Iron-catalyzed stereoselective haloamidation of amide-tethered alkynes†
Jin-Biao
Liu
 a,
Miaofeng
Ren
b,
Xiaojing
Lai
b and
Guanyinsheng
Qiu
a,
Miaofeng
Ren
b,
Xiaojing
Lai
b and
Guanyinsheng
Qiu
 *b
*b
aSchool of Metallurgical and Chemical Engineering, Jiangxi University of Science and Technology, Ganzhou 341000, China
bCollege of Biological, Chemical Sciences and Engineering, Jiaxing University, Jiaxing 314001, Zhejiang, China. E-mail: qiuguanyinsheng@mail.zjxu.edu.cn
First published on 26th March 2021
Abstract
In this work, by using N-methoxybenzamides as efficient acyl nitrene precursors, an iron-catalyzed formal cis-haloamidation of alkyne is reported. Without assistance of additives, the reaction worked well in the presence of 50 mol% FeCl3 or FeBr3, leading to a series of chloro/bromo-containing isoindolin-5-ones with high efficiency and wide reaction scope. In the reaction, the iron-facilitated haloamidation proceeds through a halo anion-participating concerted [3+2] cyclization to release the final products. The key intermediate ferric acyl nitrene A is generated in situ from a formal removal of MeOH.
Regarded as a highly reactive species, the nitrene intermediate has long attracted the attention of synthetic chemists.1 This is most likely because, via a nitrene transfer reaction, nitrogen-containing building blocks can be incorporated into target products for the construction of structurally complex motifs. To date, a series of well-recognized nitrene precursors, which predominantly include azides, sulfonamides, and iminoiodinanes, etc., enable nitrene transfer reactions for C–N bond formation through typical C–H bond insertion and aziridination.
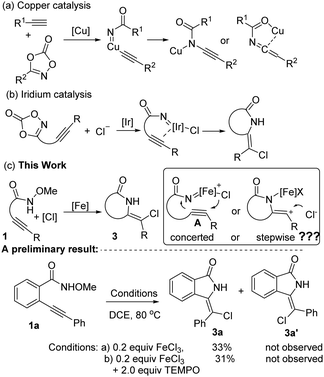
In addition to well-established C–H insertion and aziridinations,2 nitrene/alkyne metalation has attracted significant attention. A rhodium-catalyzed nitrene/alkynes metalation was initially investigated by the Blakey, Panek, Xu, and Shi groups.3 The resulting rhodium nitrene could be trapped by an intramolecular/intermolecular allylic ester,3a,b alkene/arene,3c,d intramolecular cyclopropane,3e and even by water.3f It is noteworthy that this elegant methodology employs sulfonamides as precursors and the use of a stoichiometric amount of hypervalent iodide is thus required. Notably, enantioselective reactions have also been demonstrated.3g Employing open-shell catalysis enabled by iminoiodinanes,4a,c Pérez and co-workers recently realized a Cu-catalyzed intermolecular radical functionalization of the resulting copper nitrene with alkynes.4a By adopting dioxazoles as acyl nitrene precursors, in 2019 De Bruin and co-workers found that ketenimine and ynamide species could be prepared via a copper-catalyzed acyl nitrene transfer into intramolecular C–C triple bonds (Scheme 1a).5a In the reaction, the formation of ketenimines or ynamides was ascribed to the insertion of the resulting copper acyl nitrene into the copper acetylide Cu–C bond. Distinctively, the dioxazole-derived nitrene transfer into intramolecular alkynes under iridium catalysis, developed by Chang and co-workers, proceeds through a chloro anion-participating concerted [3+2] cyclization mechanism (Scheme 1b).5b
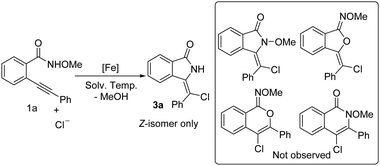
The use of amides or carbamates as acyl nitrene precursors is attracting the interest of chemists, and an array of publications have suggested that N-protecting groups play a pivotal role in the formation of acyl nitrene intermediates; metal catalysts such as rhodium and iridium salts have been employed in these reactions.6 Very recently, our group developed an iron-catalyzed acyl nitrene/alkyne metalation of N-methoxyamides and β-dicarbonyl compounds for the synthesis of pyrrolo[2,1-a]isoindol-5-ones.7 In the reaction, using iron catalysis, N-methoxyamides serve as efficient acyl nitrene precursors through a