temperature, affording disilaketenes.16–18 Featuring a Si⋯Si distance of about 3.3 Å, the two Si(II) centres in the ortho-dicarborane-based bis-NHSi CB–SiSi (1)19 (Scheme 1) are in a predestinate position to coordinate and subsequently activate CO.17 Moreover, the bis-NHSi 1 has been employed to stabilise a single zero-valent Si atom,20 monovalent boron,21 and serves in nickel-mediated catalytic transformations.19 More recently we succeeded in the synthesis of the isolobal phosphanyl-silylenyl-functionalised o-dicarborane CB–SiP (3) where one silylenyl group in 1 is replaced by a phosphanyl moiety.22 Compound 3 enabled the formation of new classes of C,C′-dicarborandiyl-silylene supported Ge2 and Ge4 species which could not be realised with 1.22 Herein, we wish to report the markedly different reactivity of 1 and 3 towards O2, N2O, and CO2, respectively.
Exposure of solutions of 1 in diethyl ether at −20 °C to O2 gas leads to immediate disappearance of the yellow color and formation of the 1,3,2,4-disiladioxetane 2 (Scheme 1). Interestingly, 2 is also formed as single product through exposure of 1 towards N2O and CO2 under the same reaction conditions. After workup, 2 has been isolated as a colorless solid; it crystallises in the monoclinic space group C2/c. The two Si centres are five-coordinated and bridged by two oxygen atoms (Fig. 1). The Si atoms adopt a distorted square-pyramidal coordination geometry with the carborane–C atom at the apical position. The Si1–O1 [1.695(1) Å] and Si1–O1′ [1.708(1) Å] distances in 2 are slightly longer than the Si–O length in octamethyl cyclotetrasiloxane (ca. 1.65 Å).23 As expected, the five-coordinate 29Si nuclei exhibit a drastic up-field shift in the 29Si{1H} NMR spectrum (δ = −98.6 ppm) when compared with the precursor 1 (δ = 18.9 ppm).19
The reactions of O2, N2O, and CO2 with various stable silylenes to give Si![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O-containing compounds or further oxygenated Si–O-containing species are well-documented.5,9,24–30 We wondered whether a transient or even isolable Si
O-containing compounds or further oxygenated Si–O-containing species are well-documented.5,9,24–30 We wondered whether a transient or even isolable Si![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O species could be detected if one silylenyl moiety in 1 is replaced by a isoelectronic phosphanyl group; thus we employed the phosphanyl-silylenyl-functionalised carborane CB–SiP (3). Indeed, under the same reaction conditions, exposure of 3 to dioxygen gas at −20 °C resulted in the formation of the CB–Si(
O species could be detected if one silylenyl moiety in 1 is replaced by a isoelectronic phosphanyl group; thus we employed the phosphanyl-silylenyl-functionalised carborane CB–SiP (3). Indeed, under the same reaction conditions, exposure of 3 to dioxygen gas at −20 °C resulted in the formation of the CB–Si(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)P(
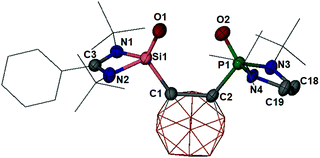
O)P(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) silanone-phosphine oxide (4) (Scheme 2 and Fig. 2), in which both P and Si atoms are mono-oxygenated. Compound 4 was isolated at −20 °C as a colorless solid in 68% yields. It is sparingly soluble in Et2O, but well soluble in THF. Solutions of 4 in ethereal solvents are only stable below −20 °C; its decomposition affords a mixture of unidentified products. In the solid state, however, 4 is stable at room temperature and decomposes above 153 °C.
O) silanone-phosphine oxide (4) (Scheme 2 and Fig. 2), in which both P and Si atoms are mono-oxygenated. Compound 4 was isolated at −20 °C as a colorless solid in 68% yields. It is sparingly soluble in Et2O, but well soluble in THF. Solutions of 4 in ethereal solvents are only stable below −20 °C; its decomposition affords a mixture of unidentified products. In the solid state, however, 4 is stable at room temperature and decomposes above 153 °C.
