Open Access Article
Open Access ArticleChiral metal down to 4.2 K - a BDH-TTP radical-cation salt with spiroboronate anion B(2-chloromandelate)2−†
Toby J.
Blundell
 a,
Michael
Brannan
a,
Hiroshi
Nishimoto
a,
Michael
Brannan
a,
Hiroshi
Nishimoto
 b,
Tomofumi
Kadoya
b,
Tomofumi
Kadoya
 b,
Jun-ichi
Yamada
b,
Hiroki
Akutsu
b,
Jun-ichi
Yamada
b,
Hiroki
Akutsu
 c,
Yasuhiro
Nakazawa
c and
Lee
Martin
c,
Yasuhiro
Nakazawa
c and
Lee
Martin
 *a
*a
aSchool of Science and Technology, Nottingham Trent University, Clifton Lane, Nottingham, NG11 8NS, UK. E-mail: lee.martin@ntu.ac.uk
bGraduate School of Material Science, University of Hyogo, Kamigori-cho, Ako-gun, Hyogo, 678-1297, Japan
cDepartment of Chemistry, Graduate School of Science, Osaka University, 1-1 Machikaneyama, Toyonaka, Osaka 560-0043, Japan
First published on 4th May 2021
Abstract
We report the first example of a chiral BDH-TTP radical-cation salt. Chirality is induced in the structure via the use of a chiral spiroboronate anion where three stereocentres are present, one on each chiral ligand and one on the boron centre. Despite starting from a labile racemic mixture of BS and BR enantiomers, only one enantiomer is present in the crystal lattice. The anions pack in a novel double anion layer which is the thickest anion layer found in a BDH-TTP salt. This material is chiral and shows metallic behaviour down to at least 4.2 K.
The combination of conductivity and chirality in the same material is not found in nature and the inclusion of chirality in conducting materials has been a hotly pursued topic since the discovery of electrical magneto-chiral anisotropy (eMChA).1 Differences in resistivity in applied magnetic fields have since been observed for chiral enantiomers of bismuth helices1 and carbon nanotubes.2 Non-reciprocal charge transport has also recently been observed in non-centrosymmetric superconductors such as WS2 nanotubes and MoS2 thin single crystals.3 However, the chiral nature of these inorganic materials cannot be controlled because they happen to crystallise in non-centrosymmetric structures. Molecular materials containing stereogenic centres on the other hand can be synthesised in both enantiomeric forms and also the racemate to allow a study of their physical properties in each form. As such, there is a huge interest in the synthesis of molecular chiral conducting materials.4
The first bulk conductor to exhibit eMChA was reported in 2014 by Pop and co-workers.5 A pair of enantiopure radical-cation salts of dimethyl-ethylenedithio-tetrathiafulvalene (DM-EDT-TTF) with ClO4− anions were crystallised in the space groups P6222 and P6422 and showed metallic behaviour down to 40 K. Chiral molecular conductors provide an excellent opportunity to study eMChA and there are several sources to introduce chirality into radical-cation salts: via chiral donors,6 chiral anions7 or chiral solvents.8
The use of chiral spiroboronate anions in radical-cation salts allows the introduction of multiple chiral centres on the same anion, and we have previously reported that this can result in chiral crystallisation where only specific enantiomers crystallise in the radical-cation salt despite a racemic anion mixture being present in solution.9
