 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Distinct photodynamics of κ-N and κ-C pseudoisomeric iron(II) complexes†‡
Philipp
Dierks
a,
Ayla
Kruse
b,
Olga S.
Bokareva
 bc,
Mohammed J.
Al-Marri
bc,
Mohammed J.
Al-Marri
 bd,
Jens
Kalmbach
e,
Marc
Baltrun
a,
Adam
Neuba
a,
Roland
Schoch
bd,
Jens
Kalmbach
e,
Marc
Baltrun
a,
Adam
Neuba
a,
Roland
Schoch
 a,
Stephan
Hohloch
a,
Stephan
Hohloch
 f,
Katja
Heinze
f,
Katja
Heinze
 g,
Michael
Seitz
g,
Michael
Seitz
 e,
Oliver
Kühn
e,
Oliver
Kühn
 b,
Stefan
Lochbrunner
b,
Stefan
Lochbrunner
 b and
Matthias
Bauer
b and
Matthias
Bauer
 *a
*a
aFaculty of Science, Chemistry Department and Centre for Sustainable Systems Design, Paderborn University, 33098 Paderborn, Germany. E-mail: matthias.bauer@upb.de
bInstitute of Physics and Department of Life, Light and Matter, University of Rostock, 18051 Rostock, Germany
cDepartment of Physical Chemistry, Kazan Federal University, 420008 Kazan, Russia
dCollege of Engineering, Qatar University, P.O. Box 2713, Doha, Qatar
eInstitute of Inorganic Chemistry, University of Tübingen, Auf der Morgenstelle 18, 72076 Tübingen, Germany
fUniversity of Innsbruck, Faculty of Chemistry and Pharmacy, Institute for General, Inorganic and Theoretical Chemistry, Innrain 80-82, Innsbruck 6020, Austria
gDepartment of Chemistry, Johannes Gutenberg, University of Mainz, 55128 Mainz, Germany
First published on 14th June 2021
Abstract
Two closely related FeII complexes with 2,6-bis(1-ethyl-1H-1,2,3-triazol-4yl)pyridine and 2,6-bis(1,2,3-triazol-5-ylidene)pyridine ligands are presented to gain new insights into the photophysics of bis(tridentate) iron(II) complexes. The [Fe(N^N^N)2]2+ pseudoisomer sensitizes singlet oxygen through a MC state with nanosecond lifetime after MLCT excitation, while the bis(tridentate) [Fe(C^N^C)2]2+ pseudoisomer possesses a similar 3MLCT lifetime as the tris(bidentate) [Fe(C^C)2(N^N)]2+ complexes with four mesoionic carbenes.
Utilization of solar energy is a current scientific challenge. Light harvesting approaches based on d6 metal complexes of RuII, OsII and IrIII with π-accepting ligands have been widely explored.1 The search for earth-abundant alternatives focusses on iron complexes.2,3 Challenge here is the small ligand field splitting, leading to short-lived MLCT (Metal to Ligand Charge Transfer) states due to MC (Metal Centred) states of lower energy. Several strategies are available to extend the MLCT lifetimes of iron complexes. The ligand field splitting can be increased by strong σ-donors like N-heterocyclic carbenes (NHCs)4 or optimization of the octahedral symmetry.5,6 MLCT states can be stabilized by π-acceptor ligands or increased excited electron delocalization.7 Following these strategies, MLCT lifetimes could be increased from 0.15 ps in [Fe(tpy)2]2+ (tpy = 2,2′:6′,2′′-terpyridine) over 9 ps in [Fe(bim)2]2+ (bim = 2,6-bis(imidazol-2-ylidene)pyridine)8 to 528 ps in [Fe(btz)3]2+ (btz = 4,4′-bis(1,2,3-triazol-5-ylidene)).9 Polypyridyl ligands are weak σ-donors, but good π-acceptors. In contrast, carbenes in [Fe(bim)2]2+ are stronger σ-donors and weaker π-acceptors. Mesoionic carbenes (MICs) like in [Fe(btz)3]2+ are both strong σ-donors and moderate π-acceptors.10–12 For NHC–iron(II)-complexes, the lifetime of 3MLCT states has shown to increase with the number of NHC and MIC units.9,13,14
