Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
The Hantzsch reaction for nitrogen-13 PET: preparation of [13N]nifedipine and derivatives†
Julia E.
Blower
 *,
Michelle T.
Ma
*,
Michelle T.
Ma
 ,
Fahad A. I.
Al-Salemee
,
Fahad A. I.
Al-Salemee
 and
Antony D.
Gee
and
Antony D.
Gee
 *
*
King's College London, School of Biomedical Engineering and Imaging Sciences, Department of Imaging Chemistry and Biology, 4th Floor Lambeth Wing, St Thomas’ Hospital, London, SE1 7EH, UK. E-mail: julia.blower@kcl.ac.uk; antony.gee@kcl.ac.uk
First published on 8th April 2021
Abstract
Nitrogen-13 is an attractive but under-used PET radionuclide for labelling molecules of biological and pharmaceutical interest, complementing other PET radionuclides. Its short half-life (t1/2 = 9.97 min) imposes synthetic challenges, but we have expanded the hitherto limited pool of 13N labelling strategies and tracers by adapting the multicomponent Hantzsch condensation reaction to prepare a library of 13N-labelled 1,4-dihydropyridines from [13N]ammonia, including the widely-used drug nifedipine. This represents a key advance in 13N PET radiochemistry, and will serve to underpin the renewed interest in clinical opportunities offered by short-lived PET tracers.
Nitrogen-13 (t1/2 = 9.97 min, 100% β+) is a potentially useful radionuclide for imaging with Positron Emission Tomography (PET). To date nitrogen-13 has been used clinically in the form of [13N]NH3 for myocardial perfusion imaging.1 However, it has been largely over-looked as a viable option for radiolabelling more complex molecules as its very short half-life poses challenges to the development of new synthetic methods.
Despite its limited use, nitrogen-13 has several attractive features. Firstly, the ubiquity of nitrogen in biological and pharmaceutical compounds allows the study of authentic radiolabelled biogenic molecules, which fluorine-18, the most commonly used PET radionuclide, and radiometals very rarely allow. Secondly, whilst many strategies enable biogenic radiolabelling of organic compounds with the PET radionuclide carbon-11, labelling with nitrogen-13 further expands the scope of radiochemical methods available, and labelling the same molecule in alternative positions may give useful metabolic information in vivo. Thirdly, unlike fluorine-18 (t1/2 = 109.7 min) the short half-life of nitrogen-13 allows repeated PET scans on the same individual within a short time period with low radiation dose to the patient. Finally, with the advent of highly sensitive total-body PET scanners that allow for low dose PET imaging, the use of radiopharmaceuticals based on very short-lived radionuclides becomes more feasible.2
Single-step multi-component reactions (MCRs) can efficiently generate structurally diverse libraries enabling high-throughput screening.3 MCRs have already had some application in the field of radiochemistry: the Ugi, Passerini, Biginelli and Groebke MCRs have been used to access complex 18F-labelled structures via [18F]fluorobenzaldehyde prosthetic groups.4 We have labelled peptidic α-aminoacyl amide derivatives and cyclic γ-lactams with nitrogen-13 via the Ugi reaction.511C-labelled sulfonyl carbamates can be accessed by combining [11C]carbon monoxide with sulfonyl azides and alcohols, and the automated synthesis of 11C-labelled amino acids via the Strecker reaction has been developed.6,7
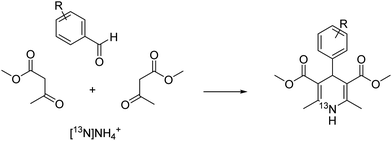
The Hantzsch dihydropyridine (DHP) synthesis is a multicomponent reaction between an aldehyde, two equivalents of a β-keto ester, and an amine (or ammonia). Clinically, 1,4-dihydropyridines including dimethyl 2,6-dimethyl-4-(2-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylate (nifedipine), are used as anti-hypertensive and anti-anginal drugs in cardiovascular disease by acting at voltage-gated calcium channels in peripheral blood vessels and the heart;8,9 these have been radiolabelled using a range of radionuclides including 3H, 14C, 11C, 125I, and 18F in order to probe modes of action, pharmacokinetics and metabolism of this class of compounds, and to study the pathophysiology of calcium channels in cardiovascular diseases.10–21
To expand the arsenal of 13N-radiolabelling tools, we explored the Hantzsch dihydropyridine synthesis as a strategy to access [13N]1,4-dihydropyridines ([13N]1,4-DHPs) using aqueous [13N]ammonia as a synthetic precursor (Fig. 1). These compounds could have clinical utility in imaging the function of calcium channels in vivo, providing mechanistic insight into cardiac abnormalities (e.g. hypertension, angina, congestive heart failure). The 1,4-DHP scaffold has also exhibited vasodilatory, bronchodilatory, anti-atherosclerotic, hepatoprotective, anti-tumour, anti-diabetic, antimalarial, anti-inflammatory and antibacterial properties.9,22 Here we report the first radio-synthesis of a library of 13N-labelled 1,4-DHPs using the Hantzsch dihydropyridine synthesis.