The most successful method for producing chiral radical-cation salts has been by means of enantiopure donors based on EDT-TTF10 or BEDT-TTF.11 Whilst still difficult to obtain highly crystalline materials, the donor tetramethyl-BEDT-TTF has produced several enantiopure salts.12 As an alternative to BEDT-TTF, we have utilised the donor 2,5-bis(1,3-dithiolan-2-ylidene)-1,3,4,6-tetrathiapentalene (BDH-TTP) which has a tendency to form a κ-type packing arrangement and show metallic behaviour.13
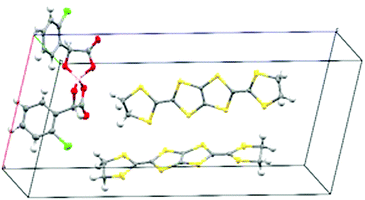
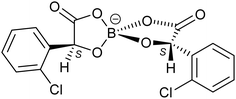
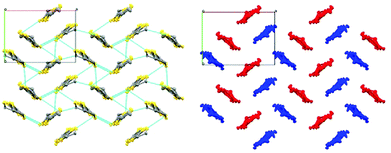
We report the first enantiopure radical-cation salt of BDH-TTP, κ-BDH-TTP2[BS-(S-ClMan)2] (ClMan = 2-(2-chlorophenyl)-2-oxidoacetate) I which crystallises in the P21 space group where the asymmetric unit contains two crystallographically independent BDH-TTP molecules and one [BS(S-ClMan)2]− anion, with no other guest or solvent molecules being present (Fig. 1).‡
The crystal lattice of the salt contains alternating layers of κ-type packed BDH-TTP and anionic layers containing the enantiopure [BS(S-ClMan)2]− anion. The anion contains three stereocentres with two enantiopure S-chloromandelate ligands bound to a boron centre via oxygens, resulting in tetrahedral coordination, and the introduction of a chiral centre at boron (Fig. 2).
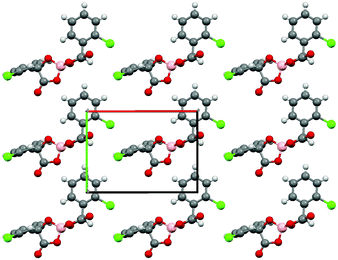
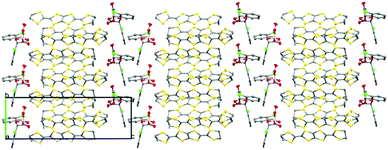
Salt I shows the presence of only a single enantiomer at boron (BS) despite the electrocrystallisation experiment starting from a labile racemic mixture of BS and BR enantiomers of the anion. We have observed this chiral selectivity in previous examples where a racemic mixture of spiroboronate anions is present in solution but a single enantiomer is preferentially crystallised during crystal growth.9 Wong et al. have reported differences in shape for the twisted BR- and the V-shaped BS-[(S-Man)2]− anions leading to preferential packing and crystallisation with various cations in their resolution studies.14 The spiroboronate forms a double anion layer, as can be seen in Fig. 3 and 4, in contrast to previous κ-type BDH-TTP salts which have a single layer of anions.13 This results in an anion layer considerably thicker than for other BDH-TTP salts with the distance between terminal ethylene moieties of adjacent BDH-TTP layers of 10.246 Å.
The donor layer of salt I contains two crystallographically independent BDH-TTP molecules (shown in red and blue in Fig. 5) which dimerise face-to-face along the ab crystallographic axis. Dimers stack in the so-called “chess-board” pattern perpendicular to each other resulting in a κ-type arrangement. In this arrangement, a pair of donors are arranged parallel to each other whilst neighbouring donor pairs are arranged perpendicular to the parallel pair (Fig. 5).
Due to the presence of mandelic acid moieties on the anions there are a number of hydrogen bond interactions between the anion oxygen and chlorides and the hydrogen atoms on the terminal ethyl groups of the BDH-TTP. The most prominent is that between O(2) and the disordered ethyl hydrogen atoms C(9), C(9a), C(10) and C(10a) (Fig. S1 and summarised in Table S2, ESI†), as well as the adjacent donor C(19), which could be responsible for the presence of disorder on the C(9)/C(10) group where none is present elsewhere.
MOPAC calculations15 indicate that the [BS(S-ClMan)2]− anion has 9.97 Debye of dipole moment, which is partially cancelled by the other anion in a unit cell. The calculation using the two anions in the unit cell elucidates that 6.03 Debye of dipole moment per one anion persists. This indicates that the crystal is not only chiral but also polar.