The 31P{1H} NMR spectrum of 4 (measured at −20 °C in d8-THF) exhibits a singlet at δ = 26.4 ppm, which is significantly up-field shifted relative to that of the precursor 3 (δ = 102.9 ppm).22 A similar up-field shift is observed for the 29Si{1H} NMR resonance of 4 (δ = −52.8 vs. 17.5 ppm for 3).22 The IR spectrum of 4 shows a very strong stretching vibration mode at ν = 1193 cm−1 for the Si![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond, which is slightly larger than the value observed for the Si
O bond, which is slightly larger than the value observed for the Si![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond in a C
O bond in a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O-supported silanone (1153 cm−1).27 A single-crystal X-ray diffraction analysis confirmed the presence of Si
O-supported silanone (1153 cm−1).27 A single-crystal X-ray diffraction analysis confirmed the presence of Si![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and P
O and P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds in 4 with a O1–Si1–P1–O2 torsion angle of 36.8° (Fig. 2). In 4 both silicon and phosphorus atoms adopt a distorted tetrahedral coordination environment. In line with the IR spectroscopic data, the Si–O distance of 1.524(3) Å in 4 is slightly shorter than that in the aforementioned keto-supported silanone adduct [1.532(2) Å],27 indicating a more pronounced Si
O bonds in 4 with a O1–Si1–P1–O2 torsion angle of 36.8° (Fig. 2). In 4 both silicon and phosphorus atoms adopt a distorted tetrahedral coordination environment. In line with the IR spectroscopic data, the Si–O distance of 1.524(3) Å in 4 is slightly shorter than that in the aforementioned keto-supported silanone adduct [1.532(2) Å],27 indicating a more pronounced Si![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond character.
O bond character.
As mentioned above, the reaction of silylenes with dioxygen usually affords disiladioxetanes or related Si–O single bond containing products. The formation of 4 as an isolable Si![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O species is apparently due to the presence of the phosphanyl moiety, acting as an oxygen atom acceptor in close proximity to the silicon atom. In order to figure out whether 3 is capable of N2O activation to furnish a Si
O species is apparently due to the presence of the phosphanyl moiety, acting as an oxygen atom acceptor in close proximity to the silicon atom. In order to figure out whether 3 is capable of N2O activation to furnish a Si![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O-(and/or P
O-(and/or P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)-containing product, we exposed solutions of 3 in Et2O to N2O gas at −20 °C. To our surprise, the oxygenation took place only at the silicon centre to form the CB-(Si
O)-containing product, we exposed solutions of 3 in Et2O to N2O gas at −20 °C. To our surprise, the oxygenation took place only at the silicon centre to form the CB-(Si![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)P silanone-phosphine (5), which could be isolated in 51% yields (Scheme 2). This is consistent with the fact, that such substituted phosphines are inert toward N2O. Akin to 4, compound 5 is labile in Et2O and THF solutions above −20 °C and decomposes to an unidentified mixture of products. However, 5 is indefinitely stable in the solid state at ambient temperature and decomposes above 176 °C.
O)P silanone-phosphine (5), which could be isolated in 51% yields (Scheme 2). This is consistent with the fact, that such substituted phosphines are inert toward N2O. Akin to 4, compound 5 is labile in Et2O and THF solutions above −20 °C and decomposes to an unidentified mixture of products. However, 5 is indefinitely stable in the solid state at ambient temperature and decomposes above 176 °C.