Since triazolylidenes as a special class of MICs, can be synthesized via methylation of the corresponding triazoles, they offer a unique possibility to address some fundamental questions in the photochemistry of FeII complexes using tridentate ligands. Such complexes usually offer higher chemical stability, but lower octahedral symmetry compared to FeII complexes of bidentate ligands. Although FeII triazole complexes are known for their spin-crossover properties15,16 their photophysics are unexplored. Moving from the triazole FeII complex Fe1 to the MIC complex Fe2 (Scheme 1) allows to address how much the degree of octahedral symmetry – in the sense of the C–Fe–C trans angle – affects the excited state landscape by comparison of bis(tridentate) with tris(bidentate) MIC complexes. Therefore two new FeII complexes with 2,6-bis(1-ethyl-1H-1,2,3-triazol-4-yl)pyridine (2) Fe1 and 2,6-bis(1,2,3-triazol-5-ylidene)pyridine (3) ligand Fe2 as a bis(tridentate) tetra-MIC type were prepared (Scheme 1). 2 is obtained by coupling of 2,6-dibromopyridine with trimethylsilylacetylene to give 1 and a azide–alkyne cyclization of 1 with in situ prepared ethylazide. Triazole 2 is methylated with methyltriflate in DCM at −80 °C to give the triazolium salt 3. Reaction of FeBr2 in degassed ethanol with 2 yields Fe1. The preparation of Fe2 requires deprotonation of 3 with LiHMDS in THF at −80 °C and addition of FeBr2.
The key structural parameters obtained from single crystal diffraction are discussed by comparing Fe1 to [Fe(tpy)2]2+ as [Fe(N^N^N)2]2+ reference. Fe2 is compared to [Fe(bim)2]2+ and [Fe(btz)2(bpy)]2+ as bis(tridentate) [Fe(C^N^C)2]2+ and tris(bidentate) [Fe(C^C)2(N^N)]2+ reference. The Fe–N bond length of 1.9539(9) Å in Fe1 and the N–Fe–N trans angle of 161.06(4)° are only slightly changed with respect to 1.987(3) Å and 161.55(12)° in [Fe(tpy)2]2+.17 Thus, the different electronic properties of the triazole and tpy ligands only slightly affect the crystal structure. A different picture can be found for Fe2 in comparison to the bis(tridentate) [Fe(bim)2]2+. The C–Fe–C trans angle of 159.68(17)° in Fe2 indicates an increased octahedral symmetry in comparison to 158.32(15)° in [Fe(bim)2]2+. The Fe–Npyridine bond length is elongated from 1.925(3) Å in [Fe(bim)2]2+ to 1.956(3) Å in Fe2 by stronger π-backdonation. This interpretation is confirmed by the shortening of the Fe–Ccarbene bond length from 1.9665(3) Å in [Fe(bim)2]2+ to 1.9403(4) Å in Fe2.8 The structural parameters, which are in line with those of [Fe(btp)2]2+ reported by Iwasaki et al.18 (btp = 2,6-bis(1-mesityl-2,3-triazol-5-ylidene)pyridine), therefore support the stronger σ-donating character of MICs in comparison to classical NHCs. Bidentate ligands are advantageous to achieve a high octahedral symmetry. Accordingly, the tris(bidentate) tetra-MIC complex [Fe(btz)2(bpy)]2+ – with the same number of MIC and N-donors as Fe2 – shows a much larger C–Fe–C trans angle of 173.0(7)° when compared to 159.68(17)° in Fe2. Although the strong σ-donating character of MICs is also reflected in the comparatively short Fe–Ccarbene bond length of 1.967(17) Å in [Fe(btz)2(bpy)]2+, this value is also shorter in the bis(tridentate) complex Fe2.