SQUID magnetometry (Fig. S2, ESI†) shows almost temperature-independent magnetic susceptibility of 4.5 × 10−4 emu mol−1, which is likely to be Pauli paramagnetism. A Curie tail is observed at the lowest temperature. The Curie–Weiss fitting indicates that 0.41% of S = 1/2 spins exist as impurities.
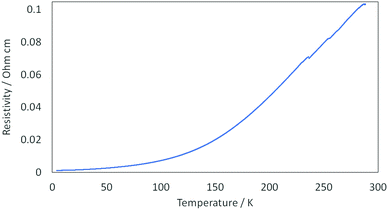
Four-probe DC transport measurements were performed on six different single crystals of I which showed metallic behaviour from room temperature down to 4.2 K (Fig. 6) with a room temperature conductivity of 9.7 S cm−1.
We calculated the band structure for I (Fig. 7) using a tight binding band structure calculation package on the basis of extended Hückel method written by Prof. Takehiko Mori.16a,b The figure of the band dispersions has a clear mid-gap, so called Mott gap, the width of which is 45 meV. This indicates that the band can be regarded as an effective half-filled band. The Fermi surfaces consist of hole pockets and quasi-1D sheets. If the overlap integrals in the κ-type donor layer are isotropic, the hole pockets and electron sheets gather to form a circle, at which no energy gaps between hole pockets and electron sheets are observed. I has a gap, 12.6% of the length of the b* axis, which is the largest in all κ-type BDH-TTP salts whose band structures have already been reported ([FeNO(CN)5]PhNO2 salt17 has 9.4% of gap and the other salts18 have no gaps), suggesting that I has the most anisotropic Fermi surfaces of all κ-type BDH-TTP salts where the interaction along a + b (n) axis is stronger than that along the b − a axis. This is perhaps because I has the largest counter-anion. In addition, a frustration parameter t/t’ of 1.24 is calculated.19
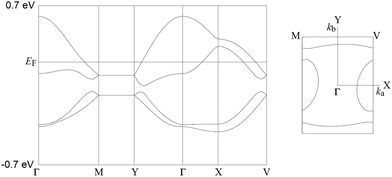 | ||
| Fig. 7 Band calculation for I at 150 K. Transfer integrals are shown in Fig. S3 (ESI†). | ||
We report the first example of a chiral BDH-TTP radical-cation salt. This salt is a chiral metal down to 4.2 K, the lowest temperature at which metallic behaviour has been observed for a chiral molecular radical-cation salt. Work is underway to prepare the opposite enantiomer and the racemate to enable a comparison of their conducting properties. There are very few examples of chiral metals20 available for the study of eMChA and the discovery of the first chiral molecular superconductor still proves illusive.21 This salt has the potential to become superconducting under negative chemical pressure using a larger halogen instead of the Cl of chloromandelate. Future work will also involve synthesis of related chiral spiroboronate salts using the pool of available ligands and performing calculations on anionic diastereomers to evaluate their relative stability to further investigate the preferential crystallisation of one enantiomeric form over the other.14
L. M. and T. B. would like to thank the Royal Society and Leverhulme Trust for financial support (RPG-2019-242).
Conflicts of interest
There are no conflicts to declare.Notes and references
- G. L. J. A. Rikken, J. Fölling and P. Wyder, Phys. Rev. Lett., 2001, 87, 236602 CrossRef CAS PubMed.
- V. Krstic, S. Roth, M. Burghard, K. Kern and G. L. J. A. Rikken, J. Chem. Phys., 2002, 117, 11315–11319 CrossRef CAS; V. Krstić and G. L. J. A. Rikken, Chem. Phys. Lett., 2002, 364, 51–56 CrossRef.
- F. Qin, W. Shi, T. Ideue, M. Yoshida, A. Zak, R. Tenne, T. Kikitsu, D. Inoue, D. Hashizume and Y. Iwasa, Nat. Commun., 2017, 8, 14465 CrossRef CAS PubMed; R. Wakatsuki, Y. Saito, S. Hoshino, Y. M. Itahashi, T. Ideue, M. Ezawa, Y. Iwasa and N. Nagaosa, Sci. Adv., 2017, 3, e1602390 CrossRef PubMed.
