Open Access Article
Open Access ArticleLayered assembly of cationic and anionic supramolecular polymers†
Jovana
Jevric
,
Simon M.
Langenegger
and
Robert
Häner
 *
*
Department of Chemistry, Biochemistry and Pharmacy, University of Bern, Freiestrasse 3, Bern CH-3012, Switzerland. E-mail: robert.haener@dcb.unibe.ch; Web: http://haener.dcb.unibe.ch
First published on 10th June 2021
Abstract
The chemical synthesis and the supramolecular assembly of an aromatic oligoamine are described. The self-assembly of the cationic oligomers in aqueous solution leads to the formation of vesicular objects. The assembly process of the oligomers is monitored by absorption and fluorescence spectroscopy and the formed vesicles are characterized by atomic force and transmission electron microscopy. The electrostatic complementarity of anionic supramolecular polymers sheets and the cationic vesicles is used for a layered assembly process.
The field of supramolecular polymers is rapidly expanding.1–4 The properties of supramolecular polymers, such as self-healing, remouldability and easy recyclability, may lead to future-oriented types of application.5–8 In addition to the commonly used organic solvents, also aqueous conditions can be used for the self-assembly of supramolecular polymers.9 In particular, the employment of water as a medium opens the possibility of using highly polar and even ionic compounds as building blocks for the assembly of supramolecular systems. Supramolecular polymers assembled in aqueous medium find applications in biomedical engineering,2,9–16 sensing,17 organic electronics,18 light harvesting19–23 and solar energy conversion.9,24 For the assembly of supramolecular structures with special spectroscopic and light-harvesting properties, π-conjugated and aromatic molecules are of particular interest.2,9 We have previously observed light-harvesting properties in various types of supramolecular assemblies of oligophosphates based on phenanthrenes and tetraphenylethylene in aqueous medium.19–21,25 Further, pyrene trimers showed interesting spectroscopic properties due to their self-assembly into nanosheets.26 These polyaromatic oligomers were joined by phosphodiester linkages and, thus, were of anionic nature.19,26–29 Besides these examples of polyphosphoester oligomers,29–32 amine-linked polyaromatic oligomers have not been studied in this context. Under physiological conditions, an amine-based connecting unit will lead to the formation of cationic oligomers with, presumably, different chemical properties. In addition, access to cationic oligomers would bring the further option of forming and studying composites with anionic phosphodiester-linked oligomers. The method of electrostatic layering was first applied in the 60's by Iler33 and Kirkland,34 who both made use of microparticles. Around 30 years later, Decher revived the method by the application on a wide range of polyelectrolytes.35,36 The method has since been used for the preparation of ultrathin-layers and is of high interest for the production of optics and sensors.37–43 Herein, we present the synthesis, the self-assembly and the spectroscopic properties of a 3,6-dialkynyl-substituted, amine-linked phenanthrene-trimer (N-Phe3, Scheme 1). Additionally, the cationic oligomer was used for the formation of nanometer-scaled aggregates in an electrostatic layered assembly36,38 in combination with an anionic supramolecular polymer.
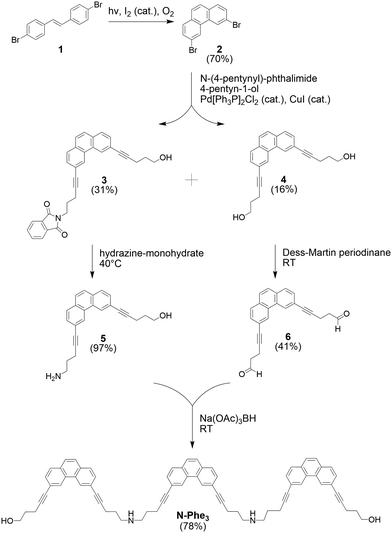
The synthesis of the target compound N-Phe3, the amino-linked trimer of 3,6-dipentynyl substituted phenanthrene, is depicted in Scheme 1. First, 4,4′-dibromo-trans-stilbene (1) was converted into 3,6-dibromophenanthrene (2) via oxidative photochemical cyclization in analogy to the literature.44 A Sonogashira cross-coupling reaction afforded a mixture of compounds 3 and 4, which were separated by flash column chromatography. Treatment of compound 3 with hydrazine afforded the amine 5. Separately, the diol 4 was converted into the bis-aldehyde 6 by a Dess–Martin oxidation. A reductive amination reaction with compounds 5 and 6 finally yielded the oligomer N-Phe3, which was purified by preparative TLC (ESI†).
