Open Access Article
Open Access ArticleRetracted Article: Nucleophilic iodonium interactions (NIIs) in 2-coordinate iodine(I) and silver(I) complexes†
Jas S.
Ward
 *a,
Antonio
Frontera
*a,
Antonio
Frontera
 b and
Kari
Rissanen
b and
Kari
Rissanen
 *a
*a
aUniversity of Jyvaskyla, Department of Chemistry, Jyväskylä 40014, Finland. E-mail: james.s.ward@jyu.fi; kari.t.rissanen@jyu.fi
bDepartment of Chemistry, Universitat de les Illes Balears, Crts de Valldemossa km 7.6, Palma de Mallorca Baleares 07122, Spain
First published on 20th April 2021
Abstract
The generality of nucleophilic iodonium interactions (NIIs) has been demonstrated by preparing a range of silver(I) and iodonium (I+) complexes and studying their 15N NMR chemical shifts, with the first example of a NII-complex involving a 2-coordinate silver(I) complex being confirmed by X-ray crystallography, and its nucleophilicity studied by DFT calculations.
Halonium complexes, the extreme case of a halogen atom that has been completely ionised to a formally cationic state (a halenium ion stabilised by a pair of Lewis bases),1 have long enjoyed pride of place for their novelty and their utility in organic transformations.2,3 Whilst first reported in the late 1960's,4,5 iodonium ion complexes [N–I–N]+ were repurposed by Barluenga and co-workers in the 1990's through his eponymous reagent, [I(pyridine)2]BF4, where he showcased their myriad of uses, including the electrophilic iodination of unactivated arenes, the promotion of C–C and C–X bond formation, and the selective direct iodination of peptides.6–8
The stability and nucleophilic behaviour of halonium ions has been less pursued in comparison to the study of their uses, though the recent report of an iodonium ion forming a strong bonding interaction with a silver(I) cation has opened up new avenues of study (Fig. 1).9,10 Computational studies on the I+⋯Ag+ interaction indicated that it is different from an argentophilic interaction, as it is comprised of electron donation from the I+ to the Ag+ and notably lacks a retro-donation component, with the I+ acting as a nucleophile.
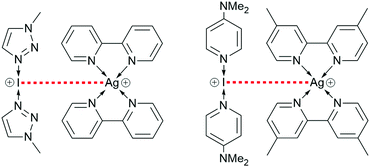 | ||
| Fig. 1 The only two known examples of nucleophilic iodonium interactions (NIIs, indicated with a dashed red line; PF6 anions omitted for clarity).9,10 | ||
The interaction was first confirmed in the solid-state by X-ray diffraction, and was undetectable in the 1H NMR studies. However, further investigation proved the interaction persisted in the solution state, detected through comparison of the 15N NMR chemicals shifts of the atoms directly bonded to the I+ and Ag+ centres determined by 1H–15N HMBC experiments.
These comparisons revealed that the pair of I+ and Ag+ complexes, when compared to its respective pure constituent Ag+ complex, demonstrated a modest but definitive difference in the 15N NMR shifts. A second example of a NII, [I(4-DMAP)2]PF6*[Ag(bpyMe2)2]PF6, has also been recently reported.10
Inspired by these two examples of Nucleophilic Iodonium Interactions (NIIs), an investigation of simpler combinations of I+ and Ag+ complexes was performed to see if other examples of this new type of interaction could be obtained.