- N. Avarvari and J. D. Wallis, J. Mater. Chem., 2009, 19, 4061–4076 RSC; F. Pop, N. Zigon and N. Avarvari, Chem. Rev., 2019, 119, 8435–8478 CrossRef CAS PubMed.
- F. Pop, P. Auban-Senzier, E. Canadell, G. L. J. A. Rikken and N. Avarvari, Nat. Commun., 2014, 5, 3757 CrossRef PubMed.
- S. Yang, F. Pop, C. Melan, A. C. Brooks, L. Martin, P. Horton, P. Auban-Senzier, G. L. J. A. Rikken, N. Avarvari and J. D. Wallis, CrystEngComm, 2014, 16, 3906–3916 RSC.
- E. Coronado, J. R. Galán-Mascarós, C. J. Gómez-García, A. Murcia-Martinez and E. Canadell, Inorg. Chem., 2004, 43, 8072 CrossRef CAS PubMed; M. Clemente-León, E. Coronado, C. J. Gómez-García, A. Soriano-Portillo, S. Constant, R. Frantz and J. Lacour, Inorg. Chim. Acta, 2007, 360, 955 CrossRef.
- L. Martin, H. Akutsu, P. N. Horton, M. B. Hursthouse, R. W. Harrington and W. Clegg, Eur. J. Inorg. Chem., 2015, 1865 CrossRef CAS; L. Martin, H. Akutsu, P. N. Horton and M. B. Hursthouse, CrystEngComm, 2015, 17, 2783 RSC.
- J. R. Lopez, L. Martin, J. D. Wallis, H. Akutsu, Y. Nakazawa, J. I. Yamada, T. Kadoya, S. J. Coles and C. Wilson, Dalton Trans., 2016, 45, 9285–9293 RSC.
- N. Mroweh, F. Pop, C. Mézière, M. Allain, P. Auban-Senzier, N. Vanthuyne, P. Alemany, E. Canadell and N. Avarvari, Cryst. Growth Des., 2020, 20, 2516–2526 CrossRef CAS; N. Mroweh, P. Auban-Senzier, N. Vanthuyne, E. Canadell and N. Avarvari, J. Mater. Chem. C, 2019, 7, 12664–12673 RSC; F. Pop, P. Auban-Senzier, E. Canadell and N. Avarvari, Chem. Commun., 2016, 52, 12438–12441 RSC.
- S. Yang, A. C. Brooks, L. Martin, P. Day, M. Pilkington, W. Clegg, R. W. Harrington, L. Russo and J. D. Wallis, Tetrahedron, 2010, 66, 6977–6989 CrossRef CAS.
- J. R. Galán-Mascarós, E. Coronado, P. A. Goddard, J. Singleton, A. I. Coldea, J. D. Wallis, S. J. Coles and A. Alberolá, J. Am. Chem. Soc., 2010, 132, 9271–9273 CrossRef CAS PubMed; F. Pop, S. Laroussi, T. Cauchy, C. J. Gomez-Garcia, J. D. Wallis and N. Avarvari, Chirality, 2013, 25, 466–474 CrossRef PubMed.
- J.-I. Yamada, H. Akutsu, H. Nishikawa and K. Kikuchi, Chem. Rev., 2004, 104, 5057–5083 CrossRef CAS PubMed; A. A. Bardin, H. Akutsu and J.-i. Yamada, Cryst. Growth Des., 2016, 16, 1228–1246 CrossRef.
- L. W.-Y. Wong, J. W.-H. Kan, T.-h. Nguyen, H. H.-Y. Sung, D. Li, A. S.-F. Au-Yeung, R. Sharma, Z. Lin and I. D. Williams, Chem. Commun., 2015, 51, 15760–15763 RSC.
