Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Tuning extreme anisotropic thermal expansion in 1D coordination polymers through metal selection and solid solutions†
Lisa M.
van Wyk
 ,
Leigh
Loots
,
Leigh
Loots
 and
Leonard J.
Barbour
and
Leonard J.
Barbour
 *
*
Department of Chemistry and Polymer Science, University of Stellenbosch, Matieland 7602, South Africa. E-mail: ljb@sun.ac.za
First published on 7th July 2021
Abstract
The thermal expansion behaviour of a series of 1D coordination polymers has been investigated. Variation of the metal centre allows tuning of the thermal expansion behaviour from colossal positive volumetric to extreme anomalous thermal expansion. Preparation of solid solutions increased the magnitude of the anomalous thermal expansion further, producing two species displaying supercolossal anisotropic thermal expansion (ZnCoCPHTαY2 = −712 MK−1, αY3 = 1632 MK−1 and ZnCdCPHTαY2 = −711 MK−1, αY3 = 1216 MK−1).
Owing to increased thermal motion of their constituent atoms, solids usually expand upon heating;1–3 this is known as positive thermal expansion (PTE).1,3,4 However, some materials display anomalous thermal expansion behaviour, including negative and zero thermal expansion (NTE and ZTE, respectively), exceptional magnitudes of PTE, as well as extreme anisotropy.1,3 Anisotropic thermal expansion is characterised by significantly different linear thermal expansion coefficients for the individual principal axes.1 Although anisotropy is expected for materials that do not possess cubic symmetry, extreme anisotropy is uncommon. There have been relatively few reports of anomalous thermal expansion behaviour,4–7 but such materials are of great interest in materials science owing to possible applications in optical devices, semiconductor materials and space exploration vehicles.1,2,8,9 Furthermore, controlled tuning of the thermal expansion behaviour of solids is desirable for tailoring materials to specific applications.1,7,8,10
Previous studies on thermal expansion of crystalline solids have explored molecular crystals, co-crystals and mineral-type compounds.3,11–13 However, recent interest in extended materials such as metal–organic frameworks, coordination polymers (CPs), and covalent and hydrogen bonded frameworks have inspired new avenues of investigation.10,14–16 Flexible one dimensional CPs have received little attention in this regard, but may prove to be excellent candidates for studies of solid-state dynamics.
Recent studies have shown that compounds with “wine-rack” or “lattice-fence” solid-state packing often exhibit anomalous thermal expansion (Fig. 1a–c)3,5,7,10,17 as a consequence of hinging and twisting around the metal nodes.3,5,10 We have also seen that solid solutions show promise for tuning thermal expansion.7,9,18 Here we describe the thermal expansion behaviour of a series of isotypical19 CPs and their solid solutions, which all employ packing reminiscent of ideal “wine-rack” or “lattice-fence” motifs (Fig. 1d).
Four CPs with the general formula [M(bpdc)(bpy)·2DMF]n (MCP, M = Zn, Ni, Co and Cd) were prepared solvothermally from a solution of metal nitrate, 4,4′-biphenyldicarboxylic acid (bpdc) and 2,2′-bipyridine (bpy) in N,N-dimethylformamide (DMF). ZnCP was the first of these to be characterised structurally, but the low-temperature (100 K) single-crystal X-ray diffraction (SCXRD) data were of poorer quality than we had expected (Fig. S8, ESI†). SCXRD at 270 K (the crystals lose solvent at room temperature) yielded better data (Fig. S8, ESI†), but notably different unit-cell parameters relative to those at 100 K. Differential scanning calorimetry (DSC) revealed that this discrepancy is due to a subtle phase transition, which occurs with an onset temperature of 250–240 K upon cooling (Fig. S17, ESI†). Comparison of the high- and low-temperature crystal structures of ZnCP (ZnCPHT and ZnCPLT, respectively) reveals that the space group (P21/n) remains unchanged and that, although highly reminiscent of one another, their corresponding unit-cell parameters differ by more than can be expected for normal thermal expansion (Table S1, ESI†). Nevertheless, the two crystal structures can be considered isotypical.19
ZnCPHT is comprised of infinite 1D “zigzag” chains (∠Zn⋯Zn⋯Zn = 125.34(3)°) that propagate along [602]. Successive metal nodes are linked by means of chelating bpdc ligands (disordered over two positions with site occupancies of 0.53 and 0.47). Each metal centre is also bound to a chelating bpy ligand (disordered over two positions with site occupancies of 0.67 and 0.33), resulting in an overall highly distorted octahedral coordination environment. The 1D strands interdigitate via offset π⋯π interactions between bpy moieties of adjacent CP strands. This arrangement produces 1D guest-accessible channels that propagate along [100] and that contain DMF guest molecules (Fig. 2 and Fig. S10, ESI†). We have explored the transient porosity20 of ZnCP as part of a separate study,21 and a view along the 1D channels serves as a convenient frame of reference with respect to comparing the crystal structures.