formal removal of MeOH. Moreover, it is interesting that the resulting ferric acyl nitrene should be in a singlet state, which differs from the previous reports implying ferric imido radical species.6f,g,8 Considering the low cost of iron catalysts and their versatility,9 we herein report the further application of N-methoxylamides10 as acyl nitrene precursors in iron-catalyzed intramolecular acyl nitrene/alkyne metalation and chlorination (Scheme 1d). In addition to developing an alternative pathway for the preparation of various isoindolin-1-ones pre-products for structural elaboration, we have also provided further evidence for the formal ferric acyl nitrene-based chloroamidation (a concerted or stepwise pathway). With the projected idea in mind, a model reaction of N-methoxybenzamide 1a in the presence of 0.2 equiv. of FeCl3 was conducted in DCE at 80 °C. The preliminary result suggested that the above intramolecular acyl nitrene/alkyne metalation takes place, leading uniquely to a chloroamidative product (Z)-3-(chloro(phenyl)methylene)isoindolin-1-one 3a in 33% isolated yield. (E)-3-(chloro(phenyl)methylene)isoindolin-1-one 3a′, a well-known product derived from 5-exo-dig chlorocyclization,11 was not observed. Furthermore, it was pleasing to find the undesired Curtius rearrangement byproduct was not observed.12 The exact structure of product 3a was identified by X-ray diffraction (CCDC†: 1992175). Additionally, a control experiment with 2.0 equiv. of TEMPO gave a similar yield (Scheme 1c), indicating the reaction probably proceeds through a non-radical pathway. Assuming that the chloroamidation is a stepwise process, it seems reasonable that the model reaction should produce a mixture of 3a and 3a′. The result that the reaction provides only (Z)-product 3a provides some evidence of a concerted chloroamidation mechanism. It is believed that this ferric acyl nitrene-based chloroamidation may serve as an important supplement for the additive-free synthesis of chloro-containing heteroisoindolin-1-ones5b and bromo-containing isoindolin-1-ones.13
The above positive result encouraged us to optimize the model reaction. To our delight, increasing the loading of FeCl3 to 0.5 equiv. drastically improved the reaction efficiency, producing the target product 3a in almost quantitative yield (entry 3, Table 1). Other N-nucleophilic or O-nucleophilic chlorocyclization byproducts were not detected.14 An increase or decrease in reaction temperature did not improve the reaction outcome, and inferior yields were obtained when the model reaction was carried out at 100 °C or 60 °C (entries 5 and 6, Table 1). No further improvement for reaction efficiency was observed when different solvents were employed (entries 8–10, Table 1). In order to reduce the FeCl3 loading, reactions with various chloro sources were conducted (entries 11–13, Table 1). To our surprise, poorer results were obtained when KCl, NH4Cl, and NaCl were used as replacements. These results suggest that the additional chloro source does not have a significant impact on reaction efficiency, thus resulting in the need for a relatively high loading of iron salt. Addition of radical scavengers did not have a significant impact on the reaction yield (entry 14, Table 1), suggesting that the reaction does not involve the generation of a ferric imido radical species.8
| Entry | [Fe] (equiv.) | Sol. | Temp. (°C) | Yield of 3ab (%) |
|---|---|---|---|---|
| a Conditions: the reaction of 1a (0.2 mmol) was carried out under air atmosphere. b Isolated yield based on 1a. c 2.0 equiv. of TEMPO was added. | ||||
| 1 | FeCl3 (0.2) | DCE | 80 | 33 |
| 2 | FeCl3 (0.4) | DCE | 80 | 71 |
| 3 | FeCl3 (0.5) | DCE | 80 | 98 |
| 4 | FeCl3 (1.0) | DCE | 80 | 91 |
| 5 | FeCl3 (0.5) | DCE | 60 | 81 |
| 6 | FeCl3 (0.5) | DCE | 100 | 74 |
| 7 | FeCl3 (0.5) | DCE | 80 | 79 |
| 8 | FeCl3 (0.5) | Toluene | 80 | 80 |