The optical absorption spectra of Fe1 and Fe2 together with TDDFT calculations with an optimally-tuned range-separated LC-BLYP functional are shown in Fig. 1.7,19–21 The intense bands below 350 nm are dominated by ligand π–π* transitions. The presence of small intensity MLCT transitions in this region should be noted. At 444 nm, Fe1 exhibits a sharp absorption maximum with an additional shoulder at higher energies. These features are assigned to MLCT transitions with some admixture of MC character, which is also known for [Fe(tpy)2]2+.13Fe2 shows a much broader MLCT absorption. The lowest energy maximum is at 593 nm and thus lower in energy than in [Fe(bim)2]2+.13Fe2 shows a MLCT shift from 593 nm to 609 nm in comparison to [Fe(btz)2(bpy)]2+.14 This band and the maximum at 411 nm have also a predominant MLCT character. The offset of the lowest bands between experiment and theory has been observed for other iron complexes, too.7 Also, the intensities are underestimated in that range. The origin of this problem is currently not clear.20 For both complexes, the lowest adiabatic triplet state has MC character with energies of 1.31 eV (946 nm) for Fe1 and 1.62 eV (765 nm) for Fe2. Both energies are sufficient for effective oxygen quenching.24 The excitation from the ground state (1GS) to the lowest triplet states is accompanied by elongation of the central Fe–N bonds by 0.16 Å (Fe1) and by 0.28 Å (Fe2).14
 | ||
| Fig. 1 Experimental and theoretical absorption spectra of Fe1 (panel A, upper part) and Fe2 (panel B, upper part) as well as density-matrix analysis21 based assignment of corresponding vertical singlet and triplet excited states (lower parts). Broadening of theoretical spectra is done by Gaussian function (FWHM 0.2 eV). The legends in panel (A) are also valid for panel (B) and vice versa. | ||
The cyclic voltammogram of Fe1 shows a reversible wave at 0.74 V (Fig. 2, all redox potentials are given vs. FcH/FcH+) which is assigned to the FeIII/II couple.7,13 The shift to more anodic potentials compared to [Fe(tpy)2]2+ (0.72 V)13 indicates a slightly reduced HOMO energy. The HOMO in Fe1 has a dπ(Fe) character. This points to a similar π-acceptability of the triazoles in comparison to tpy. At cathodic potentials, two reversible reductions at −1.79 V and −2.11 V are detected. In analogy to [Fe(tpy)2]2+, they are assigned to a stepwise reduction of the two ligands.13 The electrochemical energy gap of 2.85 eV (435 nm) is in line with the absorption maximum in the MLCT region.
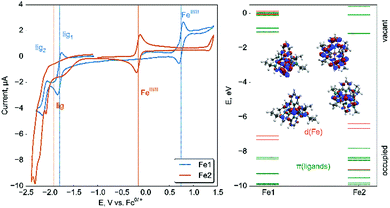 | ||
| Fig. 2 Cyclic voltammetry (left) and molecular orbital scheme (right) of Fe1 and Fe2 including shapes of HOMOs (predominantly localized on d(Fe)) and LUMOs (π*-orbitals of ligands). | ||
The FeIII/II couple in Fe2 is found at a low cathodic potential of −0.15 V. This significant shift in comparison to Fe1 and [Fe(bim)2]2+ (0.34 V) is intimately connected to the MIC coordination and agrees with reported trends.7,13 In [Fe(btz)2(bpy)]2+, the FeIII/II couple occurs at a more cathodic potential (−0.35 V). The HOMO of Fe2 has a dominating dπ(Fe) character and is destabilized by a weaker π-acceptability of the triazolylidenes in comparison to the triazoles. In comparison to imidazolylidene ligands in [Fe(bim)2]2+, the triazolylidine ligands in Fe2 might have a better π-acceptability,10,14 as the irreversible reduction in Fe2 occurs at a potential of −1.91 V.7 The electrochemical energy gap of 2.00 eV (600 nm) again proves the MLCT assignment of the 593 nm absorption (see Table 1). Electrochemical experiments allow to identify the charge-transfer excited states in Fe1 and Fe2.22
| UV [nm] (ε [M−1 cm−1]) | CV [V vs. FcH/FcH+] | ΔE1/2 | TA | Emission | |
|---|---|---|---|---|---|
| Fe1 | 444 (11.500) | 0.74 (rev.) | 2.85 eV | τ 1 ≫ 1.6 ns | 334 nm |
| 295 (44.900) | −1.79 (rev.) | (435 nm) | 2.9 ns | ||
| −2.11 (rev.) | |||||
| Fe2 | 593 (21.000) | −0.15 (rev.) | 1.76 eV | τ 1 = 0.1 ps | — |
| 411 (11.200) | −1.91 (irrev.) | (425 nm) | τ 2 = 2.7 ps | ||
| 326 (32.500) | −2.15 (irrev.) | 2.00 eV | τ 3 = 8.7 ps | ||
| (619 nm) |
These states are probed by ultrafast pump–probe spectroscopy. For Fe1, a step-like absorption change at time zero is observed, which persists beyond the experimental time window of 1.6 ns (see Fig. 3A). The transient absorption (TA) spectra reflect the ground state bleach (GSB) but deviate significantly from what is expected for a populated charge transfer state (Fig. 3A, Fe1+–Fe1).22 Beside the dominant GSB signature, excited state absorption (ESA) should be detected, which is missing in the TA spectra.