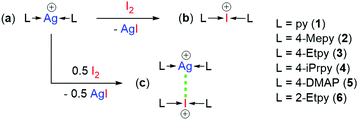
Halonium complexes, [N–X–N]+ (X = Br, I), can be readily prepared by cation exchange of their respective silver(I) complexes upon reaction with one equivalent of the elemental halogen, X2 (Scheme 1).11–14 To be able to directly access a wide range of combinations of I+ and Ag+ complexes, the potential NII-bonded pairs of complexes, or NII-complexes, in this study were synthesised by partial cation exchange using only 0.5 equivalents of I2,9 which resulted in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 product mixture of the I+
1 product mixture of the I+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ag+ NII-complexes. The use of partial cation exchange obviated the need to isolate and accurately determine the amount of the iodonium complexes, which can be difficult due to their reactivity. Similarly, the use of homoleptic (with respect to both the I+ and Ag+) complexes eliminated the effect of ligand scrambling that was demonstrated to be a possible complication when the first examples of heteroleptic iodonium complexes were reported.15,16
Ag+ NII-complexes. The use of partial cation exchange obviated the need to isolate and accurately determine the amount of the iodonium complexes, which can be difficult due to their reactivity. Similarly, the use of homoleptic (with respect to both the I+ and Ag+) complexes eliminated the effect of ligand scrambling that was demonstrated to be a possible complication when the first examples of heteroleptic iodonium complexes were reported.15,16
For this work, a series of silver(I) (1a–6a), iodonium (1b–6b), and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 I+
1 I+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ag+ NII-complexes (1c–6c) were synthesised from common substituted pyridines
Ag+ NII-complexes (1c–6c) were synthesised from common substituted pyridines![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) pyridine (py; 1), 4-methylpyridine (4-Mepy; 2), 4-ethylpyridine (4-Etpy; 3), 4-isopropylpyridine (4-iPrpy; 4), N,N-dimethylaminopyridin-4-amine (4-DMAP; 5), and 2-ethylpyridine (2-Etpy; 6).
pyridine (py; 1), 4-methylpyridine (4-Mepy; 2), 4-ethylpyridine (4-Etpy; 3), 4-isopropylpyridine (4-iPrpy; 4), N,N-dimethylaminopyridin-4-amine (4-DMAP; 5), and 2-ethylpyridine (2-Etpy; 6).
Remarkably, all of the NII-complexes tested in this study showed significant 15N NMR chemical shift changes (also known as coordination shifts), ΔδN (Table 1). These ΔδN shifts were calculated by subtraction of the 15N NMR resonance of the pure Ag+ complexes (1a–6a) from the 15N NMR resonances of the Ag–N nuclei in the NII-complexes (1c–6c), with observed ΔδN shifts ranging from 0.9 ppm for 2c, to 10.9 ppm for 6c. Three of the NII-complexes (3c–4c, 6c) gave ΔδN shifts greater than both those reported for the only two known examples of NIIs (cf. −2.9 and 2.2 ppm),9,10 which could be interpreted as those three NII-complexes exhibiting stronger NIIs in solution.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 iodonium
1 iodonium![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) silver(I) NII-complexes relative to their respective pure silver(I) complexes
silver(I) NII-complexes relative to their respective pure silver(I) complexes
| NII-complexes | ΔδN (ppm) | |
|---|---|---|
| 1c | [I(py)2]PF6*[Ag(py)2]PF6 | 2.3 |
| 2c | [I(4-Mepy)2]PF6*[Ag(4-Mepy)2]PF6 | 0.9 |
| 3c | [I(4-Etpy)2]PF6*[Ag(4-Etpy)2]PF6 | −7.8 |
| 4c | [I(4-iPrpy)2]PF6*[Ag(4-iPrpy)2]PF6 | 7.7 |
| 5c | [I(4-DMAP)2]PF6*[Ag(4-DMAP)2]PF6 | −1.6 |
| 6c | [I(2-Etpy)2]PF6*[Ag(2-Etpy)2]PF6 | 10.9 |
It should be noted that in all the NII-complexes tested (1c–6c), the ΔδN values for the I–N nuclei when compared to their respective pure I+ complexes (1b–6b) were always effectively unchanged, with all but one value having a difference of less than 0.10 ppm (Table S1, ESI†). All I–N ΔδN values were in the range of 0.0–0.4 ppm, with the largest (and only statistically valid) difference observed between the NII-complex [I(4-Etpy)2]PF6*[Ag(4-Etpy)2]PF6‡ (3c) and [I(4-Etpy)2]PF6 (3b). The unchanging I–N ΔδN values demonstrate that though the iodonium centres are acting as nucleophiles toward the Ag+, this has little to no effect on the strength of the I+ intramolecular bonds. The static I–N ΔδN values also facilitate their use as a visual NMR reference in striking contrast to the variable Ag–N ΔδN values that are heavily affected by the NIIs.