| 9 | FeCl3 (0.5) | DMF | 80 | 65 |
| 10 | FeCl3 (0.5) | THF | 80 | 74 |
| 11 | FeCl3 (0.2) + KCl (1.5) | DCE | 80 | 39 |
| 12 | FeCl3 (0.2) + NH4Cl (1.5) | DCE | 80 | 41 |
| 13 | FeCl3 (0.2) + NaCl (1.5) | DCE | 80 | 43 |
| 14c | FeCl3 (0.5) | DCE | 80 | 95 |
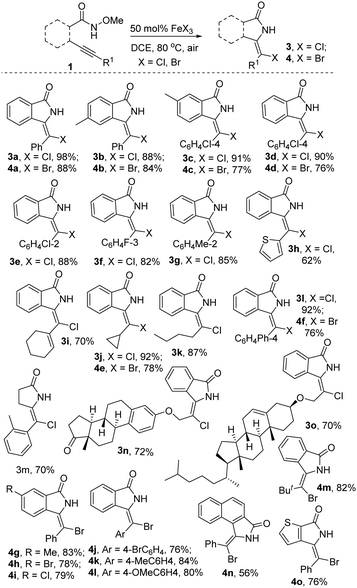
With the optimized conditions in hand, we investigated the scope of this reaction. The results are presented in Scheme 2. As shown in Scheme 2, the reaction is amenable to an array of functional groups, and a series of halo-containing isoindolin-1-ones 3a–3o and 4a–4o were obtained in good to excellent yields. For example, the reaction of N-methoxy-4-methyl-2-(phenylethynyl)benzamide 1b under the standard conditions provided the desired products 3b and 4b in 88% and 84% yields, respectively. The reaction of 2-(cyclopropylethynyl)-N-methoxybenzamide proceeded smoothly, leading to the corresponding products 3j in 92% yield and 4e in 78% yield. From investigations of the substituent effect on the alkyne, it was found that the substituents such aryl, alkyl, and heteroaryl groups were all tolerated. Some sensitive functional groups, including cyclohexyl, cyclopropyl and thiophene functionalities, were tolerated under the reaction conditions, with the formation of the desired products 3h, 3i, 3j, 4e, and 4o. It is worth noting that the substrates with complex alkynes were efficient reaction partners. For instance, the correspoding products 3n and 3o were synthesized efficiently when estrone- and cholestrol-derived substrates were used. In particular, the reaction of N-methoxy-5-(o-tolyl)pent-4-ynamide 1m also worked well, providing the desired product 3m in 70% yield when FeCl3 was used.
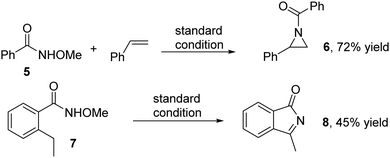
As mentioned above, it is envisioned that the reaction proceeds through a ferric acyl nitrenoid-based [3+2] cyclization pathway. It is well known that nitrenes readily react with styrene and C–H bonds, and undergo aziridination and C–H insertion. To support our assumption, aziridination of N-methoxylbenzamide 5 was carried out under the standard conditions. To our delight, the aziridinated product 6 was afforded in 72% yield. Furthermore, treatment of 2-ethyl-N-methoxybenzamide 7 with 50 mol% FeCl3 under the standard conditions gave rise to a C–H insertion and sequential oxidation reaction, leading to isoindolin-1-one 8 in moderate yield (Scheme 3).
In light of the aforementioned results and the previous findings,7,15 a plausible mechanism has been proposed (Scheme 4). In the reaction, a ferric acyl nitrene species A is generated via a formal removal of MeOH under iron catalysis. We reasoned the removal of MeOH involves 1,2-H migration15b and ligand exchange with FeX3. Subsequentially, the ferric acyl nitrene intermediate A undergoes a halo anion-participating concerted [3+2] cyclization, forming an isoindolin-1-one-containing ferric metallacyle intermediate B. Finally, protonation of the stable metallacyle intermediate B takes place to regenerate the iron catalyst and deliver the desired products 3 and 4.5b
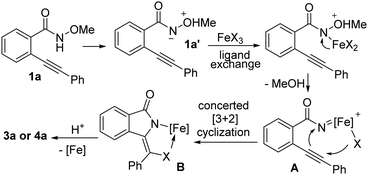 | ||
| Scheme 4 A halo anion-participating [3+2] cyclization mechanism (other ligand in iron center is omitted). | ||
In conclusion, N-methoxybenzamides have been developed as efficient acyl nitrene precursors, and an iron-catalyzed formal cis-chloroamidation for the synthesis of isoindol-1-ones has been reported. The reaction proceeds smoothly with broad functional group tolerance. Mechanistic studies suggest that the formal chloroamidation occurs via an iron-catalyzed acyl nitrene-based chloro anion-participating concerted [3+2] cyclization. Further transformations involving iron-catalyzed acyl nitrene/alkyne metalations with N-methoxybenzamides are being investigated in our laboratory, and the results will be reported in due course.