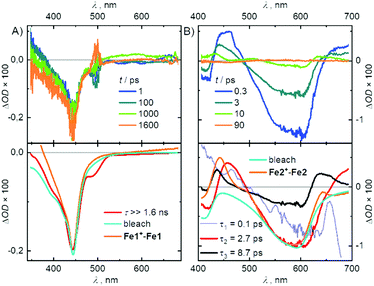 | ||
| Fig. 3 TA spectra (top) and DAS (bottom) of (A) Fe1 and (B) Fe2. Excited-state differential spectra (orange) approximated as suggested by McCusker et al.22 DAS of Fe1 was smoothed. Both bleaches and difference spectra were scaled. | ||
This and the very long lifetime of the TA signal indicate that excitation of Fe1 results in an ultrafast population of a ligand field MC state. Similar findings related to 3/5MC → 1GS ground state recovery were also reported for other [FeN6]2+ complexes.5 If the ligand π–π* transitions at 295 nm are excited, Fe1 exhibits a ligand-based emission at λem = 334 nm (ΔEphoton = 0.49 eV) with a lifetime of τem = 2.9 ns. Such an emission is not observed in Fe2. The TA of Fe2 is dominated by GSB causing a strong negative band around 600 nm and a less intense one around 420 nm (Fig. 3B). In addition, two ESA features at about 455 nm and in the red spectral region are observed. The TA disappears in less than 50 ps with an evolution, which has to be described by four exponential decay components. One component of 5 fs is an artefact at time zero. The component with a time constant of τ1 = 0.1 ps is slightly red-shifted compared to the longer living contributions, suggesting an ultrafast relaxation like vibrational redistribution or intersystem crossing (ISC). The decay associated spectra (DAS) of the dominating component with τ2 = 2.7 ps and the weaker one with τ3 = 8.7 ps consist both of the described GSB and ESA features in the blue and red of the GSB, most probably a 3MLCT feature.14 This conclusion is supported by the difference spectrum of Fe2+–Fe2, which reproduces the ESA in the blue and the GSB of the two decay components very well. In contrast, the ESA in the red is only insufficiently reflected. This may result from the fact, that for a better description of the MLCT spectrum the difference Fe2+ + Fe2−–Fe2 should be considered,22 which is impossible due to the irreversible ligand reduction.
Analogous behaviour is observed in [Fe(btz)2(bpy)]2+ and the spectral components were assigned to multiple MLCT states.14 The analysis of data from time-resolved X-ray spectroscopy on this tris(bidentate) reference associated the time constants to different transitions in a hot branching scenario.23 Transferred to Fe2 this means a vibrationally hot 3MLCT state is populated by the ultrafast ISC and vibrational redistribution leads within 0.1 ps to a relaxed 3MLCT state. In case of hot branching, the redistribution process competes with a direct channel to the 3MC state in which a large fraction of the population ends up within the first 0.1 ps. The remaining population in the relaxed 3MLCT state decays comparatively slowly (8.7 ps) to the 3MC state, which deactivates more rapidly (2.7 ps) back to the 1GS. In iron carbene complexes the 5MC state are bypassed in the relaxation cascade as strong structural rearrangement is necessary.4,7 This relaxation scenario is supported by the observation that the DAS of both, the 2.7 ps and the 8.7 ps component exhibit a strong GSB contribution. Although it is not in line with the current literature reports on similar complexes, the following second scenario could also explain the reported findings. The 3MLCT state exhibits two parallel relaxation channels, one leading directly back to the 1GS and the second one to the 3MC state. Then the 2.7 ps have to be assigned to the 3MLCT state and the 8.7 ps to the 3MC state.