The directionality of the ΔδN shifts is of interest, because it implies a shielding (negative ΔδN) or deshielding (positive ΔδN) effect on the receiving silver(I) complex relative to its pure complex. However, this might be misleading as the solid-state structures of 1a–3a exist as argentophilically-coordinated dimers (4a–6a were observed as monomers), which might persist in some form to the solution state, with the presence of this argentophilic interaction potential having an effect on the δN values for the ‘pure’ silver(I) complexes and skew the ΔδN comparisons. It should be noted that even the two previously known examples of NIIs, [I(mtz)2]PF6*[Ag(bpy)2]PF6 and [I(4-DMAP)2]PF6*[Ag(bpyMe2)2]PF6,9,10 show contrary ΔδN shifts of −2.9 and 2.2 ppm, respectively, with the positive ΔδN shift of [I(4-DMAP)2]PF6*[Ag(bpyMe2)2]PF6 possibly being a consequence of the significant structural changes observed in the solid-state for the iodonium component to accommodate this shorter and stronger NII. Therefore, the magnitude and directionality of the observed ΔδN shifts is best utilised as an indicator for the presence of NIIs, rather than a metric to quantify their effect.
To explore the possibility that the observed ΔδN values were not a result of the addition of any ion pair in general, rather than nucleophilic interactions with the iodonium complexes, experiments were conducted with [NnBu4]PF6. The [NnBu4]PF6 ion pair was chosen as a suitable candidate that would be chemically inert, would not give rise to any anion exchange effects with the silver(I) complexes, and was comfortably soluble in CD2Cl2. A solution of 2a with 1 equivalent of [NnBu4]PF6, at the same concentration as the previously recorded spectra of 2a, was prepared toward this end. As expected, the resulting spectrum gave no distinguishable ΔδN change with an observed value of −136.1 ppm (cf. pure 2a = −136.0 ppm), discounting this possibility from consideration.
To further study the NIIs, extensive attempts were made to isolate the solid-state structures of all the NII-complexes synthesised, with the belief that the stronger NIIs in solution might persist in the solid-state and facilitate their isolation. Toward this end, a number of previously unreported iodonium complexes were prepared for the purpose of eliminating their solid-state structures from consideration whilst pursuing the desired NII-complexes. The new iodonium complexes were [I(4-Mepy)2]PF6 (2b; I–N = 2.235(4), 2.268(4) Å), [I(4-iPrpy)2]PF6 (4b; I–N = 2.203(14)–2.329(13) Å), and [I(2-Etpy)2]PF6 (6b; I–N = 2.270(2) Å), all of which had I–N bond lengths in the usual range based on previously known examples,11,12,15 and were otherwise unremarkable and warrant no further comment.
Disappointingly, despite the comparable or larger shifts in the ΔδN values as compared to the two literature examples (vide supra), of the six NII-complexes explored only the structures of their respective pure Ag+ and I+ complexes were found to result from the crystallisation of NMR-confirmed 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 I+
1 I+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ag+ NII-complexes for four of them (1c, 3c, 4c, 5c).§ However, the NII-complex based on 4-Mepy, [I(4-Mepy)2]PF6*[Ag(4-Mepy)2]PF6 (2c), was successfully crystallised to reveal the solid-state NIIs present (Fig. 2). The structure was found to have two crystallographically independent NII-complexes, with one complex comprised of a partially eclipsing polymeric packing of a repeating I+*Ag+*I+*Ag+ motif and somewhat comparable I+*Ag+ separations of 3.5372(6) and 3.5739(6) Å (cf. van der Waals radius of silver and iodine = 3.70 Å). The other complex was a staggered parallel array of I+*Ag+⋯I+*Ag+⋯I+*Ag+ subunits, again in a polymeric fashion, with the shortest I+*Ag+ distance in the structure of 3.5184(7) Å and Ag+⋯I+ distance of 3.8762(7) Å. It should be noted that for the two crystallographically independent NII-complexes, the NC5 centroid-to-centroid distances were 3.73 and 3.78 Å for the 3.5184(7) Å NII, and 3.54 and 3.70 Å for the 3.5372(6) Å NII. These close intermolecular distances attributed to the I+*Ag+ NIIs are reminiscent of the well-established argentophilic (Ag+⋯Ag+) interaction observed between two molecules of 2a (3.0676(4) Å), and in sharp contrast to the closest I+⋯I+ intermolecular distance observed in 2b of 5.7969(5) Å. To date, 2c is the only known solid-state example of a NII involving a 2-coordinate silver(I) complex, and the two differing modes of packing observed in the structure, both of which demonstrate NIIs, reflects the ability of this NII-complex to accommodate this new intermolecular interaction in the solid-state.