Financial support from the Natural Science Foundation of China (21772067,21762018 and 21961014), the Natural Science Foundation of Jiangxi Province, China (20192BCBL23009 and 20202BABL203005), and The Youth Jinggang Scholars Program in Jiangxi Province is gratefully acknowledged.
Conflicts of interest
There are no conflicts to declare.Notes and references
- For recent reviews, see: (a) F.-L. Hong and L.-W. Ye, Acc. Chem. Res., 2020, 53, 2003 CrossRef CAS PubMed; (b) Y. Liu, T. You, H.-X. Wang, T. Zhou, C. Zhou and Z.-M. Che, Chem. Soc. Rev., 2020, 49, 5310 RSC; (c) S. Xie, M. Sundhoro, K. N. Houk and M. Yan, Acc. Chem. Res., 2020, 53, 937 CrossRef CAS PubMed; (d) B. Plietker and A. Roeske, Catal. Sci. Technol., 2019, 9, 4188 RSC ; For recent examples, see: ; (e) K. Lang, S. Torker, L. Wojtas and X. P. Zhang, J. Am. Chem. Soc., 2019, 141, 12388 CrossRef CAS PubMed; (f) Z. W. Davis-Gilbert, X. Wen, J. D. Goodpaster and L. A. Tonks, J. Am. Chem. Soc., 2019, 141, 7267 Search PubMed; (g) M. Lee, H. Jung, D. Kim, J.-W. Park and S. Chang, J. Am. Chem. Soc., 2020, 142, 11999 CrossRef CAS PubMed; (h) M. R. Rodriguez, M. Besora, F. Molina, F. Maseras, M. M. Diaz-Requejo and P. J. Pérez, J. Am. Chem. Soc., 2020, 142, 13062 CrossRef CAS PubMed; (i) M. Ju, E. E. Zerull, J. M. Roberts, M. Huang, L. A. Guzer and J. M. Schomaker, J. Am. Chem. Soc., 2020, 142, 12930 CrossRef CAS PubMed.
- For recent reviews, see: (a) H. Hayashi and T. Uchida, Eur. J. Org. Chem., 2020, 909 CrossRef CAS; (b) R. Singh and A. Nyjherjee, ACS Catal., 2019, 9, 3604 CrossRef CAS; (c) T. Shimbayashi, K. Sasakura, A. Eguchi, K. Okamoto and K. Ohe, Chem. – Eur. J., 2019, 25, 3156 CAS; (d) K. M. Van Vliet and B. Du Bruin, ACS Catal., 2020, 10, 4751 CrossRef CAS; (e) Y. Park, Y. Kim and S. Chang, Chem. Rev., 2017, 117, 9247 CrossRef CAS PubMed; (f) Y.-C. Wang, X.-J. Lai, K. Huang, S. Yadav, G. Qiu, L. Zhang and H. Zhou, Org. Chem. Front., 2021 10.1039/D0QO01360A.
- For rhodium catalysis: (a) A. R. Thornton and S. B. Blakey, J. Am. Chem. Soc., 2008, 130, 5020 CrossRef CAS PubMed; (b) N. Mace, A. R. Thornton and S. B. Blakey, Angew. Chem., Int. Ed., 2013, 52, 5836 CrossRef CAS PubMed; (c) A. R. Thornton, V. I. Martin and S. B. Blakey, J. Am. Chem. Soc., 2009, 131, 2434 CrossRef CAS PubMed; (d) R. A. Brawn, K. Zhu and J. S. Panek, Org. Lett., 2014, 16, 74 CrossRef CAS PubMed; (e) D. Pan, Y. Wei and M. Shi, Org. Lett., 2017, 19, 3584 CrossRef CAS PubMed; (f) D. Pan, Y. Wei and M. Shi, Org. Lett., 2018, 20, 84 CrossRef CAS PubMed; (g) K. Hong, H. Su, C. Pei, X. X. Lv, W. H. Hu, L. H. Qiu and X. F. Xu, Org. Lett., 2019, 21, 3328 CrossRef CAS PubMed; (h) K. Hong, S. Zhou, W.-H. Hu and X.-F. Xu, Org. Chem. Front., 2020, 7, 1327 RSC.
