Open Access Article
Open Access ArticleLithium halide ion-pair recognition with halogen bonding and chalcogen bonding heteroditopic macrocycles†
Yuen Cheong
Tse
,
Andrew
Docker
,
Zongyao
Zhang
and
Paul D.
Beer
 *
*
Chemistry Research Laboratory, Department of Chemistry, University of Oxford, Mansfield Road, Oxford, OX1 3TA, UK. E-mail: paul.beer@chem.ox.ac.uk
First published on 14th April 2021
Abstract
A series of halogen bonding and chalcogen bonding phenanthroline containing heteroditopic macrocyclic receptors exhibit cooperative recognition of lithium halide (LiX) ion-pairs. Quantitative 1H NMR ion-pair titration experiments in CDCl3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CD3CN (1
CD3CN (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) reveal a co-bound lithium cation switches on halide anion binding, most notably with the halogen bonding host system. The employment of bis- iodo- and telluromethyl-triazole sigma–hole donor motifs endows contrasting halide anion selectivity and binding affinity, with the halogen bonding ditopic host capable of exclusively binding lithium chloride whereas the chalcogen bonding ditopic receptor displays notable selectivity for lithium iodide over lithium bromide. Preliminary solid–liquid extraction experiments demonstrate the potential of sigma–hole mediated ion-pair recognition as a promising strategy for lithium salt recovery.
1, v/v) reveal a co-bound lithium cation switches on halide anion binding, most notably with the halogen bonding host system. The employment of bis- iodo- and telluromethyl-triazole sigma–hole donor motifs endows contrasting halide anion selectivity and binding affinity, with the halogen bonding ditopic host capable of exclusively binding lithium chloride whereas the chalcogen bonding ditopic receptor displays notable selectivity for lithium iodide over lithium bromide. Preliminary solid–liquid extraction experiments demonstrate the potential of sigma–hole mediated ion-pair recognition as a promising strategy for lithium salt recovery.
Lithium constitutes a crucial element in modern life. Its pervasive application in energy storage materials, polymer manufacture and pharmacology have stimulated an ever-increasing demand for the requisite lithium precursors. Contemporary methods of obtaining lithium typically rely on mineral reserves, such as brine and ore deposits, which by virtue of their accessibility and low processing costs have continued to dominate the global supply of lithium.1 Whilst the abundance of these natural resources presents no immediate threat, the continued exploitation of primary sources currently employed to meet this ever-growing demand raises significant environmental and ecological concerns.2–4 Indeed, despite the evident motivation for developing strategies recovering lithium from extant non-natural sources (e.g. disposed electrical devices),5–7 only <5% of lithium-ion batteries are recycled.8 The judicious exploitation of heteroditopic molecular receptors, capable of simultaneously binding cationic and anionic species, has demonstrated enormous potential in facilitating the extraction and recovery of a range of transition-9–13 and alkali-metal salts.14–18 However, reports of employing this strategy towards extracting lithium salts, in particular, remain scarce.19–23 In general, heteroditopic receptor design typically relies on crown ether-based cation recognition sites and convergent hydrogen bond donor arrays as anion binding sites.24,25 Over the last decade the emergence of sigma–hole interactions, such as halogen bonding (XB) and chalcogen bonding (ChB), have gained increasing attention in the field of anion recognition.26–35 Despite the noteworthy enhancements in anion affinity and marked contrasting selectivity behaviour frequently observed relative to hydrogen bonding (HB) analogues, the strategic integration of sigma–hole donors in heteroditopic ion-pair receptor design is extremely rare.36–41
Herein, we report a series of novel XB, ChB and HB 1,10-phenanthroline-based macrocycles, that serve as heteroditopic ion-pair hosts for the cooperative recognition and solid–liquid extraction of lithium halide salts (Fig. 1). Importantly, the incorporation of a bidentate XB donor motif dramatically increases the potency of the ditopic receptor for lithium halide recognition, remarkably facilitating the challenging stabilisation of LiCl ion-pairs in organic solvent media. In stark contrast, the ChB heteroditopic receptor displays a pronounced selectivity preference towards the ‘softer’ LiI ion-pair.