Fig. 1 shows the absorption spectra of N-Phe3 under different conditions. In ethanol, N-Phe3 exhibits a spectrum with three major absorption maxima at 253 nm, 314 nm, and 329 nm (black). N-Phe3 is soluble in ethanol and the spectrum closely resembles the one of phenanthrene monomer.19 In aqueous medium (sample preparation see ESI†), a distinct shift in the long-wavelength absorption bands is observed when the spectra are taken at elevated (75 °C) or at low (15 °C) temperature, which suggests a self-assembly process of N-Phe3 in this temperature range. At 75 °C the maxima (315 and 329 nm) correlate well with the ones observed in ethanol, which indicates that the oligomer is not assembled. Lowering the temperature leads to the self-assembly of N-Phe3 and a bathochromic shift of approximately 3 nm (317 and 332 nm). This redshift is explained by a structural change during the self-assembly of trimers upon lowering the temperature. Stepwise temperature-dependent recording of the spectrum reveals that the red-shift, and thus the self-assembly process, occurs in the range between 35 °C to 45 °C (ESI†).
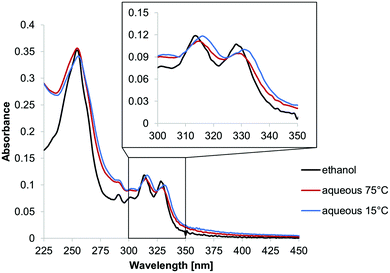 | ||
| Fig. 1 UV-vis spectra of N-Phe3 (1 μM) in ethanol (black) and in an aqueous medium (10 mM sodium acetate buffer pH 4.7, containing 10 vol% ethanol) at 75 °C (red) and 15 °C (blue). | ||
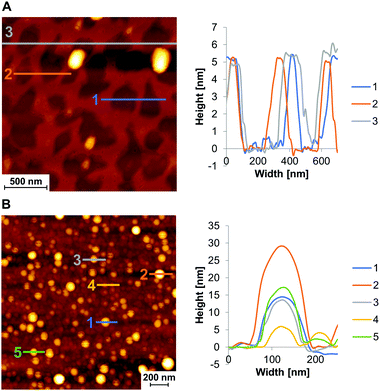
The morphology of the objects formed by self-assembly of N-Phe3 was investigated by atomic force microscopy (AFM) on mica. As shown in Fig. 2, the sample concentration has a strong effect on the course of the self-assembly. At a 1 μM concentration (Fig. 2A), the formation of a layer was observed in which coherent areas are separated by intervening holes. The average height of 5–6 nm suggests that the covered areas correspond to a bilayer of trimers (Fig. 3). After the increase of the N-Phe3 concentration to 10 μM, vesicles were observed (Fig. 2B). This type of concentration dependence in AFM imaging was already noticed in previous work.27 Apparently, vesicles formed in the solution first, form the mentioned bilayer after deposition on mica. At higher concentrations (10 μM), vesicles are further adsorbed on this preformed bilayer. The vesicles exhibit a height of 6–30 nm and a width of 50–100 nm (Fig. 2B).
 | ||
| Fig. 3 Graphical illustration of vesicle formation by N-Phe3. Blue dots indicate amines, red dots hydroxyl groups. A bilayer of trimers has a calculated height of ∼6 nm. | ||
The aqueous environment and hydrophobic effects favor stacking interactions between the phenanthrenes in the bilayer. The linkers with the amine and hydroxyl groups are pointing to the inner and outer side of the vesicles, as illustrated in Fig. 3. Under the experimental conditions (pH 4.7), the amino groups are protonated, hence the vesicles are positively charged (zeta-potential = +3.72 ± 0.14 mV).
The structural observations made by AFM were supported by transmission electron microscopy (TEM) experiments, which confirmed the formation of vesicles by N-Phe3 (Fig. 4). The vesicles exhibited a diameter range from 50 nm up to 100 nm, which closely correlates with observations made by AFM. Dynamic light scattering (DLS) measurements revealed an average particle size of around 150 nm at 20 °C (ESI†).
 | ||
| Fig. 4 TEM images of vesicles assembled from N-Phe3 (left: 3 μM; right: 5 μM). Conditions as in Fig. 2. | ||
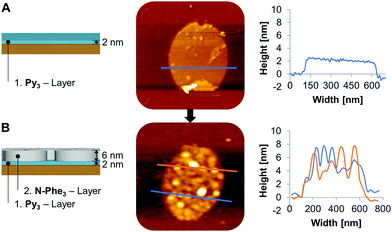
The above described positively charged vesicular assemblies of amine-linked oligomer N-Phe3 were next used for electrostatic layered assembly experiments in combination with negatively charged supramolecular nanosheets. The latter were assembled from a phosphodiester-linked, 1,6-disubstituted pyrene trimer (Py3, see ESI†), as described previously.26 The 2-dimensional shape of this supramolecular polymer was considered as an ideal starting platform for the subsequent layering process. For the electrostatic assembly, the supramolecular objects, cationic vesicles and anionic nanosheets, were prepared independently with the trimers Py3 and N-Phe3 (Fig. 5A and ESI†). Fig. 5B illustrates the formation of an electrostatic assembly, starting from an anionic Py3-nanosheet on APTES-modified mica (positively charged). This first Py3-layer is followed by a layer of positively charged N-Phe3 which adsorbs on the Py3-sheet.45 Unlike the vesicular shape on the unmodified mica (Fig. 2A), N-Phe3 appears as a layer of discs on the negatively charged Py3-sheets (Fig. 5B). We attribute this difference to the change of the surface charge.