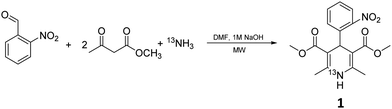
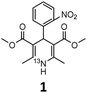
Prior to exploring the versatility of a Hantzsch MCR radiolabelling strategy, we established optimised radiosynthetic conditions for nifedipine (1, Table 1), a calcium channel blocker widely used to treat hypertension and angina. In the first instance, solutions of 2-nitrobenzaldehyde, methylacetoacetate, aqueous [13N]NH3 (100–150 MBq, cyclotron-produced via the 16O(p,α)13N nuclear reaction with 8 mL H2O/ethanol target) and NaOH were combined in DMF and heated in a microwave reactor at 100 °C for 10 min. The crude reaction solution was analysed using reverse-phase radio-HPLC. Encouragingly, the desired product [13N]nifedipine, 13N-1, was present in a radiochemical yield of 20%. This was verified by HPLC co-elution with non-radioactive nifedipine (the non-radioactive “standard” in this case).
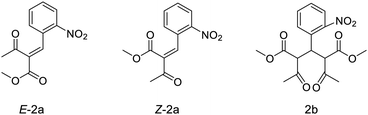
Reaction mixtures were also analysed by LC–HRMS to assess whether known intermediates of the Hantzsch MCR were present.23 LC–HRMS indicated the presence of methyl-2-(2-nitrobenzylidene)-3-oxobutanoate and dimethyl 2,4-diacetyl-3-(2-nitrophenyl)pentanedioate intermediates (Fig. 2). To probe the reaction pathway further, the reagents 2-nitrobenzaldehyde (5 mmol) and methylacetoacetate (10 mmol) were combined in solution (DMF) and allowed to react at 4 °C. Under these conditions, the rate of reaction of these reagents was sufficiently slow to observe the predicted intermediates formed prior to reaction with ammonia: stereoisomers methyl (E)-2-(2-nitrobenzylidene)-3-oxobutanoate (E-2a, Fig. 2) and methyl (Z)-2-(2-nitrobenzylidene)-3-oxobutanoate (Z-2a, Fig. 2), and dimethyl 2,4-diacetyl-3-(2-nitrophenyl)pentanedioate (2b, Fig. 2). Samples of the mixture were analysed periodically using LC–HRMS over the course of five weeks. During this time, signals corresponding to E-2a, Z-2a and 2b increased (ESI,† Fig. S1). The intermediate species E-2a and Z-2a were isolated using semi-preparative HPLC and their identity confirmed by 1H and 13C NMR. With the presence of the intermediates now confirmed in solution, the radiosynthesis of 13N-1 was repeated by combining a “precursor” solution, which contained intermediates E-2a, Z-2a and 2b, with [13N]NH3 and NaOH followed by microwave heating. Radio-HPLC analysis showed again the presence of product 13N-1 but now with a significantly improved RCY of 60%. Formation of these intermediates E-2a, Z-2a and 2b is therefore rate-limiting in the synthesis of 13N-1. Further optimisation of reaction conditions was carried out on the radiosynthesis of 13N-1 (Table 1). The reaction was observed to be temperature-dependant: an increase in reaction temperature improved yields, achieving a maximum 80% RCY at 100 °C. Heating at temperatures above 100 °C significantly reduced yields to 60% and 50% at 120 °C and 150 °C respectively. Of the short reaction times tested (8, 5 and 3 min to be compatible with the half-life of 13N), 5 minutes produced the highest RCY across all temperatures tested. The reaction was observed to be entirely dependent on the presence of sodium hydroxide: its absence resulted in a RCY of 0%. The Hantzsch synthesis typically proceeds under slightly basic conditions, usually provided inherently by the presence of stoichiometric amounts of primary amine or ammonia reagent. Here, with only sub-nanomolar quantities of no-carrier-added [13N]ammonia present, supplementing with additional base proved essential for the reaction to proceed, and importantly, under no-carrier-added conditions.