 | ||
| Fig. 1 Structure of target phenanthroline-based heteroditopic macrocycles incorporated with sigma–hole donors designed for ion-pair recognition of lithium salts. A− = anion. | ||
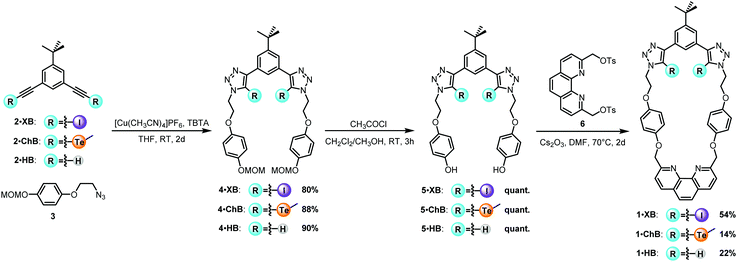
The synthesis of the target heteroditopic macrocyclic hosts is outlined in Scheme 1. The bis-triazole anion binding sites were constructed via a CuAAC42,43 ‘click’ reaction between the appropriately appended alkyne precursors 231,44 and two equivalents of azide 3 which gave the corresponding methoxymethyl (MOM) acetal protected precursors 4, in excellent yields in the range of 77–95%. Acidic deprotection afforded the bis-phenols 5 in quantitative yields. The target ditopic receptors 1·XB, 1·ChB and 1·HB were prepared via macrocyclization reactions between 1,10-phenanthroline bis-tosylate 645 and respective bis-phenols 5 in the presence of Cs2CO3 in dry DMF under high-dilution conditions in yields of up to 54% after chromatographic purification. All novel macrocycles were characterised by 1H, 13C and 125Te NMR (where relevant) and high-resolution ESI mass spectrometry.
Crystals suitable for X-ray diffraction analysis were obtained by slow evaporation of a solution of 1·XB in chloroform/methanol mixture and by vapour diffusion of pentane into a chloroform solution of 1·HB (Fig. 2).46 Inspection of the two structures reveals that the two iodo-triazole groups in 1·XB are significantly more twisted out of the plane relative to the central aromatic aryl spacer compared to the proto-triazoles in 1·HB. Notably, the potency of the XB-donor was revealed by the observation of a I⋯O short contact between the iodo-triazole donor and a methanol solvate molecule, exhibiting a distance significantly shorter than the sum of the van der Waals radii (87%).
To assess the ion-pair binding properties of the macrocycles, preliminary qualitative 1H NMR binding investigations were undertaken in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 CDCl3
1 CDCl3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CD3CN. Initial halide anion titration experiments conducted on the free macrocycles indicated no halide binding in this mixed solvent system.
CD3CN. Initial halide anion titration experiments conducted on the free macrocycles indicated no halide binding in this mixed solvent system.
The addition of one equivalent of LiClO4, followed by the sequential addition of one, two and five equivalents of tetrabutylammonium (TBA) halide salts, however, provided evidence for macrocycle phenanthroline-bound Li+ switching on halide anion binding. A representative example with 1·XB is shown in Fig. 3a. Upon addition of LiClO4, downfield shifts of phenanthroline proton signals 5–7 were indicative of Li+ complexation. Subsequent addition of TBACl caused a gradual downfield shift of internal benzene proton 2. This is consistent with the endotopic binding of Cl− occurring in the vicinity of the XB anion binding cavity of the macrocycle. Analogous experiments with TBABr and TBAI elicited similar chemical shift perturbations, indicating the concomitant binding of ion-pairs (Fig. S4-5 and S4-6, ESI†). In the case of 1·ChB·LiClO4 and 1·HB·LiClO4, the addition of TBABr and TBAI caused significant perturbations of the respective TeCH3 and triazole protons suggesting LiBr and LiI ion-pair binding. However, adding TBACl to both receptor lithium metal complex solutions resulted in LiCl salt precipitation (Fig. S4-7–S4-10, ESI†).