Fig. 6 shows the AFM images of the layering process according to Fig. 5B. The first layer consists of Py3-sheets adsorbed on APTES modified mica46 with a height of 2 nm (Fig. 6A). In a next step the second layer, N-Phe3-vesicles, is added, which results in a total height of 8 nm for the two layers (Fig. 6B). The height of 8 nm consists of the Py3-sheets (2 nm) and the N-Phe3-vesicles, which are adsorbed as bilayer discs (6 nm).
In conclusion, we have described the synthesis and self-assembly properties of a water-soluble amine-bridged phenanthrene oligomer (N-Phe3). The oligomer self-assembles into vesicles in aqueous medium as shown by atomic force and electron microscopy. In addition, the cationic vesicles give rise to novel types of electrostatically controlled layered assemblies in combination with anionic supramolecular two-dimensional polymers, which was confirmed by AFM. Electrostatically controlled deposition of supramolecular polymers enables the buildup of layered nanomaterials. With well-defined dimensions and properties of the individual tiers, such nanocomposites are of interest for the field of molecular electronics, as sensors or as components of light-harvesting materials.
We gratefully acknowledge the financial support by the Swiss National Foundation (grant 200020_188468). TEM was performed on equipment supported by the Microscopy Imaging Center (MIC), University of Bern, Switzerland.
Conflicts of interest
There are no conflicts to declare.Notes and references
- T. Aida, E. W. Meijer and S. I. Stupp, Science, 2012, 335, 813–817 Search PubMed.
- L. Yang, X. Tan, Z. Wang and X. Zhang, Chem. Rev., 2015, 115, 7196–7239 Search PubMed.
- J. F. Lutz, J. M. Lehn, E. W. Meijer and K. Matyjaszewski, Nat. Rev. Mater., 2016, 1, 1–14 Search PubMed.
- S. P. W. Wijnands, E. W. Meijer and M. Merkx, Bioconjugate Chem., 2019, 30, 1905–1914 Search PubMed.
- T. Aida and E. W. Meijer, Isr. J. Chem., 2020, 60, 33–47 Search PubMed.
- Y. Yang and M. W. Urban, Chem. Soc. Rev., 2013, 42, 7446–7467 Search PubMed.
- B. Rybtchinski, ACS Nano, 2011, 5, 6791–6818 Search PubMed.
- E. A. Appel, J. del Barrio, X. J. Loh and O. A. Scherman, Chem. Soc. Rev., 2012, 41, 6195–6214 Search PubMed.
- E. Krieg, M. M. C. Bastings, P. Besenius and B. Rybtchinski, Chem. Rev., 2016, 116, 2414–2477 Search PubMed.
- S. I. Stupp, Nano Lett., 2010, 10, 4783–4786 Search PubMed.
- R. Dong, Y. Zhou, X. Huang, X. Zhu, Y. Lu and J. Shen, Adv. Mater., 2015, 27, 498–526 Search PubMed.
- C. Gong, S. Sun, Y. Zhang, L. Sun, Z. Su, A. Wu and G. Wei, Nanoscale, 2019, 11, 4147–4182 Search PubMed.
- K. Petkau-Milroy and L. Brunsveld, Org. Biomol. Chem., 2013, 11, 219–232 Search PubMed.
- E. Stulz, Acc. Chem. Res., 2017, 50, 823–831 Search PubMed.
- S. K. Albert, M. Golla, N. Krishnan, D. Perumal and R. Varghese, Acc. Chem. Res., 2020, 53, 2668–2679 Search PubMed.
- D. Bousmail, P. Chidchob and H. F. Sleiman, J. Am. Chem. Soc., 2018, 140, 9518–9530 Search PubMed.
- C. A. E. Hauser, S. Maurer-Stroh and I. C. Martins, Chem. Soc. Rev., 2014, 43, 5326–5345 Search PubMed.
- T. M. Figueira-Duarte and K. Müllen, Chem. Rev., 2011, 111, 7260–7314 Search PubMed.
