Open Access Article
Open Access ArticleReactivity of mono- and divalent aluminium compounds towards group 15 nanoparticles†
Adrian
Hauser
 ,
Luca
Münzfeld
,
Luca
Münzfeld
 and
Peter W.
Roesky
and
Peter W.
Roesky
 *
*
Institute of Inorganic Chemistry, Karlsruhe Institute of Technology (KIT), Engesserstraße 15, Karlsruhe D-76131, Germany. E-mail: roesky@kit.edu
First published on 12th May 2021
Abstract
Herein, we present a novel approach towards organometallic group 13/15-compounds, i.e. the reaction of nanoparticular arsenic and antimony with low-valent aluminium species. The reaction of the two-electron reducing agent [AlCp*]4 (Cp* = C5Me5) with arsenic nanoparticles gave rise to a mixture of two unprecedented deca- and dodecanuclear Al–As clusters. In contrast, the analogous transformation with nanoparticular antimony yielded the already known Al–Sb compound [(AlCp*)3Sb2]. Additionally, two different dialanes [AlCp*X]2 (X = Br, I) were employed as one-electron reducing agents, forming calix like coordination compounds upon reaction with nano arsenic. The isolated species significantly enlarge the accessible structural variety of molecular group 13/15 compounds, highlighting the exceptional utility and reactivity of nanoscale group 15 precursors.
Low-valent organometallic compounds of aluminium have been known for several decades. Textbook examples are the mono valent compounds [AlCp*]4 (Chart 1, left) (Cp* = C5Me5), and [Al(DippBDI)] (DippBDI = diisopropylphenyl-β-diketiminate),1 which were studied intensively.2 Very recently, the reactivity of [Al(DippBDI)] was enhanced by deprotonating one methyl group in the ligand backbone to obtain the corresponding aluminyl-anion.3 However, the first anionic Al(I) complex exhibiting a unique reactivity was reported shortly before, establishing a new generation of Al(I)-compounds.4 These recent advances in the synthetic chemistry of molecular Al(I)-species, as well as in depth reactivity studies, have brought low-valent group 13 compounds back to the spotlight of main group organometallic chemistry.5 Besides above mentioned monovalent aluminium(I) compounds, isolable divalent aluminium compounds are well-established.4a,6 One example is [AlCp*X]2 (X = Br, I) (Chart 1, right), which can be isolated as an intermediate from the synthesis of [AlCp*]4 by the reduction of [AlCp*X(μ-X)]2 with sodium or potassium. Due to their tendency to disproportionate easily, these dialanes have only been used sporadically in dialumination reactions of organic substrates or simple oxidation reactions so far.7
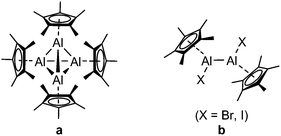 | ||
| Chart 1 Classes of low-valent aluminium compounds of relevance to this work: (a) Al(I)-tetramer [AlCp*]4 (left) and (b) Cp*-containing dialanes [AlCp*X]2 (X = Br, I) (right). | ||
In contrast, [AlCp*]4 has been reacted with a large number of organic substrates and main group elements.2i,5d,8 Thus, the aluminium group 16 heterocubanes [Cp*AlE]4 (E = O, S, Se, Te) (Chart 2, left) can be obtained by reaction of [AlCp*]4 with the corresponding group 16 elements.9,10 Similarly, [AlCp*]4 reacts with white phosphorus resulting in [P4(Cp*Al)6] (Chart 2, centre), which consists of a P4Al6 cage.11 In contrast, no reaction has been reported of an Al(I) species with the heavier group 15 elements As, Sb, Bi.