Ag+ NII-complexes for four of them (1c, 3c, 4c, 5c).§ However, the NII-complex based on 4-Mepy, [I(4-Mepy)2]PF6*[Ag(4-Mepy)2]PF6 (2c), was successfully crystallised to reveal the solid-state NIIs present (Fig. 2). The structure was found to have two crystallographically independent NII-complexes, with one complex comprised of a partially eclipsing polymeric packing of a repeating I+*Ag+*I+*Ag+ motif and somewhat comparable I+*Ag+ separations of 3.5372(6) and 3.5739(6) Å (cf. van der Waals radius of silver and iodine = 3.70 Å). The other complex was a staggered parallel array of I+*Ag+⋯I+*Ag+⋯I+*Ag+ subunits, again in a polymeric fashion, with the shortest I+*Ag+ distance in the structure of 3.5184(7) Å and Ag+⋯I+ distance of 3.8762(7) Å. It should be noted that for the two crystallographically independent NII-complexes, the NC5 centroid-to-centroid distances were 3.73 and 3.78 Å for the 3.5184(7) Å NII, and 3.54 and 3.70 Å for the 3.5372(6) Å NII. These close intermolecular distances attributed to the I+*Ag+ NIIs are reminiscent of the well-established argentophilic (Ag+⋯Ag+) interaction observed between two molecules of 2a (3.0676(4) Å), and in sharp contrast to the closest I+⋯I+ intermolecular distance observed in 2b of 5.7969(5) Å. To date, 2c is the only known solid-state example of a NII involving a 2-coordinate silver(I) complex, and the two differing modes of packing observed in the structure, both of which demonstrate NIIs, reflects the ability of this NII-complex to accommodate this new intermolecular interaction in the solid-state.
An unusual case also arose whilst pursuing the solid-state structure of the NII-complex based on 2-Etpy (6c), where a partial occupancy structure, with 74% I+ and 26% Ag+ in the central coordinated position, was observed in the solid-state. However, this structure can be seen as a somewhat unique case where the pure I+ and Ag+ complexes are effectively isostructural, allowing for possible contamination with one another whilst maintaining crystallinity. Despite the mixed occupancy of the central cation, the packing of the structure was such that there were no adjacent cation–cation interactions present with the potential to exhibit NIIs.
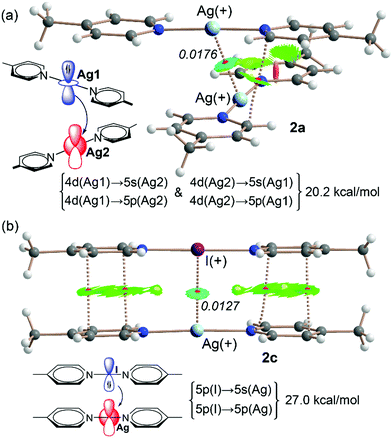
DFT calculations at the M06-2X/def2-TZVP level of theory (see ESI† for details) were carried out for both 2a and 2c compounds with the purpose to compare the nature of the Ag+⋯Ag+ interaction observed in 2a (Fig. 3a) to the I+⋯Ag+ NII in compound 2c (Fig. 3b), and to further corroborate the recent explanation given by us for this counterintuitive I+⋯Ag+ non-covalent contact where the iodonium acts as electron donor in spite of bearing a formal positive charge.9 The quantum theory of “atoms-in-molecules” QTAIM and the NCIplot index surface analyses were performed in both 2a and 2c dimers (for 2c the motif with the shortest Ag+⋯I+ distance of 3.5184(7) Å was selected, see Fig. 3 (right)).17,18 Both Ag+⋯Ag+ and Ag+⋯I+ interactions are characterised by a bond CP (red sphere) and bond path interconnecting both heavy atoms. Moreover, a bluish NCIplot surface appears between both atoms, coinciding with the location of the bond CP. The bluish colour evidences a moderately strong interaction. In the dimer 2a, the combined NCIplot/QTAIM analysis reveals the existence of weak van der Waals interactions between the aromatic rings. The small size of the surfaces and their green colour suggest that these ancillary interactions are not particularly relevant to the formation of the dimer.
