Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Targeted pH switched europium complexes monitoring receptor internalisation in living cells†
Jack D.
Fradgley
a,
Matthieu
Starck
 a,
Michel
Laget
b,
Emmanuel
Bourrier
b,
Elodie
Dupuis
b,
Laurent
Lamarque
b,
Eric
Trinquet
b,
Jurriaan M.
Zwier
b and
David
Parker
a,
Michel
Laget
b,
Emmanuel
Bourrier
b,
Elodie
Dupuis
b,
Laurent
Lamarque
b,
Eric
Trinquet
b,
Jurriaan M.
Zwier
b and
David
Parker
 *a
*a
aDepartment of Chemistry, Durham University, South Road, Durham DH1 3LE, UK. E-mail: david.parker@dur.ac.uk
bCisbio Bioassays, BP 84175, 30200 Codolet, France
First published on 4th May 2021
Abstract
We report the design and evaluation of pH responsive luminescent europium(III) probes that allow conjugation to targeting vectors to monitor receptor internalisation in cells. The approach adopted here can be used to tag proteins selectively and to monitor uptake into more acidic organelles, thereby enhancing the performance of time-resolved internalisation assays that require pH monitoring in real time.
During the ageing process of endosomes and phagosomes, the pH tends to reduce with time and endosomes eventually may evolve into lysosomes. The cytosolic pH of healthy cells is around 7.2, whereas endosomal pH varies from 6.5 to 5.5; in mature lysosomes the pH is typically around 4.5. Receptor internalisation and endosomal uptake1 can be monitored provided that the species that is internalised (receptor or substrate) is labelled with a pH sensitive dye, whose emission intensity or lifetime varies significantly with pH.2–5
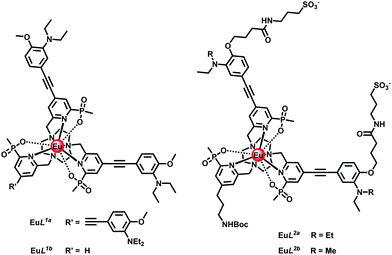
Recently, we have identified europium(III) complexes that show a pH dependent change in lifetime of a factor of 3 and an emission intensity pH switching ratio of around two orders of magnitude, using time-gated acquisition methods.6 Each of these Eu(III) complexes is C3 symmetric and contains strongly absorbing alkynyl-aryl chromophores, e.g.EuL1a with a brightness of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 M−1 cm−1 (332 nm) in acidic conditions. With a conjugated diethylamino group the pKa value is 6.2 (295 K) and falls by one unit as a methyl group replaces one of the ethyl substituents. The absorption spectrum changes with pH because acidification perturbs the energy of the ligand centred and internal charge transfer transitions that exist in these EuroTrackerTM dyes and related series of complexes.7–12
200 M−1 cm−1 (332 nm) in acidic conditions. With a conjugated diethylamino group the pKa value is 6.2 (295 K) and falls by one unit as a methyl group replaces one of the ethyl substituents. The absorption spectrum changes with pH because acidification perturbs the energy of the ligand centred and internal charge transfer transitions that exist in these EuroTrackerTM dyes and related series of complexes.7–12