Further insights into the presence of long-lived triplet states is gained by 1O2 sensitization experiments (see ESI‡).24Fe2 does not show any sensitization activity. In contrast, Fe1 sensitizes oxygen efficiently after excitation with 480 nm and 275 nm as deduced from energy transfer reaction of formed 1O2 with 1,3-diphenyl-isobenzofuran and detection of the 1O2 emission signal at 1270 nm. A significant 3MC contribution to the relaxation pathway is thus concluded by energy transfer experiments and lowest triplet state optimization, although a 5MC contribution cannot be fully ruled out. McCusker et al. recently reported spectroscopical evidence of 5MC states taking part in electron transfer reactions25 and reactivity of MC-states is also known in chromium(III)- and cobalt(III) complexes.26,27 The deduced relaxation cascades for Fe1 and Fe2 are compared in Fig. 4.
 | ||
| Fig. 4 Proposed relaxation cascade following MLCT excitation of Fe1 resulting from the experimental findings and of Fe2 according to the hot branching scenario by Wärnmark et al.14,23 | ||
A pair of two pseudo-constitutional FeII complex isomers with the same ligand core motif, but in form of a triazole [Fe(N^N^N)2]2+ (N^N^N = 2,6-bis(1-ethyl-1H-1,2,3-triazol-4-yl)pyridine) (Fe1) and of a pyridine-MIC [Fe(C^N^C)2]2+ (C^N^C = 2,6-bis(1-ethyl-2,3-triazol-5-ylidene)pyridine) (Fe2) is presented. Fe1 shows a similar excited state behaviour as other [Fe(N^N^N)2]2+ complexes, such as [Fe(tpy)2]2+ with a long-lived 3MC, identified by its transient absorption and 1O2 sensitizing activity. This bis(triazolyl)pyridine complex offers thus a potential new class of FeII complexes that enable energy transfer reactions by excitation, making use of metal centred states. Despite a significantly reduced octahedral symmetry, the bis(tridentate) [Fe(C^N^C)2]2+ complex Fe2 shows a very similar behaviour as the tris(bidentate) [Fe(C^C)2(N^N)]2+ complex [Fe(btz)2(bpy)]2+. Transient absorption of Fe2 suggests analogous hot branching dynamics as in [Fe(btz)2(bpy)]2+. Comparing the photophysics of tetra-MIC Fe2 and [Fe(btz)2(bpy)]2+ – which show both a net coordination of two nitrogen and four carbon ligand atoms – the bis(tridentate) [Fe(C^N^C)2]2+ form appears to be equivalent or even slightly superior to the tris(bidentate) [Fe(C^C)2(N^N)]2+ in terms of excited state lifetimes.
Financial support from the Deutsche Forschungsgemeinschaft [DFG, Priority Program SPP 2102] “Light-controlled reactivity of metal complexes” (BA 4467/7-1, LO 714/11-1, KU 952/12-1, SE 1448/8-1, HE 2778/14-1) is gratefully acknowledged by M. B., O. K., S. L., M. S. and K. H. P. D. thanks the Fonds der Chemischen Industrie for a Kekulé grant. O. B. thanks Grant No. 14.Y26.31.0019 from Ministry of Education and Science of Russian Federation for financial support.
Conflicts of interest
There are no conflicts to declare.References
- V. Balzani, G. Bergamini, S. Campagna and F. Puntoriero, Photochemistry and Photophysics of Coordination Compounds I, Springer, 2007, pp. 1–36 Search PubMed.
- C. Förster and K. Heinze, Chem. Soc. Rev., 2020, 49, 1057 Search PubMed.
- O. S. Wenger, Chem. – Eur. J., 2019, 25, 6043 Search PubMed.
- Y. Liu, P. Persson, V. Sundström and K. Wärnmark, Acc. Chem. Res., 2016, 49, 1477 Search PubMed.
- L. L. Jamula, A. M. Brown, D. Guo and J. K. McCusker, Inorg. Chem., 2014, 53, 15 Search PubMed.
- A. K. C. Mengel, C. Förster, A. Breivogel, K. Mack, J. R. Ochsmann, F. Laquai, V. Ksenofontov and K. Heinze, Chem. – Eur. J., 2015, 21, 704 Search PubMed.
- P. Dierks, A. Päpcke, O. S. Bokareva, B. Altenburger, T. Reuter, K. Heinze, O. Kühn, S. Lochbrunner and M. Bauer, Inorg. Chem., 2020, 59, 14746 Search PubMed.