The phase transition from ZnCPHT to ZnCPLT (based on the crystal structures determined at 270 and 100 K, respectively) involves a relatively minor structural rearrangement. The connectivity of ZnCPHT is maintained in ZnCPLT, and the disorder of the ligands remains similar (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 and 62
50 and 62![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 38 for bpdc and bpy, respectively). However, after the phase change the zigzag angle is markedly different. These changes lead to slight distortion of the 1D channel, with lengthening along [010] and shortening along [001] (Fig. 2 and Fig. S9 and S14, ESI†).
38 for bpdc and bpy, respectively). However, after the phase change the zigzag angle is markedly different. These changes lead to slight distortion of the 1D channel, with lengthening along [010] and shortening along [001] (Fig. 2 and Fig. S9 and S14, ESI†).
The phase change experienced by ZnCP upon cooling is due to an abrupt decrease in the M⋯M⋯M zigzag angle from 123.89(3)° at 250 K to 121.66(3)° at 240 K (Fig. S16 and Table S13, ESI†). This is accompanied by elongation of the channels along [010], with concomitant narrowing along the vector perpendicular to (001) (cf.Fig. 2 and Fig. S8, ESI†). These changes are manifested as elongation of the crystallographic a and b axes, a small increase in the β angle, and shortening of the c axis (Fig. S13, ESI†).
Determination of unit-cell parameters at 10 K intervals from 270 to 100 K shows that both phases of ZnCP undergo dramatic anisotropic thermal expansion (Fig. S14, ESI†). Respectively, the linear thermal expansion coefficients22αy1, αy2 and αy3 for ZnCPHT are 100, −462 and 882 MK−1, while those for ZnCPLT are 69, −57 and 275 MK−1. The matrices relating the principal axes to the unit-cell axes are given in Fig. S25, S26 and S39 of the ESI.† Taken together, the linear thermal expansion coefficients of ZnCPHT represent some of the most extreme anisotropic values recorded to date for any known crystalline materials (Table S17, ESI†). Moreover, both phases also undergo colossal (α > 100 MK−1) volumetric PTE (αV = 575 and 292 MK−1, respectively). Since the 1D channels propagate along [100] (Fig. 2), we can infer that most of the thermal expansion anisotropy is due to distortion of the host around the channels. In ZnCPHT the expansion coefficient approximately along [100] (i.e., αY1) is relatively modest, while that along [010] (i.e., αY2) is highly negative. The expansion coefficient normal to (001) is very highly positive in both cases. These observations comport with gradual narrowing (see ESI†) of the zigzag angle with decreasing temperature, as also occurs abruptly during the phase transition.
Previously we23,24 and others3,6,25–27 were successful in tuning the thermal expansion behaviour of structural analogues by modulating their composition. In this context we explored metal node substitution for facile modification of the coordination geometries about various metal centres; a series of MCP analogues was prepared with M = Ni, Co and Cd. Using SCXRD, we established that all of these materials are isotypes19 of ZnCP (Fig. 2 and Table S1, ESI†). They also undergo subtle low temperature phase transitions in the ranges 250–240, 260–250 and 200–190 K, respectively (Fig. S14, ESI†).
Multiple attempts were made to determine successive crystal structures across the phase change with slow cooling. However, in all cases the crystals fractured during the transition, thus yielding poor quality diffraction data from which it was only possible to determine unit cell parameters, the space group and estimates of M⋯M⋯M angles (with the exception of CoCP).
Upon cooling, the changes in the unit-cell parameters of NiCP across the phase change are similar to those of ZnCP, with expansion along [010] and concomitant contraction along [001]. Moreover, the M⋯M⋯M zigzag angle in NiCP also narrows across the phase change (from 123.31(3)° at 250 K to 116.38(3)° at 240 K, Table S13 and Fig. S16b, ESI†). Interestingly, in the cases of CoCP and CdCP, cooling across the phase-change results in shortening of the b axis and narrowing of the β angle, with elongation of the c axis (Fig. S14, ESI†). Therefore, with regard to these parameters, the phase changes in CoCP and CdCP are inversely related to those of ZnCP and NiCP. This is also reflected in the increase of the M⋯M⋯M zigzag angle in CdCP from 140.24(1)° at 200 K to 145.29(1)° at 190 K (Table S13 and Fig. S16, ESI†). The phase change temperatures for CoCP and CdCP are significantly different (ca. 260 and 200 K, respectively).