With this background in mind, we set about preparing complexes suitable for use in cellulo that are hydrophilic and appropriately substituted to enable conjugation to targeting vectors. Thus, in EuL2a/2b (Scheme S2, ESI†), peripheral sulfonate groups on the two conjugated chromophores were introduced in order to minimise non-specific binding of the complex to proteins.12 In addition, the simple pyridine group was functionalised with a primary amino-propyl group (N-Boc protected), in order to permit subsequent site-selective conjugation to different targeting vectors.13 The synthesis of the Eu(III) complexes of L1b and L2a/2b was undertaken in a similar manner, (Scheme 1 and Scheme S2, ESI†), from mono-Boc-1,4,7-triazacyclononane. The syntheses involved a series of N-alkylation and simple deprotection steps, and the final complexes were purified by reverse phase HPLC. Under the strongly acidic conditions of the Boc removal step, protonation of the proximate dialkylamino group inhibits any unwanted acid catalysed alkyne hydration. Reaction of the mesylate 1,6 with mono-Boc-1,4,7-triazacyclononane gave the carbamate 2, followed by treatment with TFA in CH2Cl2 to yield the amine 3. Alkylation with the disubstituted pyridine mesylate 4,14 afforded the ester 5, and basic hydrolysis in aqueous methanol followed by complexation with EuCl3 at pH 6 gave the desired neutral complex, EuL.1b
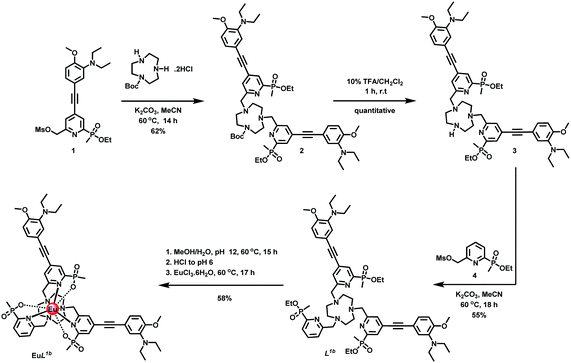 | ||
| Scheme 1 Synthesis of complex EuL1b | ||
The absorption spectrum of EuL2b showed a pH dependence associated with reversible protonation of the dialkylamino group that was characterised by the appearance of two isosbestic points at 284 and 332 nm, (Fig. 1). Similar behaviour was found with the other two complexes (Fig. S1 and S3–S5, ESI†). In each case, protonation switched on europium luminescence and the emission lifetime and intensity were measured as a function of pH (Fig. 2, Tables 1 and 2). Thus, with EuL2a excitation at the isosbestic point led to an increase in lifetime from 0.24 (pH 8) to 1.02 ms (pH 4) with a 50-fold increase in emission intensity (Fig. S6, ESI†). Monitoring changes in emission lifetime or intensity allowed pKa values to be estimated. In 0.1 M NaCl solution, the N,N-diethyl complexes had similar values around 6.2, reducing to 5.34 for the N-methylethyl analogue, EuL2b (Fig. S10–S12, ESI†). These pKa values are sensitive to the nature of the medium and reduced slightly in cell lysate solution, highlighting the need to calibrate the pH dependence in the medium of interest.
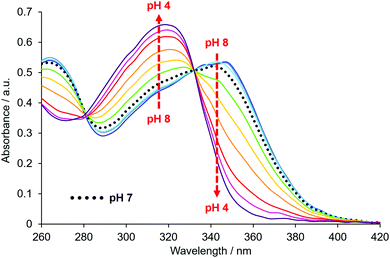 | ||
| Fig. 1 pH Variation of the absorbance spectrum of EuL2b (295 K, 0.1 M NaCl), showing isosbestic points at 284 and 332 nm. | ||
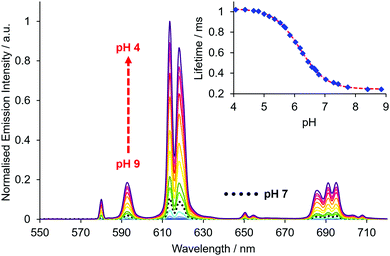 | ||
| Fig. 2 pH Variation of the europium emission spectrum of EuL2a (λexc 332 nm, 295 K, c = 20 μM, 0.1 M NaCl); the inset shows the variation of the europium emission lifetime with pH (λexc 332 nm, λem 613 nm). A pKa value of 6.18 was estimated by non-linear least squares regression analysis. | ||
| Complex | Conditions | pKa |
|---|---|---|
| a pKa values given are the mean of three replicates, at 295 K. | ||
| EuL 1a | 0.1 M NaCl | 6.21 (03) |
| NIH-3T3 cell lysate | 5.92 (04) | |
| EuL 1b | 0.1 M NaCl | 6.30 (03) |
| NIH-3T3 cell lysate | 6.25 (04) | |
| EuL 2a | 0.1 M NaCl | 6.18 (03) |
| NIH-3T3 cell lysate | 6.00 (04) | |
| EuL 2b | 0.1 M NaCl | 5.34 (03) |