For the dimer 2c four additional bond CPs and bond paths interconnect the aromatic rings, thus evidencing the existence of π–π stacking interactions, which is also confirmed by the presence of green and extended NCIplot surfaces located between the aromatic rings. Further discussion of the π–stacking interactions is provided in the ESI† (see Fig. S35).
Thirdly, the existence and relative importance of orbital interactions in both 2a and 2c dimers were evaluated by using the natural bond orbital analysis (NBO) and focusing on the second order perturbation analysis.19 This methodology has been successfully used to investigate the stabilisation associated to orbital donor–acceptor interactions in similar complexes.9,20,21 The results of the NBO calculations are also gathered in Fig. 3 and they evidence the importance of orbital effects. In the dimer of 2a, two relevant orbital donor–acceptor contributions (10.1 kcal mol−1) exist, derived from the electron donation from the 4d atomic orbital of Ag(1) to the empty 5s and 5p orbitals of Ag(2). In addition, an equivalent “back-donation” of 4d(Ag2) → 5s(Ag1) and 4d(Ag2) → 5s(Ag1) also exists (see Fig. 3a). Consequently, the total stabilisation energy is 20.2 kcal mol−1, which is comparable to other calculations involving Ag dimers.21,22
In line with our previous investigation on NIIs,9 the NBO analysis of compound 2c shows two relevant orbital contributions, which correspond to the electron donation from the lone pair (LP) orbital of iodine (5p orbital) to the 5s and 5p atomic orbitals of Ag with a total stabilisation energy of 27.0 kcal mol−1. It is worth mentioning that, there is no back-donation from silver to iodine. Therefore, the NBO analysis corroborates the nucleophilic role of I+ in the nucleophilic iodonium interaction. The total stabilisation energy is greater (27 kcal mol−1) in the dimer of 2c than that in the dimer of 2a (22.2 kcal mol−1), thus suggesting that NII interaction is much stronger that its analogous argentophilic interaction, even though it exhibits a longer distance (3.514 Å in 2c and 3.068 Å in 2a). This also supports the formation of the I+⋯Ag+ complex in solution upon mixing complexes 2a and 2b. Moreover, the fact that 2b does not form I+⋯I+ dimers in the solid state agrees well with the distinctive nucleophilic role of iodonium in N–I+–N complexes via its available lone pairs.
All of the six different NII-complexes explored in this study exhibited nucleophilic iodonium interactions (NIIs) in solution, with one of those being further confirmed in the solid-state as the first example of a 2-coordinate silver(I) complex exhibiting a NII. This demonstrates the rapidly expanding scope of complexes found to be capable of this new interaction. The discovery that these NIIs are much more common than their (lack of) occurrence in the literature would suggest opens up the possibility of exploiting iodonium complexes in new and exciting ways, and it is expected that the methodology presented herein will lead to further examples of NIIs and insights regarding their potential uses.
We gratefully acknowledge the Finnish Cultural Foundation Central Fund (J. S. W. grant number 00201148), the Magnus Ehrnrooth Foundation (J. S. W.), the MICIU/AEI of Spain (A. F. project CTQ2017-85821-R FEDER), the Academy of Finland (K. R. grant no. 317259), and the University of Jyvaskyla, Finland for financial support.
Conflicts of interest
There are no conflicts to declare.Notes and references
- G. R. Desiraju, P. S. Ho, L. Kloo, A. C. Legon, R. Marquardt, P. Metrangolo, P. Politzer, G. Resnati and K. Rissanen, Pure Appl. Chem., 2013, 85, 1711–1713 CAS.
- G. Cavallo, P. Metrangolo, R. Milani, T. Pilati, A. Priimagi, G. Resnati and G. Terraneo, Chem. Rev., 2016, 116, 2478–2601 CrossRef CAS PubMed.