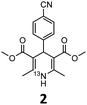
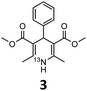
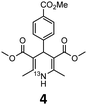
These optimised conditions (100 °C, 5 min) were applied to the synthesis of a library of seven [13N]1,4-DHPs containing varying aromatic substituents. In all cases, reaction mixtures containing the respective benzaldehyde derivative and β-keto ester were prepared in DMF, allowed to react, and the presence of the respective E/Z-enone and diketone intermediates confirmed via LC–HRMS, prior to radiolabelling. Subsequent addition of [13N]NH3 (100 μL, 100–150 MBq) and sodium hydroxide to these mixtures under the optimised reaction conditions resulted in insertion of 13N to furnish the dihydropyridine ring, forming the final 13N-labelled 1,4-DHP products 1–7. All seven 1,4-DHPs were successfully radiolabelled with nitrogen-13 using this adapted Hantzsch synthesis, each confirmed by co-elution with non-radioactive standards, that were either commercially available or prepared using Hantzsch methodology and characterised by 1H-NMR, 13C-NMR and LC–HRMS (ESI). Excellent radiochemical yields of 1–7 were achieved in the range of 62–95% (Table 2). The labelling of [13N]nifedipine (1) exhibited a RCY of 80%.
| 1,4-DHP | t R (min) | t R ref (min) | Opt. RCYc (%) | Scale-up RCYd (%) |
|---|---|---|---|---|
| a Retention time of 13N-labelled 1,4-DHP on C18 analytical HPLC column. b Retention time of 1,4-DHP reference standard on C18 analytical HPLC column. c Optimum radiochemical yield (before scale-up), decay-corrected. d Scaled-up radiochemical yield, decay-corrected. | ||||
|
7.85 | 7.68 | 80 | 50 |
|
7.98 | 7.45 | 95 | 50 |
|
7.90 | 7.73 | 90 | 40 |
|
7.83 | 7.55 | 95 | 40 |
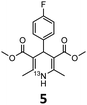
|
8.22 | 8.03 | 91 | 50 |
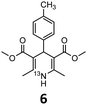
|
8.50 | 8.22 | 73 | 40 |
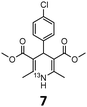
|
8.82 | 8.63 | 62 | 37 |
To achieve sufficient quantities of tracer suitable for preclinical PET imaging, the radiosynthesis required further modification. However, simply increasing the aqueous volume of [13N]NH3 added to the reaction severely reduced the RCY of the final product. To overcome this, [13N]NH4+ was first concentrated further into a smaller volume by solid-phase extraction into a higher concentration of aqueous sodium chloride (1 M).
A modified radiosynthesis was tested: addition of 250 μL aqueous [13N]NH3 (∼850 MBq) to the solution of reaction intermediates (200 μL) under the revised reaction conditions produced 13N-1 in a reduced RCY of 50%. These conditions were subsequently applied to all reactions to produce 13N-labelled products 1–7 with lower RCY (37–50%) (Table 2), but with higher overall amounts of activity, sufficient for preclinical PET imaging. Reversed-phase C18 HPLC purification provided 1–7 in 100% radiochemical purity (ESI,† Fig. S3), and for 13N-1, a molar activity of 174 MBq mmol−1 in the final formulation. This radiosynthesis, starting with 850 MBq, took 18 min from start of synthesis to final formulation. However, this “manual” synthesis could be readily adapted to an automated system, enabling use of larger amounts of activity, and shorter overall synthesis and purification times. This would provide [13N]nifedipine in higher activities, compatible with clinical use.