Analogous qualitative 1H NMR titrations of 1·XB with one equivalent of NaClO4 or KClO4 gave similar perturbations in the phenanthroline signals indicative of successful metal cation complexation. Subsequent addition of TBACl, however, caused salt precipitation and recovery of the free macrocycle, highlighting the preference for the Li+ cation which is particularly impressive considering the sizeable lattice enthalpy of LiCl (834 kJ mol−1) driving salt recombination (Fig. S4-11 and S4-12, ESI†).47
Quantitative analysis of the lithium halide ion-pair binding properties of the ditopic receptors was carried out in the same solvent system by monitoring the proton chemical shift perturbation of the Li+ complexed macrocycles as a function of halide anion concentration (Fig. 3b and Fig. S4-13–S4-18, ESI†). Bindfit analysis of the titration binding isotherm data determined 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometric anion association constants (Table 1).48 Importantly, Table 1 reveals a significant lithium cation-halide anion ion-pair binding cooperativity effect via a combination of favourable co-bound metal cation–anion electrostatic attractions and macrocycle preorganisation resulting from Li+ complexation. Comparing the halide anion association constant data, 1·XB is the most potent ion-pair receptor for all halides investigated, exhibiting two- to seven-fold enhancements in Br− and I− affinities relative to ChB and HB analogues. Notably, the potency of the XB donor motif is further illustrated by the ability of 1·XB to simultaneously complex the ‘hard’ LiCl ion-pair, while analogous experiments with 1·ChB and 1·HB resulted in LiCl salt precipitation. This may be rationalised by the strong XB-driven complexation of Cl−, thereby inhibiting its recombination with the co-bound Li+. Interestingly, 1·XB demonstrates similar strong ion-pair binding for both the heavier halides with a modest preference over chloride. By stark contrast, the ChB macrocyclic receptor, 1·ChB, displays significant selectivity for the ‘softer’ LiI ion-pair over LiBr (Ka(I−)/Ka(Br−) = 3.5) which may be attributed to a combination of factors including heteroditopic host-ion-pair guest size complementarity and favourable ChB interactions between the Te atoms and the larger I− anion.49,50
1 stoichiometric anion association constants (Table 1).48 Importantly, Table 1 reveals a significant lithium cation-halide anion ion-pair binding cooperativity effect via a combination of favourable co-bound metal cation–anion electrostatic attractions and macrocycle preorganisation resulting from Li+ complexation. Comparing the halide anion association constant data, 1·XB is the most potent ion-pair receptor for all halides investigated, exhibiting two- to seven-fold enhancements in Br− and I− affinities relative to ChB and HB analogues. Notably, the potency of the XB donor motif is further illustrated by the ability of 1·XB to simultaneously complex the ‘hard’ LiCl ion-pair, while analogous experiments with 1·ChB and 1·HB resulted in LiCl salt precipitation. This may be rationalised by the strong XB-driven complexation of Cl−, thereby inhibiting its recombination with the co-bound Li+. Interestingly, 1·XB demonstrates similar strong ion-pair binding for both the heavier halides with a modest preference over chloride. By stark contrast, the ChB macrocyclic receptor, 1·ChB, displays significant selectivity for the ‘softer’ LiI ion-pair over LiBr (Ka(I−)/Ka(Br−) = 3.5) which may be attributed to a combination of factors including heteroditopic host-ion-pair guest size complementarity and favourable ChB interactions between the Te atoms and the larger I− anion.49,50
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 CDCl3
1 CDCl3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CD3CNa
CD3CNa
| Anion | Cation | 1·XB | 1·ChB | 1·HB |
|---|---|---|---|---|
a
K
a values calculated using Bindfit software using a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 host–guest binding model. Errors (%) are in parenthesis. Lithium cation added as LiClO4. All anions added as their TBA salts. Solvent = 1 1 host–guest binding model. Errors (%) are in parenthesis. Lithium cation added as LiClO4. All anions added as their TBA salts. Solvent = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 CDCl3 1 CDCl3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CD3CN. T = 298 K.
b Salt recombination. CD3CN. T = 298 K.
b Salt recombination.