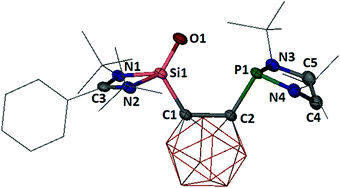
The molecular structure of 5 established by a single-crystal X-ray diffraction analysis revealed a four-coordinate silicon atom with a distorted tetrahedral coordination geometry (Fig. 3), while the phosphorus atom remains unchanged with respect to the precursor 3. The Si![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O distance of 1.524(1) Å in 5 is identical to that in 4. In line with that, the IR spectrum of 5 exhibits a Si
O distance of 1.524(1) Å in 5 is identical to that in 4. In line with that, the IR spectrum of 5 exhibits a Si![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching frequency of 1187 cm−1, which is very close to the value observed for 4 (ν = 1193 cm−1). The four-coordinate Si atom in 5 shows a resonance at δ = −51.9 ppm in the 29Si{1H} NMR spectrum (measured at −30 °C in d8-THF), matching well with that for 4 (δ = −52.8 ppm). In contrast, the marginally different chemical shift at δ = 114.2 ppm in the 31P{1H} NMR spectrum of 5 (vs. δ = 102.9 ppm of its precursor 3)22 confirms the presence of the unchanged phosphine moiety.
O stretching frequency of 1187 cm−1, which is very close to the value observed for 4 (ν = 1193 cm−1). The four-coordinate Si atom in 5 shows a resonance at δ = −51.9 ppm in the 29Si{1H} NMR spectrum (measured at −30 °C in d8-THF), matching well with that for 4 (δ = −52.8 ppm). In contrast, the marginally different chemical shift at δ = 114.2 ppm in the 31P{1H} NMR spectrum of 5 (vs. δ = 102.9 ppm of its precursor 3)22 confirms the presence of the unchanged phosphine moiety.
As aforementioned, previous studies on CO2 activation toward silylenes revealed mono-oxygen transfer and liberation of CO to form either Si![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O26,28–30 or dimeric disiladioxetane species.29,31 in less cases, silicon carbonates31–34 could be isolated which resulted from trapping reaction of Si
O26,28–30 or dimeric disiladioxetane species.29,31 in less cases, silicon carbonates31–34 could be isolated which resulted from trapping reaction of Si![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O intermediate with CO2. Similar to the reactivity of 3 towards N2O, 3 reacts with CO2 in diethyl ether at −20 °C only at the Si(II) site to yield the CB-(SiO2C
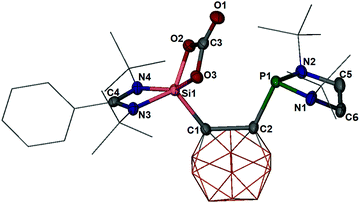
O intermediate with CO2. Similar to the reactivity of 3 towards N2O, 3 reacts with CO2 in diethyl ether at −20 °C only at the Si(II) site to yield the CB-(SiO2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)P silicon carbonate-phosphine (6) (Scheme 2). The latter product was isolated in 77% yields after workup at low temperature. The 31P{1H} NMR spectrum of 6 shows a signal at δ = 115.5 ppm (measured at −10 °C in d8-THF), indicating the presence of the unchanged phosphine moiety, whereas the 29Si{1H} NMR spectrum shows a resonance at δ = −93.1 ppm, reminiscent of the five-coordinate 29Si nuclei attached to a ferrocene spacer (δ = −92.1 ppm).29 The IR spectrum of 6 exhibits a stretching vibration mode at ν = 1813 cm−1 for the C
O)P silicon carbonate-phosphine (6) (Scheme 2). The latter product was isolated in 77% yields after workup at low temperature. The 31P{1H} NMR spectrum of 6 shows a signal at δ = 115.5 ppm (measured at −10 °C in d8-THF), indicating the presence of the unchanged phosphine moiety, whereas the 29Si{1H} NMR spectrum shows a resonance at δ = −93.1 ppm, reminiscent of the five-coordinate 29Si nuclei attached to a ferrocene spacer (δ = −92.1 ppm).29 The IR spectrum of 6 exhibits a stretching vibration mode at ν = 1813 cm−1 for the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group, comparable to that observed in a bis-NHC-supported silicon dicarbonate (1746 cm−1).35 A single-crystal X-ray diffraction analysis confirmed the molecular structure of 6 (Fig. 4), which features a five-coordinate silicon atom in a strongly distorted square-pyramidal coordination geometry with the carborane–C atom at the apical position. The Si–O distances of 1.718(1) and 1.770(1) Å in 6 are significantly longer than the Si