| NIH-3T3 cell lysate | 5.28 (04) | |
| Complex | λ exc/nm | ε/M−1 cm−1 | τ/mse | Φ/%e | q | B/M−1 cm−1f |
|---|---|---|---|---|---|---|
| a Value at the isosbestic point. b Value at pH = 8. c Value at pH = 4. d Data from Ref. 6. e Errors in lifetime measurements are ±10% and in quantum yields ±15%. f Brightness values are given at the stated excitation wavelength. | ||||||
| EuL 1a | 331 | 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000a,d 000a,d |
0.25b | 0.1b | 0 | 46b |
| 0.84c | 17c | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200c 200c |
||||
| EuL 1b | 328 | 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000a 000a |
0.34b | 0.2a | 0 | 70b |
| 1.00c | 18b | 6300c | ||||
| EuL 2a | 332 | 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000a 000a |
0.24b | 0.3a | 0 | 117b |
| 1.02c | 16b | 6240c | ||||
| EuL 2b | 332 | 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000a 000a |
0.28b | 0.01a | 0 | 4b |
| 1.04c | 15b | 5850c | ||||
In each case, the increases in quantum yield and brightness (Table 2) following acidification are in accord with a strongly emissive and longer-lived protonated complex (see the invariance of the form of the excitation spectra with pH: Fig. S4, S7 and S13, ESI†), wherein the photo-induced electron transfer process that quenches the europium excited state is suppressed. By varying both the delay time and time window for signal acquisition, the ‘switch-on’ can be increased, as signal from the more emissive complex is favoured. For example, by using a delay time of 1.5 ms and acquiring signal for one millisecond (1500–2500 μs acquisition period), intensity ratios for pH 4/pH 8 solutions of around 500 were found (Table 3 and Fig. 3; Fig. S8/9; Table S1, ESI†), with a progressive diminution in the apparent pKa value as the delay time was increased.
| 60–460 μs | 1000–2000 μs | 1500–2500 μs | |
|---|---|---|---|
| I rel: pH 4/pH 8 | 34/32 | 266/227 | 527/465 |
| Apparent pKa | 6.21/6.57 | 5.96/6.26 | 5.86/6.16 |
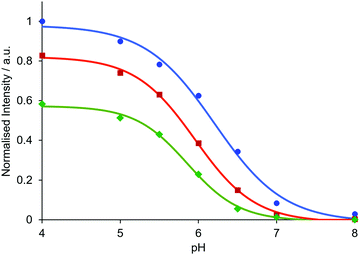 | ||
| Fig. 3 Relative emission intensity of EuL1b(λem 613 nm) as a function of pH for different time periods of signal acquisition (blue = 60–460 μs, red = 1000–2000 μs, green = 1500–2500 μs). Data are normalised to a 60–460 μs time window at pH 4. Measurements were made in aqueous solutions of NH4OAc (pH 4 and 5), MES (pH 5.5, 6, 6.5), HEPES (pH 7) and NH4HCO3 (pH 8) buffers (c = 20 μM, 0.1 M buffer in 0.1 M NaCl). | ||
In a proof-of-concept study for membrane receptor labelling and trafficking, the benzylguanine (BG) derivative EuL,2c was prepared from EuL2a using established methodology (Scheme S3, ESI†).12 The BG derivative serves to label the SNAP-tag (ST), a self-labelling suicide enzyme15 often used to study G-Protein Coupled Receptors (GPCRs). Previous studies have shown that Eu(III)12 and Tb(III) derivatives16,17 can be used to label SNAP tagged receptors. Using the non-pH sensitive Lumi4-Tb complex,18 the Tag-lite technology® emerged and Lumi4-Tb labelled GPCR can be used to signal receptor internalisation. Therefore, such a model is well suited to test the behaviour of these new europium pH responsive probes.