|
||||
| Cl− | Li+ | 1147 (3) | —b | —b |
| Br− | Li+ | 1214 (3) | 186 (6) | 211 (6) |
| I− | Li+ | 1236 (13) | 662 (10) | 121 (6) |
The ability for the heteroditopic macrocycles to solubilise inorganic lithium halide salts into organic solvent media was investigated by preliminary solid–liquid extraction studies. In a typical experiment, excess solid lithium halide salt was added to a solution of macrocycle 1·XB in CDCl3 (600 μL) and the mixture was vigorously sonicated for 1 hour. The excess salt was subsequently filtered off and CD3CN (200 μL) was added to improve the resolution of the post-extraction 1H NMR spectra. Inspection of the 1H NMR spectra (Fig. 4) confirmed the solubilisation of all three lithium halides by 1·XB as evidenced by significant downfield perturbations of the macrocycle's phenanthroline aromatic protons 5–7 and internal benzene proton 2, analogous to the 1H NMR spectra obtained from sequential addition of equimolar of LiClO4 and TBA halide salt (Fig. 2). Likewise, 1·ChB and 1·HB solubilised all three lithium halides as revealed by similar downfield proton chemical shifts in the respective 1H NMR spectra (Fig. S5-2 and 3, ESI†). These preliminary observations highlight the real potential for sigma–hole heteroditopic macrocycles to act as solid–liquid extractants for lithium salts.
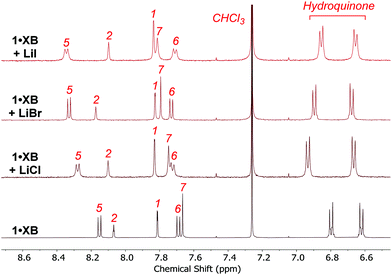 | ||
Fig. 4 Comparative pre- and post-extraction 1H NMR spectra of 1·XB with excess solid LiCl, LiBr and LiI (500 MHz, 298 K, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 CDCl3 1 CDCl3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CD3CN). CD3CN). | ||
In conclusion, a series of XB, ChB and HB heteroditopic macrocyclic receptors consisting of a 1,10-phenanthroline cation binding site and XB/ChB/HB donors for binding anions were synthesised for lithium halide salt ion-pair recognition. Extensive 1H NMR titration experiments reveal the co-complexation of lithium cation switches on sigma–hole XB and ChB halide recognition. The XB heteroditopic macrocycle proved to be the most potent LiX ion-pair receptor, impressively facilitating the binding of the ‘hard’ LiCl ion-pair species. Furthermore the incorporation of ChB donors significantly enhanced selectivity towards the ‘softer’ lithium halide salts namely LiI over LiBr. In general, the HB heteroditopic macrocycle analogue demonstrated inferior affinity for LiBr and LiI halide ion pairs relative to the sigma–hole hosts. Preliminary lithium halide solid–liquid extraction studies revealed the potential for these heteroditopic macrocycles to solubilise solid lithium halide salts into organic solvent mixtures. Importantly, these results demonstrate the exciting potential of sigma–hole mediated ion-pair recognition for modulating both ion-pair affinity and selectivity.
Y. C. T. thanks the Croucher Foundation for a scholarship, A. D. thanks the EPSRC for a studentship (Grant reference number EP/N509711/1) and Z. Z. thanks China Scholarship Council and University of Oxford for a scholarship.
Conflicts of interest
There are no conflicts to declare.Notes and references
- G. Martin, L. Rentsch, M. Höck and M. Bertau, Energy Storage Mater., 2017, 6, 171–179 CrossRef
.
-
J. Nriagu, Encylopedia of Environmental Health, Elsevier, 2nd edn, 2019 Search PubMed
.
- V. Flexer, C. F. Baspineiro and C. I. Galli, Sci. Total Environ, 2018, 639, 1188–1204 CrossRef CAS PubMed
.
- W. Liu, D. B. Agusdinata and S. W. Myint, Int. J. Appl. Earth Obs. Geoinf., 2019, 80, 145–156 CrossRef
.
- M. Kamenica, R. Kothur, A. Willows, B. Patel and P. Cragg, Sensors, 2017, 17, 2430 CrossRef PubMed
.