 | ||
| Chart 2 Products from the reaction of [AlCp*]4 with (a) elements of group 16 (left) (b) white phosphorus (centre) and (c) group 15 organyls (right). | ||
The corresponding derivatives [Pn2(AlCp*)3] (Pn = As, Sb, Bi) (Chart 2, right) can only be obtained by reaction of [AlCp*]4 with organometallic precursors as arsenic, antimony and bismuth source since the direct reaction with the elements was not observed.12 Recently, we reported on elemental arsenic (As0(nano)) and antimony (Sb0(nano)) nanoparticles, which react with samarocene as highly reactive elemental group 15 precursors.13 Herein, we showcase that this concept can be extended to low-valent main group chemistry. Due to the drastically increased surface-to-volume ratio compared to often unreactive bulk material the nanoparticles used in this work exhibit increased reactivity.14 In addition, compared to yellow arsenic (As4), which is highly photosensitive and rapidly decomposes to the thermodynamically more stable grey arsenic,15 As0(nano) as well as Sb0(nano) are employable in redox-chemistry, while being readily storable and handleable.
Herein, we report the reactivity of nanoparticular arsenic and antimony towards [AlCp*]4, which led to the formation of new aluminium–arsenic compounds exhibiting unexpected structural motifs, as well as a reported aluminium–antimony compound. Furthermore, we used the divalent aluminium species [AlCp*X]2 (X = Br, I) for the first time in a purely inorganic transformation as a one-electron reducing agent.
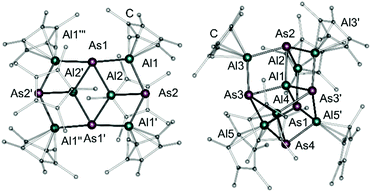
The reaction of [AlCp*]4 with an excess of arsenic nanoparticles at 70 °C in toluene for several days resulted in two different compounds. Despite several attempts and variation of the reaction conditions as well as the stoichiometry, [(Cp*Al)6As4] (1) and [(Cp*Al)6As5Al] (2) were only obtainable as a mixture of yellow (1) and red crystals (2) (Scheme 1). Additionally, the similar solubility of both species in common organic solvents prevented fractional crystallization. Nevertheless, both compounds could be characterised by manually separating the crystalline products. During the formation of compound 1, formally one and a half [AlCp*]4 moieties are reacting with arsenic nanoparticles. The aluminium atoms are oxidized to give six [AlIII]-centres, each capped by one Cp*-ligand. The respective As atoms are present in oxidation state -3 building up an As4Al6 cage structure, which is known for the lighter phosphorus congener.11
Fig. 1 (left) shows the molecular structure of compound 1 in the solid state. The As4Al6 framework can be described by two face-sharing Al4As4 cubes, each missing one arsenic atom on the opposite corners. Formally, 1 can be considered as fusion of two [(AlCp*)3As2]16 molecules. The four outer Al1 atoms are each bearing a Cp*-ligand in a η5-coordination mode with an Al1-CtCp* (CtCp* = centroid of the Cp* moiety) distance of 1.9066(8) Å, while the inner two Al2 atoms are only η1-coordinated by their corresponding Cp*-ligands with an Al2–C bond length of 2.067(3) Å. The bond lengths of the Al–As bonds vary from 2.4186(7) Å for Al1–As2 to 2.5019(6) Å for Al2–As1, which is within the expected range of comparable compounds with Al–As single bonds.16,17 As expected, the Al–As bonds are longer compared to the Al–P bonds of the isostructural phosphorus compound, while the Al–C distances are consistent with reported ones.11
As mentioned above, another type of crystals obtained from the same reaction of [AlCp*]4 with As0(nano) could be characterised. This revealed the formation of another, previously unknown aluminium arsenic cage complex (Fig. 1, right). As illustrated in Fig. 1, compound 2 consists of an As5Al7 cage, which can be described as two distorted face-sharing cubes lacking only one arsenic atom at one corner. In addition, the arsenic atoms As1 and As2 are four-fold coordinated and bridged by one more aluminium atom (Al2). All other As atoms are three-fold coordinated by aluminium atoms, which are all in a trivalent oxidation state. For Al2, Al3, and Al4 the Cp*-ligands are η5-coordinated (Al2–CtCp* 1.896(2) Å, Al3–CtCp* 1.904(2) Å, Al4–CtCp* 2.092(2) Å), while Al5 exhibits a η3-coordination mode (Al1–CtCp* 2.360(2) Å) of the five membered ring. In contrast, the central aluminium atom (Al1) is not bound to a Cp*-ligand. Instead, Al1 is exclusively tetrahedrally coordinated by four arsenic atoms. The average Al1–As bond length is 2.4647 Å, which is comparable to values observed in compound 1 and further Al–As single bonds known from literature.16–18 The other Al–As bonds in compound 2 are in a similar range.