Variable-temperature unit-cell determinations for CdCP were carried out as for ZnCP. Owing to facile desolvation and relatively high phase-change temperatures of CoCPHT and NiCPHT (both ca. 250 K), an environmental solvent cell was used for unit cell determinations in the range 280 to 310 K (see ESI†). NiCPHT exhibits colossal linear PTE along Y2 and Y3, while CoCPHT exhibits linear NTE along Y3 (Fig. 3 and Fig. S27, Table S14, ESI†). Both exhibit colossal volumetric PTE (αV = 371 MK−1 for NiCPHT and 239 MK−1CoCPHT). In CoCPHT both linear PTE and NTE occur in the ac plane between adjacent 1D strands. The magnitudes of thermal expansion (both linear and volumetric) of NiCPHT and CoCPHT are substantially lower than that of ZnCP. Both phases of CdCP exhibit extreme anisotropic thermal expansion (Fig. 3 and Fig. S40, Table S16, ESI†), similar to ZnCP (CdCPHTαY2 = 778 MK−1, αY3 = −389 MK−1 and αV = 531 MK−1).
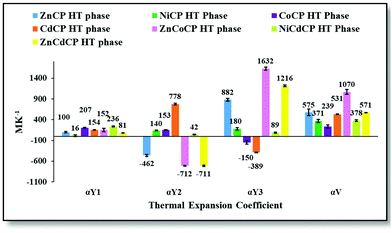 | ||
| Fig. 3 Thermal expansion coefficients for the high-temperature phases of the CPs. Error bars and specific values are indicated. | ||
Tuning of thermal expansion behaviour in metal containing materials may also be achieved by the preparation of solid solutions.7–9,18,28 Three solid solutions were prepared by the addition of equimolar amounts of two metal nitrates to the crystallisation solution (MM′CP, MM′ = ZnCo, ZnCd, NiCd). However, energy dispersive X-ray analysis showed that each of the resulting materials contains substantially different amounts of metal ions (Table S18, ESI†). All of the solid solutions have similar packing arrangements and phase change behaviour to those observed for ZnCP. As expected, the structure of ZnCoCP ([Zn0.92Co0.08(bpdc)(bpy)·2DMF]n) and ZnCdCP ([Zn0.92Cd0.08(bpdc)(bpy)·2DMF]n) are most similar to that of ZnCP; the packing is likely directed by the major metal component. This holds true for both the HT and LT phases of these materials. The structure of NiCdCPHT ([Ni0.83Cd0.17(bpdc)(bpy)·2DMF]n) is also closer to that of NiCPHT. However, owing to the notable difference in packing between NiCPLT and CdCPLT (Fig. S9, ESI†), NiCdCPLT adopts an intermediate packing arrangement, which is similar to that of the other solid solutions.
Variable-temperature unit-cell determinations were carried out to determine the thermal behaviour of the solid solutions. With regard to changes in their unit-cell parameters, all three solid solutions exhibit phase-change behaviour similar to that of ZnCP, albeit at different onset temperatures. ZnCoCPHT exhibits colossal linear NTE along Y2 (αY2 = −712 MK−1) and supercolossal (an order of magnitude larger than colossal)29 PTE along Y3 (αY3 = 1632 MK−1). ZnCoCPHT also displays supercolossal volumetric PTE (αV = 1070 MK−1, Fig. 3 and Table S16, ESI†). Both αV and the degree of anisotropy are far greater for ZnCoCPHT than for ZnCPHT or CoCPHT, and both Y2 and Y3 are nearly twice those for ZnCPHT and many times larger than for CoCPHT. Interestingly, the thermal expansion behaviour of ZnCoCPLT is more similar to that of CoCPLT; it undergoes linear ZTE along Y2 and PTE along the other principal axes (Table S16 and Fig. S40, ESI†), resulting in colossal volumetric PTE.