O group, comparable to that observed in a bis-NHC-supported silicon dicarbonate (1746 cm−1).35 A single-crystal X-ray diffraction analysis confirmed the molecular structure of 6 (Fig. 4), which features a five-coordinate silicon atom in a strongly distorted square-pyramidal coordination geometry with the carborane–C atom at the apical position. The Si–O distances of 1.718(1) and 1.770(1) Å in 6 are significantly longer than the Si![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O lengths in 4 and 5, respectively, but close to the Si–O bonds in 2 [1.695(1), 1.708(1) Å]. The C3–O1 distance of 1.194(2) Å is considerably shorter than those of C3–O2 (1.362(2) Å) and C3–O3 (1.340(2) Å), implying a C
O lengths in 4 and 5, respectively, but close to the Si–O bonds in 2 [1.695(1), 1.708(1) Å]. The C3–O1 distance of 1.194(2) Å is considerably shorter than those of C3–O2 (1.362(2) Å) and C3–O3 (1.340(2) Å), implying a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond between C3 and O1.
O bond between C3 and O1.
Although the mechanism of 6 from 3 with CO2 is still unknown, we propose that the initial step of the reaction is the formation of 5 with a Si![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O moiety under release of CO. To prove this, we conducted the reaction of 5, obtained from 3 and N2O, with CO2 at −20 °C (Scheme 2). Indeed, a clean formation of 6 confirmed this by multinuclear NMR spectroscopy measurements.
O moiety under release of CO. To prove this, we conducted the reaction of 5, obtained from 3 and N2O, with CO2 at −20 °C (Scheme 2). Indeed, a clean formation of 6 confirmed this by multinuclear NMR spectroscopy measurements.
While 6 is stable at room temperature in the solid state, it isomerises quantitatively at room temperature in ethereal solvents to give 7 in the course of 24 hours (Scheme 2). Its molecular structure has been established by a single-crystal X-ray diffraction analysis. As depicted in Fig. 5, the silicon atom in 7 is five-coordinate and in a distorted trigonal-bipyramidal coordination environment with the N1 and O2 atoms in the axial positions. Thus, the Si1–O2 distance of 1.773(2) Å is longer than that of Si1–O3 (1.624(2) Å). The driving force of the isomerization of 6 to 7 is attributed to the oxygen affinity of phosphorus that induces CB C–P bond scission and CB C–CO bond formation. In addition, the steric congestion in 6 between the phosphine group and the CB cage is released after migration of the phosphine moiety from the carborane–C2 to the O3 atom. As a result, the C1–C2 distance of 1.652(3) Å in 7 is significantly shorter than that in 6 (1.738(3) Å). In accordance with the molecular structure of 7, its 29Si{1H} NMR spectrum shows a doublet at δ = −110.4 ppm (2J(Si,P) = 9.4 Hz) comparable to that of 6 (δ = −93.1 ppm), and the 31P nucleus resonates at δ = 119.6 ppm in the 31P{1H} NMR spectrum reminiscent of that for 6 (δ = 115.5 ppm).
In summary, we have described the markedly different reactivity of the bis-NHSi dicarborane 1 and its isolobal phosphanyl-NHSi-functionalised dicarborane analogue 3 towards O2, N2O, and CO2. While all reactions of 1 with O2, N2O, and CO2 let to the same dioxygenation product CB–Si(μ-O)2Si2, the activation products with 3 turned out to furnish different products featuring Si![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, P
O, P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, or C
O, or C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O functionalities.
O functionalities.
Financial support by the Deutsche Forschungsgemeinschaft (Germany's Excellence Strategy–EXC 2008–390540038–UniSysCat and DR 226/21-1) and the Czech Science Foundation (A. R., project no. 21-02964S) is gratefully acknowledged.