- (a) M. R. Rodriguez, A. Beltran, A. L. Mudarra, E. Alvarez, F. Maseras, M. M. Diaz-Requejo and J. Pérez, Angew. Chem., Int. Ed., 2017, 56, 12842 CrossRef CAS PubMed; (b) A. Saito, Y. Kambara, T. Yagyu, K. Noguchi, A. Yoshimura and V. V. Zhdankin, Adv. Synth. Catal., 2015, 357, 667 CrossRef CAS; (c) E. Haldón, m Besora, I. Cano, X. C. Cambeiro, M. A. Pericàs, F. Maseras, M. C. Nicasio and P. J. Pérez, Chem. – Eur. J., 2014, 20, 3463 CrossRef PubMed.
- (a) K. M. Van Vliet, L. H. Polak, M. A. Siegler, J. I. van der Vlugt, C. F. Guerra and B. de Bruin, J. Am. Chem. Soc., 2019, 141, 15240 CrossRef CAS PubMed; (b) S. Y. Hong, J. Son, D. Kim and S. Chang, J. Am. Chem. Soc., 2018, 140, 12359 CrossRef CAS PubMed.
- For examples on the reactions with olefins, see: (a) J. H. Conway and T. Rovis, J. Am. Chem. Soc., 2017, 140, 135 CrossRef PubMed; (b) H. Jung, H. Keum, J. Kweon and S. Chang, J. Am. Chem. Soc., 2020, 142, 5811 CrossRef CAS PubMed; (c) T. Zhang, X. Hu, X. Dong, G. Li and H. Lu, Org. Lett., 2018, 20, 6260 CrossRef CAS PubMed; (d) L. A. Combee, S. L. Johnson, J. E. Laudenschlager and M. K. Hilinski, Org. Lett., 2019, 21, 2307 CrossRef CAS PubMed; (e) Y. Tan, F. Han, M. Hemmiing, J. Wang, K. Harms, X. Xie and E. Meggers, Org. Lett., 2020, 22, 6653 CrossRef CAS PubMed; (f) G. Liu, Y. Zhang, Y. Yuan and H. Xu, J. Am. Chem. Soc., 2013, 135, 3343 CrossRef CAS PubMed; (g) D. Lu, C. Zhu, Z. Jia and H. Xu, J. Am. Chem. Soc., 2014, 136, 13186 CrossRef CAS PubMed.
- X.-J. Lai, J.-B. Liu and Y.-C. Wang, Chem. Commun., 2021, 57, 2077 RSC.
- For recent examples, see: (a) S. K. Das, S. Roy, H. Khatuda and B. Chattopadhyyay, J. Am. Chem. Soc., 2020, 142, 16211 CrossRef CAS PubMed; (b) X. Lu, X.-X. Li, Y.-M. Lee, Y. Jang, M.-S. Seo, S. Hong, K.-B. Cho, S. Fukuzumi and W. Nam, J. Am. Chem. Soc., 2020, 142, 3891 CrossRef CAS PubMed; (c) S. Liang, X. Zhao, T. Yang and W. Yu, Org. Lett., 2020, 22, 1961 CrossRef CAS PubMed; (d) X. Zhao, S. Liang, X. Fan, T. Yang and W. Yu, Org. Lett., 2019, 21, 1559 CrossRef CAS PubMed; (e) S. A. Fayassal, A. Giungi, F. Berhal and G. Prestal, Org. Process Res. Dev., 2020, 24, 695 CrossRef; (f) Z. Lim, N. C. Thacker, T. Sawano, T. Drake, P. Ji, G. Lan, L. Guo, S. Liu, C. Wang and W. Lin, Chem. Sci., 2018, 9, 143 RSC; (g) E. T. Hennessy, R.-Y. Liu, D. Iovan, R. A. Duncan and T. A. Betley, Chem. Sci., 2014, 5, 1526 RSC.
- Selected reviews on iron catalyzed-nitrene transfer reaction C-H amination and aziridination, see: (a) A. Casnati, M. Lanzi and G. Cera, Molecules, 2020, 25, 3889 CrossRef CAS PubMed; (b) P. Wang and L. Deng, Chin. J. Chem., 2018, 36, 1222 CrossRef CAS.