The glucagon-like peptide-1 receptor (GLP-1R) is a receptor targeted for anti-diabetic drugs since it is involved in the metabolic pathway for insulin production. Tools to study its agonist-induced internalisation19,20 are therefore in high demand. After labelling a HEK-293 cell line that stably expresses GLP-1R-ST with 200 nM of EuL2c at pH 7.4, the Eu time-gated luminescence was observed (Fig. 4A: 620 nm, ΔJ = 2 band, pH 4.5, acetate buffer; ESI†). Since labelling to non-transfected HEK-293 cells is negligible under the same conditions, this demonstrates that labelling of the GLP-1R with EuL2c on the cell surface is specific, and the compound does not accumulate in endosomal particles, as this would give rise to an observable luminescence signal. As has been shown for related Eu complexes,12 the introduction of peripheral anionic sulfonate groups in the probe structure suppresses any non-specific labelling to cells.
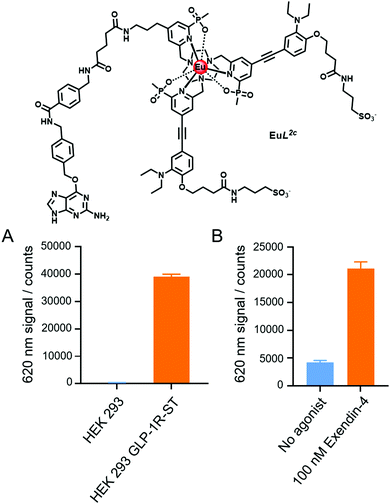 | ||
| Fig. 4 Time gated measurements of the Eu ΔJ = 2 band intensity of: (A) GLP-1R-ST receptor labelling, using EuL2c (200 nM) on non-transfected and GLP-1R-ST expressing HEK293 cells; (B) GLP-1R-ST receptor internalisation, 1 h after addition of 100 nM of the peptide agonist, Exendin-4, versus a control with no agonist added (see ESI† for experimental details). | ||
To follow receptor internalisation by monitoring endosomal acidification, 100 nM of the GLP-1R peptide agonist Exendin-4 was added to the EuL2c labelled receptors at pH 7.4. Measuring the time gated luminescence at 620 nm after a 1 h incubation revealed a 5-fold increase in luminescence intensity, as a result of both receptor internalisation and the subsequent endosomal acidification (Fig. 4B). Further pharmacological studies are underway on GLP-1R and other membrane receptors to reveal whether these promising europium pH probes have a bright future for studying receptor internalisation and its time dependence, both in vitro and in live cell assays.
We thank EPSRC (EP/L01212X/1) for grant and partial studentship support (JDF).
Conflicts of interest
Authors state that there are no conflicts to declare.Notes and references
- M. Kaksonen and A. Roux, Nat. Rev. Mol. Cell Biol., 2018, 19, 313–326 CrossRef CAS PubMed.
- Y. Urano, D. Asanuma, Y. Hama, Y. Koyama, T. Barrett, M. Kamiya, T. Nagano, T. Watanabe, A. Hasegawa, P. L. Choyke and H. Kobayashi, Nat. Med., 2009, 15, 104–109 CrossRef CAS PubMed.
- D. Asanuma, Y. Takaoka, S. Namiki, K. Takikawa, M. Kamiya, T. Nagano, Y. Urano and K. Hirose, Angew. Chem., Int. Ed., 2014, 53, 6085–6089 CrossRef CAS PubMed.
- A. Grover, B. F. Schmidt, R. D. Salter, S. C. Watkins, A. S. Waggoner and M. P. Bruchez, Angew. Chem., Int. Ed., 2012, 51, 4838–4842 CrossRef CAS PubMed.
- M. Isa, D. Asanuma, S. Namiki, K. Kumagai, H. Kojima, T. Okabe, T. Nagano and K. Hirose, ACS Chem. Biol., 2014, 9, 2237–2241 CrossRef CAS PubMed.
- M. Starck, J. D. Fradgley, R. Pal, J. M. Zwier, L. Lamarque and D. Parker, Chem. – Eur. J., 2021, 27, 766–777 CrossRef CAS PubMed.