- C. B. Winiger, S. Li, G. R. Kumar, S. M. Langenegger and R. Häner, Angew. Chem., Int. Ed., 2014, 53, 13609–13613 Search PubMed.
- S. Rothenbühler, I. Iacovache, S. M. Langenegger, B. Zuber and R. Häner, Nanoscale, 2020, 12, 21118–21123 Search PubMed.
- J. Jevric, S. M. Langenegger and R. Häner, Eur. J. Org. Chem., 2020, 4677–4680 Search PubMed.
- J. G. Woller, J. K. Hannestad and B. Albinsson, J. Am. Chem. Soc., 2013, 135, 2759–2768 Search PubMed.
- H. Bui, S. A. Díaz, J. Fontana, M. Chiriboga, R. Veneziano and I. L. Medintz, Adv. Opt. Mater., 2019, 7, 1900562 Search PubMed.
- Y. Zeng, J. Chen, T. Yu, G. Yang and Y. Li, ACS Energy Lett., 2017, 2, 357–363 Search PubMed.
- M. Kownacki, S. M. Langenegger, S. X. Liu and R. Häner, Angew. Chem., Int. Ed., 2019, 58, 751–755 Search PubMed.
- M. Vybornyi, A. V. Rudnev, S. M. Langenegger, T. Wandlowski, G. Calzaferri and R. Häner, Angew. Chem., Int. Ed., 2013, 52, 11488–11493 Search PubMed.
- C. D. Bösch, J. Jevric, N. Bürki, M. Probst, S. M. Langenegger and R. Häner, Bioconjugate Chem., 2018, 29, 1505–1509 Search PubMed.
- R. Szweda, M. Tschopp, O. Felix, G. Decher and J. F. Lutz, Angew. Chem., Int. Ed., 2018, 57, 15817–15821 Search PubMed.
- N. Appukutti and C. J. Serpell, Polym. Chem., 2018, 9, 2210–2226 Search PubMed.
- M. Vybornyi, Y. Vyborna and R. Häner, Chem. Soc. Rev., 2019, 48, 4347–4360 Search PubMed.
- R. Häner, F. Garo, D. Wenger and V. L. Malinovskii, J. Am. Chem. Soc., 2010, 132, 7466–7471 Search PubMed.
- N. F. König, S. Telitel, S. Poyer, L. Charles and J. F. Lutz, Macromol. Rapid Commun., 2017, 38, 1–5 Search PubMed.
- R. K. Iler, J. Colloid Interface Sci., 1966, 21, 569–594 Search PubMed.
- J. J. Kirkland, Anal. Chem., 1965, 37, 1458–1461 Search PubMed.
- G. Decher and J. Hong, Makromol. Chem., Macromol. Symp., 1991, 46, 321–327 Search PubMed.
- G. Decher and J. D. Hong, Ber. Bunsen-Ges. Phys. Chem., 1991, 95, 1430–1434 Search PubMed.
- J. J. Richardson, J. Cui, M. Björnmalm, J. A. Braunger, H. Ejima and F. Caruso, Chem. Rev., 2016, 116, 14828–14867 Search PubMed.
- Y. Wang and X. Zhan, Adv. Energy Mater., 2016, 6, 1–18 Search PubMed.
- U. Akiba, D. Minaki and J. I. Anzai, Polymers, 2017, 9, 553 Search PubMed.
- D. Rawtani and Y. K. Agrawal, Nanobiomedicine, 2014, vol. 1, p. 8 Search PubMed.
- J. J. Richardson, M. Björnmalm and F. Caruso, Science, 2015, 348, aaa2491 Search PubMed.
- R. M. Iost and F. N. Crespilho, Biosens. Bioelectron., 2012, 31, 1–10 Search PubMed.
- K. Ariga, M. Nishikawa, T. Mori, J. Takeya, L. K. Shrestha and J. P. Hill, Sci. Technol. Adv. Mater., 2019, 20, 51–95 Search PubMed.
- H. R. Talele, A. R. Chaudhary, P. R. Patel and A. V. Bedekar, ARKIVOC, 2011, 2011, 15–37 Search PubMed.
- E. I. Goksu, J. M. Vanegas, C. D. Blanchette, W. Lin and M. L. Longo, Biochim. Biophys. Acta, Biomembr., 2009, 1788, 254–266 Search PubMed.
- L. S. Shlyakhtenko, A. A. Gall and Y. L. Lyubchenko, Methods Mol. Biol., 2013, 931, 1–20 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, NMR spectra, UV-vis and fluorescence spectra, additional AFM and TEM images, DLS data. See DOI: 10.1039/d1cc01466h |
| This journal is © The Royal Society of Chemistry 2021 |