- X. Zheng, Z. Zhu, X. Lin, Y. Zhang, Y. He, H. Cao and Z. Sun, Engineering, 2018, 4, 361–370 CrossRef CAS
.
- C. Liu, J. Lin, H. Cao, Y. Zhang and Z. Sun, J. Cleaner Prod., 2019, 228, 801–813 CrossRef CAS
.
- B. Swain, Sep. Purif. Technol., 2017, 172, 388–403 CrossRef CAS
.
- D. J. White, N. Laing, H. Miller, S. Parsons, P. A. Tasker and S. Coles, Chem. Commun., 1999, 2077–2078 RSC
.
- K. Maity, D. K. Panda, R. J. Gallup, C. K. Choudhury and S. Saha, Org. Lett., 2018, 20, 962–965 CrossRef CAS PubMed
.
- Y. Chen, D.-X. Wang, Z.-T. Huang and M.-X. Wang, Chem. Commun., 2011, 47, 8112 RSC
.
- B. Hua, L. Shao, Z. Zhang, J. Liu and F. Huang, J. Am. Chem. Soc., 2019, 141, 15008–15012 CrossRef CAS PubMed
.
- M. Alfonso, A. Espinosa, A. Tárraga and P. Molina, Org. Lett., 2011, 13, 2078–2081 CrossRef CAS PubMed
.
- P. D. Beer, P. K. Hopkins and J. D. McKinney, Chem. Commun., 1999, 1253–1254 RSC
.
- H. Jiang, C. Dolain, J.-M. Léger, H. Gornitzka and I. Huc, J. Am. Chem. Soc., 2004, 126, 1034–1035 CrossRef CAS PubMed
.
- J. M. Mahoney, K. A. Stucker, H. Jiang, I. Carmichael, N. R. Brinkmann, A. M. Beatty, B. C. Noll and B. D. Smith, J. Am. Chem. Soc., 2005, 127, 2922–2928 CrossRef CAS PubMed
.
- Z.-H. Sun, F.-F. Pan, Triyanti, M. Albrecht and G. Raabe, Eur. J. Org. Chem., 2013, 7922–7932 CrossRef CAS
.
- B. Akhuli and P. Ghosh, Chem. Commun., 2015, 51, 16514–16517 RSC
.
- S. Tsuchiya, Y. Nakatani, R. Ibrahim and S. Ogawa, J. Am. Chem. Soc., 2002, 124, 4936–4937 CrossRef CAS PubMed
.
- J. M. Mahoney, A. M. Beatty and B. D. Smith, Inorg. Chem., 2004, 43, 7617–7621 CrossRef CAS PubMed
.
- Q. He, Z. Zhang, J. T. Brewster, V. M. Lynch, S. K. Kim and J. L. Sessler, J. Am. Chem. Soc., 2016, 138, 9779–9782 CrossRef CAS PubMed
.
- Q. He, N. J. Williams, J. H. Oh, V. M. Lynch, S. K. Kim, B. A. Moyer and J. L. Sessler, Angew. Chem., Int. Ed., 2018, 57, 11924–11928 CrossRef CAS PubMed
.
- K.-I. Hong, H. Kim, Y. Kim, M.-G. Choi and W.-D. Jang, Chem. Commun., 2020, 56, 10541–10544 RSC
.
- Q. He, G. I. Vargas-Zúñiga, S. H. Kim, S. K. Kim and J. L. Sessler, Chem. Rev., 2019, 119, 9753–9835 CrossRef CAS PubMed
.
- A. McConnell, A. Docker and P. Beer, ChemPlusChem, 2020, 85, 1824–1841 CrossRef CAS PubMed
.
- T. A. Barendt, A. Docker, I. Marques, V. Félix and P. D. Beer, Angew. Chem., Int. Ed., 2016, 55, 11069–11076 CrossRef CAS PubMed
.
- Z.-X. Liu, Y. Sun, Y. Feng, H. Chen, Y.-M. He and Q.-H. Fan, Chem. Commun., 2016, 52, 2269–2272 RSC
.
- C. J. Massena, N. B. Wageling, D. A. Decato, E. Martin Rodriguez, A. M. Rose and O. B. Berryman, Angew. Chem., Int. Ed., 2016, 55, 12398–12402 CrossRef CAS PubMed
.