The thermal expansion of ZnCdCPHT is analogous to that of the ZnCoCPHT (Fig. 3 and Table S16, ESI†). Similarly, to ZnCoCPHT the linear NTE along Y2 is colossal and the PTE along Y3 is supercolossal (αY2 = −711 MK−1, αY3 = 1216 MK−1). However, its volumetric PTE is colossal (αV = 571 MK−1). The orientations of the principal axes and thus the mechanism for thermal expansion in ZnCdCPHT are analogous to those of ZnCoCPHT (Fig. S39, ESI†). The linear thermal expansion behaviour of NiCdCPHT differs from that of both NiCPHT and CdCPHT (Fig. 3); the magnitudes of the coefficients along Y2 and Y3 for NiCdCPHT are notably smaller than those for NiCPHT and CdCPHT.
During the course of this study we noted a discrepancy between the unit-cell parameters at 100 K obtained after incremental cooling (periodic unit-cell determinations) and flash-cooling the crystal (rapid cooling from ambient to 100 K)–see Tables S3–S9, S14 (ESI†). The thermal expansion behaviour appears to be attenuated when the crystal is flash-cooled. This observation was further investigated by means of differential scanning calorimetry at different cooling rates and in triplicate, which supports the assertion that higher cooling rates lead to attenuation of the phase-change (see ESI†).
All of the CPs display interesting thermal expansion behaviour, ranging from colossal volumetric PTE to supercolossal linear thermal expansion. Their thermal expansion coefficients are comparable to the highest reported values for anisotropic thermal expansion (Table 1 and Table S17, ESI†). An overview of reports of colossal (and supercolossal) anisotropic thermal expansion is presented in Table 1 and a more comprehensive survey of the literature is given in Table S17 (ESI†). Almost all of the materials in this study exceed reports of both linear and volumetric thermal expansion in 1D CPs (Table S17, ESI†). The only material exceeding (dramatically) the values reported here requires a spin-crossover transition to effect exceptional thermal expansion of a 2D framework.7 It is interesting to note that the best performing materials in this study (ZnCoCPHT and ZnCdCPHT) and two of the four best performing materials in the literature are all solid solutions. Therefore, solid solutions should be considered a viable method of increasing the thermal expansion properties of a given class of material.
| Material | α Y1 (MK−1) | α Y2 (MK−1) | α Y3 (MK−1) | α V (MK−1) | Ref. |
|---|---|---|---|---|---|
| CA-Pyz | −1375 | 196 | 1524 | 245 | 29 |
| [Zn2(fu-L1)2dabco]n | −380 | 1161 | 14.6 | 837 | 6 |
| [Fe0.84Ni0.16(bpac) (Au(CN)2)2]·2EtOH | −3200 | 5200 | 1500 | 3200 | 7 |
| (Mn0.95Ni0.05)CoGe | −1804 | 1265 | 46 | −624 | 18 |
| ZnCoCPHT | 152 | −712 | 1632 | 1070 | This work |
| ZnCdCPHT | 81 | −711 | 1216 | 570 | This work |
We thank the National Research Foundation (NRF) of South Africa for financial support and the Central Analytical Facilities at Stellenbosch University for EDX analyses.
Conflicts of interest
There are no conflicts to declare.Notes and references
- J. Chen, L. Hu, J. Deng and X. Xing, Chem. Soc. Rev., 2015, 44, 3522–3567 RSC.
- K. Takenaka, Sci. Technol. Adv. Mater., 2012, 13, 013001 CrossRef PubMed.
- Z. Liu, Q. Gao, J. Chen, J. Deng, K. Lin and X. Xing, Chem. Commun., 2018, 54, 5164–5176 RSC.
- D. Das, T. Jacobs and L. J. Barbour, Nat. Mater., 2010, 9, 36–39 CrossRef CAS PubMed.
- W. Cai and A. Katrusiak, Nat. Commun., 2014, 5, 4337 CrossRef CAS PubMed.
- S. Henke, A. Schneemann and R. A. Fischer, Adv. Funct. Mater., 2013, 23, 5990–5996 CrossRef CAS.
- B. R. Mullaney, L. Goux-Capes, D. J. Price, G. Chastanet, J.-F. Létard and C. J. Kepert, Nat. Commun., 2017, 8, 1053 CrossRef PubMed.
- J. Chen, Q. Gao, A. Sanson, X. Jiang, Q. Huang, A. Carnera, C. G. Rodriguez, L. Olivi, L. Wang, L. Hu, K. Lin, Y. Ren, Z. Lin, C. Wang, L. Gu, J. Deng, J. P. Attfield and X. Xing, Nat. Commun., 2017, 8, 14441 CrossRef CAS PubMed.