- For recent examples using N-methoxylamide as amidating agent, see: (a) W.-J. Cui, Z.-J. Wu, Q. Gu and S.-L. You, J. Am. Chem. Soc., 2020, 142, 7379 CrossRef CAS PubMed; (b) K. M. Nakafuku, S. C. Fosu and D. A. Nagib, J. Am. Chem. Soc., 2018, 140, 11202 CrossRef CAS PubMed; (c) C. Zhou, J. Zhao, W. Guo, J. Jiang and J. Wang, Org. Lett., 2019, 21, 9315 CrossRef CAS PubMed; (d) Q. Li, M. Zhang, S. Zhan and Z. Gu, Org. Lett., 2019, 21, 6374 CrossRef CAS PubMed; (e) D. Yan, G. Wang, F. Xiong, W.-Y. Sun, Z. Shi, Y. Lu, S. Li and J. Zhao, Nat. Commun., 2018, 9, 4293 CrossRef PubMed; (f) C. Feng and T.-P. Loh, Org. Lett., 2014, 16, 3444 CrossRef CAS PubMed; (g) C. Zheng and C. J. Chou, Org. Lett., 2016, 18, 5512 CrossRef PubMed; (h) S. Banjo, E. Nakasuji, T. Meguro, T. Sato and N. Chida, Chem. – Eur. J., 2019, 25, 7941 CrossRef CAS PubMed.
- For selected examples on 5-exo-dig cyclization, see: (a) T. Yao, Z. Guo, X. Liang and L. Qi, J. Org. Chem., 2018, 83, 13370 CrossRef CAS PubMed; (b) R.-X. Wang, S.-T. Yuan, J.-B. Liu, J. Wu and G. Qiu, Org. Biomol. Chem., 2018, 16, 4501 RSC; (c) D. Brahmchari, A. K. Verma and S. Mehta, J. Org. Chem., 2018, 83, 3339 CrossRef CAS PubMed; (d) M. Jithunsa, M. Ueda and O. Miyata, Org. Lett., 2011, 13, 518 CrossRef CAS PubMed; (e) S. Mehta and D. Brahmchari, J. Org. Chem., 2019, 84, 5492 CrossRef CAS PubMed; (f) R. Liu, M. Yang, W. Xie, W. Dong, H. Zhou, S. Yadav, V. I. Potkin and G. Qiu, J. Org. Chem., 2020, 85, 5312 CrossRef CAS PubMed; (g) X. Bantreil, A. Bourderioux, P. Mateo, C. E. Hagerman, M. Selkti, E. Brachet and P. Belmont, Org. Lett., 2016, 18, 4814 CrossRef CAS PubMed; (h) Y.-C. Wang, K. Huang, X. Lai, Z. Shi, J.-B. Liu and G. Qiu, Org. Biomol. Chem., 2021, 19, 1940 RSC; (i) K. Zhou, J. Huang, J. Wu and G. Qiu, Chin. Chem. Lett., 2021, 32, 37 CrossRef CAS; (j) Y. Shan, L. Su, D. Chen, M. Yang, W. Xie and G. Qiu, Chin. Chem. Lett., 2021, 32, 437 CrossRef CAS.
- (a) A. Das, Y. S. Chen, J. H. Reibenspies and D. C. Powers, J. Am. Chem. Soc., 2019, 141, 16232 CrossRef CAS PubMed; (b) D. Li, T. Wu, K. Liang and C. Xia, Org. Lett., 2016, 18, 2228 CrossRef CAS PubMed for reviews, see: ; (c) C. Wentrup, Acc. Chem. Res., 2011, 44, 393 CrossRef CAS PubMed; (d) W. M. Jones, Org. Chem., 1980, 42, 95 CAS.
- H. Danielec, J. Klugge, B. Schlummer and T. Bach, Synthesis, 2006, 551 CAS.
- For selected examples on 6-endo-dig cyclization,see: (a) R. Liu, M. Li, W. Xie, H. Zhou, Y. Zhang and G. Qiu, J. Org. Chem., 2019, 84, 11763 CrossRef CAS PubMed; (b) Y. Yang, Y. Liu, R. Zhu, C. Liu and D. Zhang, J. Org. Chem., 2019, 84, 9705 CrossRef CAS PubMed; (c) D. Ding, T. Mou, J. Xue and X. Jiang, X, Chem. Commun., 2017, 53, 5279 RSC; (d) R.-X. Wang, Z. Fang, G. Qiu, W. Xie and J.-B. Liu, Synthesis, 2019, 4058 Search PubMed.
- (a) S. Ishii, S. Zhap and P. Helquist, J. Am. Chem. Soc., 2000, 122, 5897 CrossRef CAS; (b) R. Liu, M. Yang, G. Qiu, L. Zhang and J. Luo, Chin. J. Org. Chem., 2020, 40, 2071 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1992175. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d1cc00870f |
| This journal is © The Royal Society of Chemistry 2021 |