- J. J. P. Stewart, MOPAC Stewart Computational Chemistry, Colorado Springs, CO, USA, 2016, http://HTTP://OpenMOPAC.net/https://winmostar.com/en/ Search PubMed.
- (a) T. Mori, Bull. Chem. Soc. Jpn., 1998, 71, 2509–2526 CrossRef CAS; (b) Program Library of Energy Band Calculation for Molecular Conductors, Takehiko Mori, Tokyo Institute of Technology, Japan, http://www.op.titech.ac.jp/lab/mori/lib/program.html.
- I. Shevyakova, L. Buravov, V. Tkacheva, L. Zorina, S. Khasanov, S. Simonov, J. Yamada, E. Canadell, R. Shibaeva and E. Yagubskii, Adv. Funct. Mater., 2004, 14, 660–668 CrossRef CAS.
- K. Sugii, K. Takai, S. Uji, T. Terashima, H. Akutsu, A. Wada, S. Ichikawa, J. I. Yamada, T. Mori and T. Enoki, J. Phys. Soc. Jpn., 2013, 82, 054706 CrossRef CAS; N. D. Kushch, A. V. Kazakova, L. I. Buravov, E. B. Yagubskii, S. V. Simonov, L. V. Zorina, S. S. Khasanov, R. P. Shibaeva, E. Canadell, H. Son and J. Yamada, Synth. Met., 2005, 155, 588–594 CrossRef.
- M. Tamura, H. Tajima, K. Yakushi, H. Kuroda, A. Kobayashi, R. Kato and H. Kobayashi, J. Phys. Soc. Jpn., 2020, 60, 3861 CrossRef.
- J. Short, T. J. Blundell, S. Krivickas, S. Yang, J. D. Wallis, H. Akutsu, Y. Nakazawa and L. Martin, Chem. Commun., 2020, 56, 9497–9500 RSC.
- N. Mroweh, C. Mézière, F. Pop, P. Auban-Senzeir, P. Alemany, E. Canadell and N. Avarvari, Adv. Mater., 2020, 32(36), 2002811 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Fig. S1–S3 and Tables S1, S2. CCDC 2069593. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d1cc01441b |
‡ S-2-chloromandelic acid (0.94 g, 5 mmol) was added to a solution of boric acid (0.16 mg, 2.5 mmol) and triethylamine (2 mL, 10 mmol) in toluene (50 mL) and heated to reflux for 4 hours. The supernatant was removed in vacuo resulting in a clear pale yellow oil of [NEt3H][B(2-chloromandelate)2] which was used without further purification. BDH-TTP was obtained from Prof. Jun-ichi Yamada's Functional Materials II lab in University of Hyogo.12 A mixture of BDH-TTP (10 mg, 26 μmol) and BR/S(S-ClMan)2 (100 mg, 260 μmol) in chlorobenzene (10 mL) was filtered into a H-shaped electrochemical cell (Fig. S4). Platinum electrodes were inserted and a current of 0.5 μA was passed through the solution for a period of 18 days at room temperature resulting in dark black plates of κ-BDH-TTP2[BS-(S-ClMan)2] I. Crystal data at 150 K: C36H26BO6S16Cl2, M = 1149.24, black plate, a = 11.0733(7), b = 8.0039(4), c = 24.5775(11) Å, β = 99.546(5)°, U = 2148.1(2) Å3T = 150.00(10) K, space group P21, Z = 2, μ = 0.978 mm−1, Flack parameter = −0.01(5), reflections collected = 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 309, independent reflections = 8751, R1 = 0.0412, wR2 = 0.0861 [F2 > 2σ(F2)], R1 = 0.0485, wR2 = 0.0901 (all data). CCDC 2069593. 309, independent reflections = 8751, R1 = 0.0412, wR2 = 0.0861 [F2 > 2σ(F2)], R1 = 0.0485, wR2 = 0.0901 (all data). CCDC 2069593. |
| This journal is © The Royal Society of Chemistry 2021 |