- L. Turunen and M. Erdélyi, Chem. Soc. Rev., 2020, 49, 2688–2700 RSC.
- J. A. Creighton, I. Haque and J. L. Wood, Chem. Commun., 1966, 229 RSC.
- I. Haque and J. L. Wood, J. Mol. Struct., 1968, 2, 217–238 CrossRef CAS.
- J. Barluenga, J. M. González, M. A. Garcia-Martin, P. J. Campos and G. Asensio, J. Chem. Soc., Chem. Commun., 1992, 1016–1017 RSC.
- J. Ezquerra, A. C. Pedregal, C. Lamas, J. Barluenga, M. Pérez, A. M. Angel García-Martín and J. M. González, J. Org. Chem., 1996, 61, 5804–5812 CrossRef CAS.
- G. Espuña, G. Arsequell, G. Valencia, J. Barluenga, M. Pérez and J. M. González, Chem. Commun., 2000, 1307–1308 RSC.
- S. Yu, P. Kumar, J. S. Ward, A. Frontera and K. Rissanen, Chemistry, 2021, 7, 948–958 CrossRef CAS.
- J. S. Ward, A. Frontera and K. Rissanen, Inorg. Chem., 2021, 60, 5383–5390 CrossRef CAS PubMed.
- M. Bedin, A. Karim, M. Reitti, A.-C. C. Carlsson, F. Topić, M. Cetina, F. Pan, V. Havel, F. Al-Ameri, V. Sindelar, K. Rissanen, J. Gräfenstein and M. Erdélyi, Chem. Sci., 2015, 6, 3746–3756 RSC.
- A.-C. C. Carlsson, K. Mehmeti, M. Uhrbom, A. Karim, M. Bedin, R. Puttreddy, R. Kleinmaier, A. A. Neverov, B. Nekoueishahraki, J. Gräfenstein, K. Rissanen and M. Erdélyi, J. Am. Chem. Soc., 2016, 138, 9853–9863 CrossRef CAS PubMed.
- L. Turunen, U. Warzok, R. Puttreddy, N. K. Beyeh, C. A. Schalley and K. Rissanen, Angew. Chem., Int. Ed., 2016, 55, 14033–14036 CrossRef CAS PubMed.
- L. Turunen, U. Warzok, C. A. Schalley and K. Rissanen, Chemistry, 2017, 3, 861–869 CrossRef CAS.
- J. S. Ward, G. Fiorini, A. Frontera and K. Rissanen, Chem. Commun., 2020, 56, 8428–8431 RSC.
- D. von der Heiden, K. Rissanen and M. Erdélyi, Chem. Commun., 2020, 56, 14431–14434 RSC.
- R. F. W. Bader, Chem. Rev., 1991, 91, 893–928 CrossRef CAS.
- J. Contreras-García, E. R. Johnson, S. Keinan, R. Chaudret, J.-P. Piquemal, D. N. Beratan and W. Yang, J. Chem. Theory Comput., 2011, 7, 625–632 CrossRef PubMed.
- E. D. Glendening, C. R. Landis and F. Weinhold, Wiley Interdiscip. Rev.: Comput. Mol. Sci., 2012, 2, 1–42 CAS.
- H. Schmidbaur and A. Schier, Angew. Chem., Int. Ed., 2015, 54, 746–784 CrossRef CAS PubMed.
- A. Terrón, B. Moreno-Vachiano, A. Bauzá, A. García-Raso, J. J. Fiol, M. Barceló-Oliver, E. Molins and A. Frontera, Chem. – Eur. J., 2017, 23, 2103–2108 CrossRef PubMed.
- B. Pinter, L. Broeckaert, J. Turek, A. Růžička and F. De Proft, Chem. – Eur. J., 2014, 20, 734–744 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Synthesis, NMR and computational details. CCDC 2064891–2064898. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d1cc01505b |
| ‡ Following the notation introduced for the first example of a NII, which was represented by an asterisk.9,10 |
| § With the exception of [I(4-Etpy)2]PF6 for which the pure solid-state structure is currently unknown. This complex has however been serendipitously reported as a solvate within a larger silver(I) structure.10 |
| This journal is © The Royal Society of Chemistry 2021 |