After the successful reduction of As0(nano) with [AlCp*]4, we decided to examine the reactivity towards Sb0(nano). Actually, we isolated the already reported compound [(AlCp*)3Sb2] (3) (Scheme 1) instead of a compound isostructural to 1 or 2. The formation of compound 3 was confirmed by X-ray diffraction and NMR studies. In the 1H NMR, the resonance for the Cp* methyl protons was observed at δ = 2.08 ppm while in the 13C{1H} NMR two resonances, one for the methyl carbon at δ = 12.0 ppm and one for the ring carbon at δ = 116.1 ppm were detected, which is consistent with the literature.12a,12c However, we could show once again, that Sb0(nano) is a highly reactive source for elemental antinomy to form organometallic compounds, which are not accessible from bulk antimony.12a
Our next challenge consisted in studying the reactivity of the nanoparticles towards Al(II)-species. In contrast to [AlCp*]4, these species usually act as a one-electron reducing agent. As reactive precursor we used the dialanes [AlCp*X]2 (X = Br, I) (Chart 1). By using two different halogens we had the opportunity to study the influence of the substituents on the reactivity.19 For both species a reaction with arsenic nanoparticles was observed after stirring the reaction mixture in toluene at 80 °C for four days, yielding [(Cp*AlBr)3As] (4) and [(Cp*AlI)3As] (5) (Scheme 2). These reactions are, to the best of our knowledge, the first purely inorganic transformations of this type of dialanes. Formally, elemental As is triply reduced to [As−III] upon reaction, while the [AlII] atoms are oxidized to [AlIII]. During this process the Al–Al bond is cleaved. The observed differences in the yields (4 = 51%; 5 = 29%) could stem from the less stable Al–Al bond in the iodine compound, which could favor some unidentified side reactions.19 In contrast, no reaction with Sb0(nano), even under harsh conditions, was observed, indicating a slightly lower reactivity compared to As0(nano).
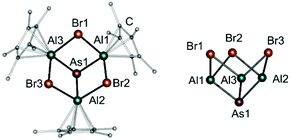
As shown in Fig. 2, compound 4 exhibits a calix-like structural motif with an [As−III] centre, bonded to three [AlIIICp*] units, which in turn are bridged to the adjacent aluminium atoms via one bromine atom each. Every [AlIII] centre is capped by a η5-coordinated Cp*-ligand with an average Al–CtCp* distance of 1.9367 Å. The As1–Al bond lengths vary from 2.4118(13) Å to 2.4374(12) Å, which is consistent with previously reported compounds.16–18 As expected, the bond lengths for the bridging bromine atoms (e.g. Al1–Br2 2.5569(13) Å and Al2–Br2 2.6091(13) Å) are slightly longer than for terminal Al–Br bonds (2.31 Å–2.41 Å) and in the same range as other Al–Br bond lengths in a bridging bonding mode (e.g. 2.513 Å–2.516 Å).2h,7c,20 The calix-like structure with an opening angle (angle between Al1–Al2—Al3 plane and As1–Al1–Al3–Br1 plane, Fig. S19, ESI†) of 124.12°, is the result of the trigonal pyramidal coordination of the central As atom (As1). The As–Al–Br angles are between 95.79(4)° and 98.33(5)°.