- M. Soulié, F. Latzko, E. Bourrier, V. Placide, S. J. Butler, R. Pal, J. W. Walton, P. L. Baldeck, B. Le Guennic, C. Andraud, J. M. Zwier, L. Lamarque, D. Parker and O. Maury, Chem. – Eur. J., 2014, 20, 8636–8646 CrossRef PubMed.
- S. J. Butler, M. Delbianco, L. Lamarque, B. K. McMahon, E. R. Neil, R. Pal, D. Parker, J. W. Walton and J. M. Zwier, Dalton Trans., 2015, 44, 4791–4803 RSC.
- M. Starck, R. Pal and D. Parker, Chem. – Eur. J., 2016, 22, 570–580 CrossRef CAS PubMed.
- S. J. Butler, L. Lamarque, R. Pal and D. Parker, Chem. Sci., 2014, 5, 1750–1756 RSC.
- S. Shuvaev, M. Starck and D. Parker, Chem. – Eur. J., 2017, 23, 9974–9989 CrossRef CAS PubMed.
- M. Delbianco, V. Sadovnikova, E. Bourrier, G. Mathis, L. Lamarque, J. M. Zwier and D. Parker, Angew. Chem., Int. Ed., 2014, 53, 10718–10722 CrossRef CAS PubMed.
- M. Starck, J. D. Fradgley, S. Di Vita, J. A. Mosely, R. Pal and D. Parker, Bioconjugate Chem., 2020, 31, 229–240 CrossRef CAS PubMed.
- J. W. Walton, R. Carr, N. H. Evans, A. M. Funk, A. M. Kenwright, D. Parker, D. S. Yufit, M. Botta, S. De Pinto and K.-L. Wong, Inorg. Chem., 2012, 51, 8042–8056 CrossRef CAS PubMed.
- A. Keppler, S. Gendreizig, T. Gronemeyer, H. Pick, H. Vogel and K. Johnsson, Nat. Biotechnol., 2003, 21, 86–89 CrossRef CAS PubMed.
- J. M. Zwier, H. Bazin, L. Lamarque and G. Mathis, Inorg. Chem., 2014, 53, 1854–1866 CrossRef CAS PubMed.
- J. M. Zwier, T. Roux, M. Cottet, T. Durroux, S. Douzon, S. Bdioui, N. Gregor, E. Bourrier, N. Oueslati, L. Nicolas, N. Tinel, C. Boisseau, P. Yverneau, F. Charrier-Savournin, M. Fink and E. Trinquet, J. Biomol. Screen., 2010, 15, 1248–1259 CrossRef CAS PubMed.
- J. Xu, T. M. Corneillie, E. G. Moore, G.-L. Law, N. G. Butlin and K. N. Raymond, J. Am. Chem. Soc., 2011, 133, 19900–19910 CrossRef CAS PubMed.
- (a) D. Donnelly, Br. J. Pharmacol., 2012, 166, 27–41 CrossRef CAS PubMed; (b) S. N. Roed, P. Wismann, C. R. Underwood, N. Kulahin, H. Iversenn, K. A. Cappelen, L. Schäffer, J. Lehtonen, J. Hecksher-Soerensen, A. Secher, J. M. Mathiesen, H. Bräuner-Osborne, J. L. Whistler, S. M. Knudsen and M. Waldhoer, Mol. Cell. Endocrinol., 2014, 382, 938–949 CrossRef CAS PubMed.
- W. J. C. van der Velden, F. X. Smit, C. B. Christiansen, T. C. Møller, G. M. Hjortø, O. Larsen, S. P. Schiellerup, H. Bräuner-Osborne, J. J. Holst, B. Hartmann, T. M. Frimurer and M. M. Rosenkilde, ACS Pharmacol. Transl. Sci., 2021, 4, 296–313 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Ligand and complex synthesis; photophysical data and spectra; cell experiments. See DOI: 10.1039/d1cc01029h |
| This journal is © The Royal Society of Chemistry 2021 |