Conflicts of interest
There are no conflicts to declare.Notes and references
- P. P. Power, Nature, 2010, 463, 171–177 CrossRef CAS PubMed.
- T. Chu and G. I. Nikonov, Chem. Rev., 2018, 118, 3608–3680 CrossRef CAS PubMed.
- S. Yadav, S. Saha and S. S. Sen, ChemCatChem, 2016, 8, 486–501 CrossRef CAS.
- C. Weetman and S. Inoue, ChemCatChem, 2018, 10, 4213–4228 CrossRef CAS.
- C. Shan, S. Yao and M. Driess, Chem. Soc. Rev., 2020, 49, 6733–6754 RSC.
- R. L. Melen, Science, 2019, 363, 479–484 CrossRef CAS.
- A. V. Protchenko, K. H. Birjkumar, D. Dange, A. D. Schwarz, D. Vidovic, C. Jones, N. Kaltsoyannis, P. Mountford and S. Aldridge, J. Am. Chem. Soc., 2012, 134, 6500–6503 CrossRef CAS PubMed.
- A. V. Protchenko, A. D. Schwarz, M. P. Blake, C. Jones, N. Kaltsoyannis, P. Mountford and S. Aldridge, Angew. Chem., Int. Ed., 2013, 52, 568–571 CrossRef CAS PubMed.
- D. Reiter, R. Holzner, A. Porzelt, P. J. Altmann, P. Frisch and S. Inoue, J. Am. Chem. Soc., 2019, 141, 13536–13546 CrossRef CAS PubMed.
- S. Takahashi, E. Bellan, A. Baceiredo, N. Saffon-Merceron, S. Massou, N. Nakata, D. Hashizume, V. Branchadell and T. Kato, Angew. Chem., Int. Ed., 2019, 58, 10310–10314 CrossRef CAS PubMed.
- M.-A. Legare, G. Belanger-Chabot, R. D. Dewhurst, E. Welz, I. Krummenacher, B. Engels and H. Braunschweig, Science, 2018, 359, 896–900 CrossRef CAS PubMed.
- M.-A. Legare, M. Rang, G. Belanger-Chabot, J. I. Schweizer, I. Krummenacher, R. Bertermann, M. Arrowsmith, M. C. Holthausen and H. Braunschweig, Science, 2019, 1332, 1329–1332 CrossRef PubMed.
- Y.-P. Zhou and M. Driess, Angew. Chem., Int. Ed., 2019, 58, 3715–3728 CrossRef CAS PubMed.
- S. Raoufmoghaddam, Y. P. Zhou, Y. Wang and M. Driess, J. Organomet. Chem., 2017, 829, 2–10 CrossRef CAS.
- B. Blom, D. Gallego and M. Driess, Inorg. Chem. Front., 2014, 1, 134–148 RSC.
- Y. Wang, A. Kostenko, T. J. Hadlington, M.-P. Luecke, S. Yao and M. Driess, J. Am. Chem. Soc., 2019, 141, 626–634 CrossRef CAS PubMed.
- Y. Xiong, S. Yao, T. Szilva, A. Ruzicka and M. Driess, Chem. Commun., 2020, 56, 747–750 RSC.
- A. Kostenko and M. Driess, J. Am. Chem. Soc., 2018, 140, 16962–16966 CrossRef CAS PubMed.
- Y.-P. Zhou, S. Raoufmoghaddam, T. Szilvási and M. Driess, Angew. Chem., Int. Ed., 2016, 55, 12868–12872 CrossRef CAS PubMed.
- S. Yao, A. Kostenko, Y. Xiong, A. Ruzicka and M. Driess, J. Am. Chem. Soc., 2020, 142, 12608–12612 CrossRef CAS PubMed.
- H. Wang, L. Wu, Z. Lin and Z. Xie, J. Am. Chem. Soc., 2017, 139, 13680–13683 CrossRef CAS PubMed.