In the solid state, compound 5 (Fig. 3) forms a calix-like structure similar to compound 4 with an opening angle of 125.59° (angle between Al1–Al1′–Al1′′ plane and As1–Al1–Al1′–I1 plane, Fig. S21, ESI†). However, the bonding situation of the halide is different. While the Al1–I1 distance (2.706(2) Å) is in the range of Al–I single bonds,7a the Al1′–I1 distance (3.088(2) Å) is beyond these values. However, it is still shorter than the sums of the van der Waals radii.21 Therefore, a weak interaction may occur (shown as dashed lines in Scheme 2 and Fig. 3), which results in an asymmetrically bridged Al–halide bond. Compared to compound 4, the As–Al–X (X = I) angle (102.29(5)°) is wider, which can be explained by the asymmetrically, rather than symmetrically bridged, Al–halide bonding situation. The Al1–C distances in 5 are similar (2.141(6) Å to 2.434(5) Å) compared to compound 4, resulting in a η5-coordination of the Cp*-ligand (Al1–CtCp* 1.946(3) Å). The As1–Al1 bond length is 2.407(2) Å, which is, as expected, in the same range as for compounds 1, 2 and 4.
In summary, we investigated the reactivity of Al(I)- and Al(II)-compounds towards elemental As and Sb nanoparticles. By using As0(nano) new products, which are not accessible by the classic route (decomposition of organoarsenides) were synthesised. Thus, the As4Al6 cage 1 and the unusual aluminium arsenic As5Al7 cage 2 were obtained by our new synthetic approach. Furthermore, the dialanes [Cp*AlX]2 (X = Br, I) were applied as single electron reducing agents for the reduction of As0(nano). The resulting compounds 4 and 5 were isolated in a calix-like structural motif in the solid state. In addition, the conversion of Sb0(nano) with [AlCp*]4 confirmed the high reactivity of the nanoparticles by isolating compound 3. In conclusion, we have successfully broadened the application of group 15 nanoparticles as reactive source for these elements and used them for the first time in main group chemistry.
All authors have given approval to the final version of the manuscript. A. H. synthesized and analysed all compounds with support from L. M., A. H. and L. M. conducted X-ray experiments. PWR originated the idea, supervised the work, and interpreted the results. All authors contributed to the preparation of the manuscript.
We are grateful to the Deutsche Forschungsgemeinschaft (DFG) (No. 266153560, Ro 2008/17-2) for financial support.
Conflicts of interest
There are no conflicts to declare.Notes and references
- (a) C. Dohmeier, C. Robl, M. Tacke and H. Schnöckel, Angew. Chem., Int. Ed. Engl., 1991, 30, 564–565 CrossRef; (b) S. Schulz, H. W. Roesky, H. J. Koch, G. M. Sheldrick, D. Stalke and A. Kuhn, Angew. Chem., Int. Ed. Engl., 1993, 32, 1729–1731 CrossRef; (c) C. Cui, H. W. Roesky, H.-G. Schmidt, M. Noltemeyer, H. Hao and F. Cimpoesu, Angew. Chem., Int. Ed., 2000, 39, 4274–4276 CrossRef CAS.
- (a) J. Gauss, U. Schneider, R. Ahlrichs, C. Dohmeier and H. Schnoeckel, J. Am. Chem. Soc., 1993, 115, 2402–2408 CrossRef CAS; (b) C. Dohmeier, H. Krautscheid and H. Schnöckel, Angew. Chem., Int. Ed. Engl., 1995, 33, 2482–2483 CrossRef; (c) C. Dohmeier, D. Loos and H. Schnöckel, Angew. Chem., Int. Ed. Engl., 1996, 35, 129–149 CrossRef CAS; (d) A. Purath, C. Dohmeier, A. Ecker, H. Schnöckel, K. Amelunxen, T. Passler and N. Wiberg, Organometallics, 1998, 17, 1894–1896 CrossRef CAS; (e) C. Schnitter, H. W. Roesky, C. Röpken, R. Herbst-Irmer, H.-G. Schmidt and M. Noltemeyer, Angew. Chem., Int. Ed., 1998, 37, 1952–1955 CrossRef CAS; (f) H. Sitzmann, M. F. Lappert, C. Dohmeier, C. Üffing and H. Schnöckel, J. Organomet. Chem., 1998, 561, 203–208 CrossRef CAS; (g) A. Purath, R. Köppe and H. Schnöckel, Angew. Chem., Int. Ed., 1999, 38, 2926–2928 CrossRef CAS; (h) M. Schormann, K. S. Klimek, H. Hatop, S. P. Varkey, H. W. Roesky, C. Lehmann, C. Röpken, R. Herbst-Irmer and M. Noltemeyer, J. Solid State Chem., 2001, 162, 225–236 CrossRef CAS; (i) H. W. Roesky and S. S. Kumar, Chem. Commun., 2005, 4027–4038 RSC.