- D. Mungalpara, S. Stegmüller and S. Kubik, Chem. Commun., 2017, 53, 5095–5098 RSC
.
- J. Y. C. Lim, I. Marques, V. Félix and P. D. Beer, Chem. Commun., 2018, 54, 10851–10854 RSC
.
- A. Borissov, I. Marques, J. Y. C. Lim, V. Félix, M. D. Smith and P. D. Beer, J. Am. Chem. Soc., 2019, 141, 4119–4129 CrossRef CAS PubMed
.
- T. Bunchuay, A. Docker, A. J. Martinez-Martinez and P. D. Beer, Angew. Chem., Int. Ed., 2019, 58, 13823–13827 CrossRef CAS PubMed
.
- J. Y. C. Lim and P. D. Beer, Chemistry, 2018, 4, 731–783 CrossRef CAS
.
- J. Pancholi and P. D. Beer, Coord. Chem. Rev., 2020, 416, 213281 CrossRef CAS
.
- M. S. Taylor, Coord. Chem. Rev., 2020, 413, 213270 CrossRef CAS
.
- A. Mele, P. Metrangolo, H. Neukirch, T. Pilati and G. Resnati, J. Am. Chem. Soc., 2005, 127, 14972–14973 CrossRef CAS PubMed
.
- R. Tepper, B. Schulze, P. Bellstedt, J. Heidler, H. Görls, M. Jäger and U. S. Schubert, Chem. Commun., 2017, 53, 2260–2263 RSC
.
- A. Brown, K. M. Mennie, O. Mason, N. G. White and P. D. Beer, Dalton Trans., 2017, 46, 13376–13385 RSC
.
- T. Bunchuay, A. Docker, U. Eiamprasert, P. Surawatanawong, A. Brown and P. D. Beer, Angew. Chem., Int. Ed., 2020, 59, 12007–12012 CrossRef CAS PubMed
.
- P. Sabater, F. Zapata, B. López, I. Fernández, A. Caballero and P. Molina, Dalton Trans., 2018, 47, 15941–15947 RSC
.
- A. Docker, T. Bunchuay, M. Ahrens, A. J. Martinez-Martinez and P. D. Beer, Chem. – Eur. J., 2021 DOI:10.1002/chem.202100579
.
- J. E. Hein, J. C. Tripp, L. B. Krasnova, K. B. Sharpless and V. V. Fokin, Angew. Chem., Int. Ed., 2009, 48, 8018–8021 CrossRef CAS PubMed
.
- H. A. Stefani, S. N. S. Vasconcelos, F. Manarin, D. M. Leal, F. B. Souza, L. S. Madureira, J. Zukerman-Schpector, M. N. Eberlin, M. N. Godoi and R. de Souza Galaverna, Eur. J. Org. Chem., 2013, 3780–3785 CrossRef CAS
.
- T. Nishikawa, S. Shibuya, S. Hosokawa and M. Isobe, Synlett, 1994, 485–486 CrossRef CAS
.
- C. J. Chandler, L. W. Deady and J. A. Reiss, J. Heterocycl. Chem., 1981, 18, 599–601 CrossRef CAS
.
- CCDC 2069372 (1·XB) and 2068373 (1·HB) contain the supplementary crystallographic data for this paper†.
-
J. R. Rumble, CRC Handbook of Chemsistry and Physics, CRC Press, 99th edn, 2018 Search PubMed
.
- P. Thordarson, Chem. Soc. Rev., 2011, 40, 1305–1323 RSC
.
- J. Y. C. Lim, I. Marques, A. L. Thompson, K. E. Christensen, V. Félix and P. D. Beer, J. Am. Chem. Soc., 2017, 139, 3122–3133 CrossRef CAS PubMed
.
- D. J. Pascoe, K. B. Ling and S. L. Cockroft, J. Am. Chem. Soc., 2017, 139, 15160–15167 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis, structural characterisation, additional figures. CCDC 2069372 and 2068373. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d1cc01287h |
| This journal is © The Royal Society of Chemistry 2021 |