- Y. Xiong, D. Chen, S. Yao, J. Zhu, A. Ruzicka and M. Driess, J. Am. Chem. Soc., 2021, 143, 6229–6237 CrossRef CAS.
- H. Steinfink, B. Post and I. Fankuchen, Acta Crystallogr., 1955, 8, 420–424 CrossRef CAS.
- M. Asay, C. Jones and M. Driess, Chem. Rev., 2011, 111, 354–396 CrossRef CAS PubMed.
- (a) R. Rodriguez, T. Troadec, D. Gau, N. Saffon-Merceron, D. Hashizume, K. Miqueu, J.-M. Sotiropoulos, A. Baceiredo and T. Kato, Angew. Chem., 2013, 52, 4426–4430 CrossRef CAS PubMed; (b) A. C. Filippou, B. Baars, O. Chernov, Y. N. Lebedev and G. Schnakenburg, Angew. Chem., Int. Ed., 2014, 53, 565–570 CrossRef CAS PubMed; (c) D. C. H. Do, A. V. Protchenko, M. Angeles Fuentes, J. Hicks, E. L. Kolychev, P. Vasko and S. Aldridge, Angew. Chem., Int. Ed., 2018, 57, 13907–13911 CrossRef CAS PubMed; (d) R. Kobayashi, S. Ishida and T. Iwamoto, Angew. Chem., Int. Ed., 2019, 58, 9425–9428 CrossRef CAS PubMed; (e) S. Takahashi, K. Nakaya, M. Frutos, A. Baceiredo, N. Saffon-Merceron, S. Massou, N. Nakata, D. Hashizume, V. Branchadell and T. Kato, Angew. Chem., Int. Ed., 2020, 59, 15937–15941 CrossRef CAS PubMed.
- S. Yao, Y. Xiong, M. Brym and M. Driess, J. Am. Chem. Soc., 2007, 129, 7268–7269 CrossRef CAS PubMed.
- Y. Xiong, S. Yao, R. Müller, M. Kaupp and M. Driess, Nat. Chem., 2010, 2, 577–580 CrossRef CAS.
- S. U. Ahmad, T. Szilvási, E. Irran and S. Inoue, J. Am. Chem. Soc., 2015, 137, 5828–5836 CrossRef CAS PubMed.
- M. P. Luecke, E. Pens, S. Yao and M. Driess, Chem. – Eur. J., 2020, 26, 4500–4504 CrossRef CAS PubMed.
- Z. Mo, T. Szilvási, Y. P. Zhou, S. Yao and M. Driess, Angew. Chem., Int. Ed., 2017, 56, 3699–3702 CrossRef CAS PubMed.
- F. M. Mück, J. A. Baus, M. Nutz, C. Burschka, J. Poater, F. M. Bickelhaupt and R. Tacke, Chem. – Eur. J., 2015, 21, 16665–16672 CrossRef PubMed.
- D. Wendel, A. Porzelt, F. A. D. Herz, D. Sarkar, C. Jandl, S. Inoue and B. Rieger, J. Am. Chem. Soc., 2017, 139, 8134–8137 CrossRef CAS.
- P. Jutzi, D. Eikenberg, A. Möhrke, B. Neumann and H. G. Stammler, Organometallics, 1996, 15, 753–759 CrossRef CAS.
- C. Yan, Z. Xu, X. Q. Xiao, Z. Li, Q. Lu, G. Lai and M. Kira, Organometallics, 2016, 35, 1323–1328 CrossRef CAS.
- A. Burchert, S. Yao, R. Müller, C. Schattenberg, Y. Xiong, M. Kaupp and M. Driess, Angew. Chem., Int. Ed., 2017, 56, 1894–1897 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental methods, crystallographic details. CCDC 2075598 (compound 2), 2075596 (compound 4), 2075600 (compound 5), 2075599 (compound 6), and 2075597 (compound 7). For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d1cc01939b |
| This journal is © The Royal Society of Chemistry 2021 |