- S. Grams, J. Eyselein, J. Langer, C. Färber and S. Harder, Angew. Chem., Int. Ed., 2020, 59, 15982–15986 CrossRef CAS PubMed.
- (a) J. Hicks, P. Vasko, J. M. Goicoechea and S. Aldridge, Nature, 2018, 557, 92–95 CrossRef CAS; (b) J. Hicks, P. Vasko, J. M. Goicoechea and S. Aldridge, J. Am. Chem. Soc., 2019, 141, 11000–11003 CrossRef CAS PubMed; (c) R. J. Schwamm, M. D. Anker, M. Lein and M. P. Coles, Angew. Chem., Int. Ed., 2019, 58, 1489–1493 CrossRef CAS PubMed; (d) R. J. Schwamm, M. P. Coles, M. S. Hill, M. F. Mahon, C. L. McMullin, N. A. Rajabi and A. S. S. Wilson, Angew. Chem., Int. Ed., 2020, 59, 3928–3932 CrossRef CAS PubMed; (e) S. Kurumada, S. Takamori and M. Yamashita, Nat. Chem., 2020, 12, 36–39 CrossRef CAS; (f) K. Koshino and R. Kinjo, J. Am. Chem. Soc., 2020, 142, 9057–9062 CrossRef CAS PubMed.
- (a) K. Hobson, C. J. Carmalt and C. Bakewell, Chem. Sci., 2020, 11, 6942–6956 RSC; (b) J. Hicks, P. Vasko, J. M. Goicoechea and S. Aldridge, Angew. Chem., Int. Ed., 2021, 60, 1702–1713 CrossRef CAS PubMed; (c) A. Hofmann, T. Tröster, T. Kupfer and H. Braunschweig, Chem. Sci., 2019, 10, 3421–3428 RSC; (d) P. Bag, C. Weetman and S. Inoue, Angew. Chem., Int. Ed., 2018, 57, 14394–14413 CrossRef CAS; (e) R. Yadav, T. Simler, B. Goswami, C. Schoo, R. Köppe, S. Dey and P. W. Roesky, Angew. Chem., Int. Ed., 2020, 59, 9443–9447 CrossRef CAS PubMed.
- (a) W. Uhl, Angew. Chem., Int. Ed. Engl., 1993, 32, 1386–1397 CrossRef; (b) N. Wiberg, K. Amelunxen, T. Blank, H. Nöth and J. Knizek, Organometallics, 1998, 17, 5431–5433 CrossRef CAS; (c) R. J. Wright, A. D. Phillips and P. P. Power, J. Am. Chem. Soc., 2003, 125, 10784–10785 CrossRef CAS PubMed; (d) C. Cui, X. Li, C. Wang, J. Zhang, J. Cheng and X. Zhu, Angew. Chem., Int. Ed., 2006, 45, 2245–2247 CrossRef CAS PubMed; (e) T. Agou, K. Nagata, T. Sasamori and N. Tokitoh, Phosphorus, Sulfur Silicon Relat. Elem., 2016, 191, 588–590 CrossRef CAS.
- (a) S. G. Minasian and J. Arnold, Chem. Commun., 2008, 4043–4045 RSC; (b) A. Hofmann, A. Lamprecht, O. F. González-Belman, R. D. Dewhurst, J. O. C. Jiménez-Halla, S. Kachel and H. Braunschweig, Chem. Commun., 2018, 54, 1639–1642 RSC; (c) A. Hofmann, A. Lamprecht, J. O. C. Jiménez-Halla, T. Tröster, R. D. Dewhurst, C. Lenczyk and H. Braunschweig, Chem. – Eur. J., 2018, 24, 11795–11802 CrossRef CAS PubMed.