- H. Yamamoto, T. Imai, Y. Sakai and M. Azuma, Angew. Chem., Int. Ed., 2018, 57, 8170–8173 CrossRef CAS PubMed.
- L. D. Devries, P. M. Barron, E. P. Hurley, C. Hu and W. Choe, J. Am. Chem. Soc., 2011, 133, 14848–14851 CrossRef CAS PubMed.
- T. A. Mary, J. S. Evans, T. Vogt and A. W. Sleight, Science, 1996, 272, 90–92 CrossRef CAS.
- J. S. O. Evans, Z. Hu, J. D. Jorgensen, D. N. Argyriou, S. Short and A. W. Sleight, Science, 1997, 275, 61–65 CrossRef CAS PubMed.
- A. L. Goodwin, M. Calleja, M. J. Conterio, M. T. Dove, J. S. O. Evans, D. A. Keen, L. Peters and M. G. Tucker, Science, 2008, 319, 794–797 CrossRef CAS PubMed.
- J. P. Zhang, P. Q. Liao, H. L. Zhou, R. B. Lin and X. M. Chen, Chem. Soc. Rev., 2014, 43, 5789–5814 RSC.
- Z. Chang, D. H. Yang, J. Xu, T. L. Hu and X. H. Bu, Adv. Mater., 2015, 27, 5432–5441 CrossRef CAS PubMed.
- S. R. Batten, N. R. Champness, X. Chen, J. Garcia-Martinez, S. Kitagawa, L. Ohrstrom, M. O’Keeffe, M. Paik Suh and J. Reedijk, CrystEngComm, 2012, 14, 3001–3004 RSC.
- S. J. Hunt, M. J. Cliffe, J. A. Hill, A. B. Cairns, N. P. Funnell and A. L. Goodwin, CrystEngComm, 2015, 17, 361–369 RSC.
- Q. Ren, W. Hutchison, J. Wang, A. Studer, G. Wang, H. Zhou, J. Ma and S. J. Campbell, ACS Appl. Mater. Interfaces, 2019, 11, 17531–17538 CrossRef CAS PubMed.
- L. J. Barbour, D. Das, T. Jacobs, G. O. Lloyd and V. J. Smith, in Supramolecular Chemistry: from Molecules to Nanomaterials, ed. P. A. Gale and J. W. Steed, John Wiley & Sons, Ltd., Hoboken, 2012 Search PubMed.
- L. J. Barbour, Chem. Commun., 2006, 1163 RSC.
- L. M. van Wyk and L. J. Barbour, Cryst. Growth Des., 2021, 21, 3056–3062 CrossRef CAS.
- M. J. Cliffe and A. L. Goodwin, J. Appl. Crystallogr., 2012, 45, 1321–1329 CrossRef CAS.
- I. Grobler, V. J. Smith, P. M. Bhatt, S. A. Herbert and L. J. Barbour, J. Am. Chem. Soc., 2013, 135, 6411–6414 CrossRef CAS PubMed.
- E. R. Engel, V. J. Smith, C. X. Bezuidenhout and L. J. Barbour, Chem. Mater., 2016, 28, 5073–5079 CrossRef CAS.
- L. Hu, J. Chen, J. Xu, N. Wang, F. Han, Y. Ren, Z. Pan, Y. Rong, R. Huang, J. Deng, L. Li and X. Xing, J. Am. Chem. Soc., 2016, 138, 14530–14533 CrossRef CAS PubMed.
- J. S. Ovens and D. B. Leznoff, Inorg. Chem., 2017, 56, 7332–7343 CrossRef CAS PubMed.
- H. L. Zhou, R. B. Lin, C. T. He, Y. B. Zhang, N. Feng, Q. Wang, F. Deng, J. P. Zhang and X. M. Chen, Nat. Commun., 2013, 4, 1–8 Search PubMed.
- H.-L. Zhou, J.-P. Zhang and X.-M. Chen, Front. Chem., 2018, 6, 1–7 CrossRef PubMed.
- H. Liu, M. J. Gutmann, H. T. Stokes, B. J. Campbell, I. R. Evans and J. S. O. Evans, Chem. Mater., 2019, 31, 4514–4523 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic procedure, TGA, additional figures, SCXRD, PXRD, DSC and PAS results. CCDC 1940578, 2036084–2036096. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d1cc01717a |
| This journal is © The Royal Society of Chemistry 2021 |