- Y. Liu, T. Harlang, S. E. Canton, P. Chábera, K. Suárez-Alcántara, A. Fleckhaus, D. A. Vithanage, E. Göransson, A. Corani, R. Lomoth, V. Sundström and K. Wärnmark, Chem. Commun., 2013, 49, 6412 Search PubMed.
- P. Chábera, K. S. Kjaer, O. Prakash, A. Honarfar, Y. Liu, L. A. Fredin, T. C. B. Harlang, S. Lidin, J. Uhlig, V. Sundström, R. Lomoth, P. Persson and K. Wärnmark, J. Phys. Chem. Lett., 2018, 9, 459 Search PubMed.
- D. G. Brown, N. Sanguantrakun, B. Schulze, U. S. Schubert and C. P. Berlinguette, J. Am. Chem. Soc., 2012, 134, 12354 Search PubMed.
- J. Beerhues, H. Aberhan, T.-N. Streit and B. Sarkar, Organometallics, 2020, 39, 4557 Search PubMed.
- D. Schweinfurth, L. Hettmanczyk, L. Suntrup and B. Sarkar, Z. Anorg. Allg. Chem., 2017, 643, 554 Search PubMed.
- P. Zimmer, L. Burkhardt, A. Friedrich, J. Steube, A. Neuba, R. Schepper, P. Müller, U. Flörke, M. Huber, S. Lochbrunner and M. Bauer, Inorg. Chem., 2018, 57, 360 Search PubMed.
- Y. Liu, K. S. Kjaer, L. A. Fredin, P. Chábera, T. Harlang, S. E. Canton, S. Lidin, J. Zhang, R. Lomoth, K.-E. Bergquist, P. Persson, K. Wärnmark and V. Sundström, Chem. – Eur. J., 2015, 21, 3628 Search PubMed.
- M. Ostermeier, M.-A. Berlin, R. M. Meudtner, S. Demeshko, F. Meyer, C. Limberg and S. Hecht, Chem. – Eur. J., 2010, 16, 10202 Search PubMed.
- D. Schweinfurth, S. Demeshko, S. Hohloch, M. Steinmetz, J. G. Brandenburg, S. Dechert, F. Meyer, S. Grimme and B. Sarkar, Inorg. Chem., 2014, 53, 8203 Search PubMed.
- H. Oshio, H. Spiering, V. Ksenofontov, F. Renz and P. Gütlich, Inorg. Chem., 2001, 40, 1143 Search PubMed.
- H. Iwasaki, Y. Koga and K. Matsubara, Org. Chem.: Curr. Res., 2016, 5, 161 Search PubMed.
- T. Möhle, O. S. Bokareva, G. Grell, O. Kühn and S. I. Bokarev, J. Chem. Theory Comput., 2018, 14, 5870 Search PubMed.
- O. S. Bokareva, O. Baig, M. J. Al-Marri, O. Kühn and L. González, Phys. Chem. Chem. Phys., 2020, 22, 27605 Search PubMed.
- F. Plasser, J. Chem. Phys., 2020, 152, 84108 Search PubMed.
- A. M. Brown, C. E. McCusker and J. K. McCusker, Dalton Trans., 2014, 43, 17635 Search PubMed.
- H. Tatsuno, et al. , Angew. Chem., Int. Ed., 2020, 59, 364 Search PubMed.
- C. Schweitzer and R. Schmidt, Chem. Rev., 2003, 103, 1685 Search PubMed.
- M. D. Woodhouse and J. K. McCusker, J. Am. Chem. Soc., 2020, 142, 16229 Search PubMed.
- S. Otto, M. Dorn, C. Förster, M. Bauer, M. Seitz and K. Heinze, Coord. Chem. Rev., 2018, 359, 102 Search PubMed.
- S. Kaufholt, N. W. Rosemann, P. Chábera, L. Lindh, I. Bolaño Losada, J. Uhlig, T. Pascher, D. Strand, K. Wärnmark, A. Yartsev and P. Persson, J. Am. Chem. Soc., 2021, 143, 1307 Search PubMed.
Footnotes |
| † Dedicated to the memory of Prof. Dr Markus Gerhards. |
| ‡ Electronic supplementary information (ESI) available: Synthetic procedures, NMR spectra, MS data, crystallographic data, information on computational studies, experimental details of the transient absorption and 1O2 sensitization experiments. CCDC 2049729 and 2050317. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d1cc01716k |
| This journal is © The Royal Society of Chemistry 2021 |