- (a) H. W. Roesky, Inorg. Chem., 2004, 43, 7284–7293 CrossRef CAS PubMed; (b) T. Chu and G. I. Nikonov, Chem. Rev., 2018, 118, 3608–3680 CrossRef CAS PubMed; (c) S. J. Urwin, G. S. Nichol and M. J. Cowley, Chem. Commun., 2018, 54, 378–380 RSC; (d) V. Nesterov, D. Reiter, P. Bag, P. Frisch, R. Holzner, A. Porzelt and S. Inoue, Chem. Rev., 2018, 118, 9678–9842 CrossRef CAS.
- S. Schulz, H. W. Roesky, H. J. Koch, G. M. Sheldrick, D. Stalke and A. Kuhn, Angew. Chem., Int. Ed. Engl., 1993, 32, 1729–1731 CrossRef.
- A. C. Stelzer, P. Hrobárik, T. Braun, M. Kaupp and B. Braun-Cula, Inorg. Chem., 2016, 55, 4915–4923 CrossRef CAS.
- C. Dohmeier, H. Schnöckel, C. Robl, U. Schneider and R. Ahlrichs, Angew. Chem., Int. Ed. Engl., 1994, 33, 199–200 CrossRef.
- (a) S. Schulz, T. Schoop, H. W. Roesky, L. Häming, A. Steiner and R. Herbst-Irmer, Angew. Chem., Int. Ed. Engl., 1995, 34, 919–920 CrossRef CAS; (b) C. K. F. von Hänisch, C. Üffing, M. A. Junker, A. Ecker, B. O. Kneisel and H. Schnöckel, Angew. Chem., Int. Ed. Engl., 1996, 35, 2875–2877 CrossRef; (c) C. Ganesamoorthy, J. Krüger, E. Glöckler, C. Helling, L. John, W. Frank, C. Wölper and S. Schulz, Inorg. Chem., 2018, 57, 9495–9503 CrossRef CAS PubMed.
- (a) C. Schoo, S. Bestgen, A. Egeberg, S. Klementyeva, C. Feldmann, S. N. Konchenko and P. W. Roesky, Angew. Chem., Int. Ed., 2018, 57, 5912–5916 CrossRef CAS PubMed; (b) C. Schoo, S. Bestgen, A. Egeberg, J. Seibert, S. N. Konchenko, C. Feldmann and P. W. Roesky, Angew. Chem., Int. Ed., 2019, 58, 4386–4389 CrossRef CAS PubMed.
- C. Schöttle, P. Bockstaller, R. Popescu, D. Gerthsen and C. Feldmann, Angew. Chem., Int. Ed., 2015, 54, 9866–9870 CrossRef PubMed.
- (a) A. F. Hollemann and E. Wiberg, Inorganic chemistry, Walter de Gruyter, Berlin, 102 edn, 2007 Search PubMed; (b) M. Seidl, G. Balázs and M. Scheer, Chem. Rev., 2019, 119, 8406–8434 CrossRef CAS PubMed.
- C. K. F. von Hänisch, C. Üffing, M. A. Junker, A. Ecker, B. O. Kneisel and H. Schnöckel, Angew. Chem., Int. Ed. Engl., 1996, 35, 2875–2877 CrossRef.
- R. J. Wehmschulte and P. P. Power, J. Am. Chem. Soc., 1996, 118, 791–797 CrossRef CAS.
- R. L. Wells, A. T. McPhail and T. M. Speer, Organometallics, 1992, 11, 960–963 CrossRef CAS.
- F. Lu, X. Li, Z. Sun, Y. Zeng and L. Meng, Dalton Trans., 2015, 44, 14092–14100 RSC.
- D. D. Eley, J. H. Taylor and S. C. Wallwork, J. Chem. Soc., 1961, 3867–3873 RSC.
- A. Bondi, J. Phys. Chem., 1964, 68, 441–451 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental and crystallographic details. CCDC 2071388–2071391. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d1cc01475g |
| This journal is © The Royal Society of Chemistry 2021 |