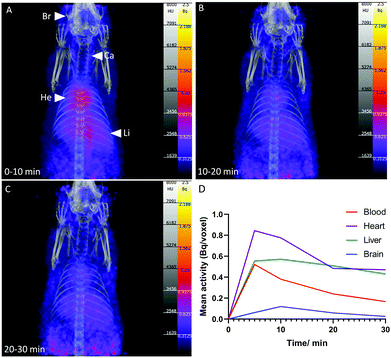
To demonstrate the feasibility of [13N]1,4-DHPs for PET imaging, [13N]nifedipine (1, 4 MBq, 800 μL in ethanol/aqueous saline solution) was administered intravenously to a healthy female Wistar rat, and PET/CT acquired over 1 h post-injection (Fig. 3). Accumulation was observed in the blood pool, heart, lung, liver and brain. At 10 min post-injection, radioactivity concentration in the heart was twice that observed in the blood pool, indicating accumulation and retention of [13N]nifedipine in myocardial tissue, consistent with the known specific uptake of nifedipine by calcium channels abundant in myocardial tissue.24 Uptake in heart, lung and brain also correlated with the known distribution of calcium channels in these organs.24–26 Accumulation in the liver was consistent with the known hepatic metabolic pathway of nifedipine in vivo: conversion to a nitropyridine analogue via oxidative cytochrome P450 3A isozymes.27,28 This is followed by further breakdown into polar metabolites most likely excreted via the kidneys (not in the field of view).29 Broadly, the biodistribution of [13N]nifedipine appeared comparable to the behaviour of an 18F-labelled nifedipine analogue (in which an [18F]CH2F group is incorporated in place of one of the CH3 groups on the pyridyl ring), with both tracers exhibiting uptake in heart, lung, liver and brain.30 Bone uptake was not observed with [13N]nifedipine, in contrast with the 18F-labelled analogue which showed activity in the skeleton increasing over the course of the imaging procedure, indicative of de-fluorination and release of bone-seeking [18F]fluoride. This highlights the advantage of using nitrogen-13 to radiolabel drugs where it is essential to maintain the authentic chemical structure to understand its precise behaviour in vivo – even small changes in molecular structure can have large and confounding effects on drug biodistribution and metabolism.
We have shown that the Hantzsch multi-component reaction is suitably versatile for 13N-labelling: various substituents can be incorporated on the benzaldehyde ring enabling the rapid development of seven [13N]1,4-DHP derivatives from readily available reagents. In these experiments it was convenient to combine the reaction precursors (aldehyde and β-keto ester in DMF) to pre-form enone and diketone intermediates prior to radiolabelling as this led to improvements in radiochemical yield. However, the preparation and isolation of enone and/or diketone intermediates (Fig. 2) could give access to even higher-yielding robust and routine radiochemical syntheses.
The development of new radiosynthetic methods for labelling with biologically and pharmaceutically significant radionuclides like nitrogen-13 and carbon-11 offers opportunities for imaging the whole-body pharmacokinetics of new and existing drugs using authentic radiolabelled biogenic molecules, to inform their early clinical development, which most other radionuclides do not offer. The Hantzsch reaction allows the rapid, no-carrier-added radiosynthesis of 1,4-DHPs from aqueous cyclotron-produced [13N]NH3, further expanding the arsenal of new 13N-labelling methods.
JEB and ADG jointly contributed to the study conception; JEB designed and executed the study; MTM contributed LC-HRMS and NMR acquisition and analysis; FAIA contributed imaging data analysis; JEB wrote the original draft of the manuscript; all authors contributed to manuscript review and editing, and approved the final submission.
The authors would like to thank Dr JB Torres and Mr S Clarke for their assistance with preclinical imaging. This research was supported by the PET Centre at St Thomas’ Hospital and the Department of Health via the National Institute for Health Research (NIHR) comprehensive Biomedical Research Centre award to Guy's & St Thomas’ NHS Foundation Trust in partnership with King's College London and King's College Hospital NHS Foundation Trust; a Cancer Research UK Career Establishment Award [C63178/A24959]; the Wellcome Trust funded ‘Multi-User radioanalytical facility for molecular imaging and radionuclide therapy research’ [212885/Z/18/Z]; Wellcome/EPSRC Centre for Medical Engineering at King's College London [WT 203148/Z/16/Z]; the EPSRC programme for next generation molecular imaging and therapy with radionuclides [EP/S019901/1]. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the DoH.
Conflicts of interest
There are no conflicts of interest to declare.References
- R. Klein, E. Celiker-Guler, B. H. Rotstein and R. A. deKemp, Semin. Nucl. Med., 2020, 50, 208–218 CrossRef PubMed.
- S. R. Cherry, T. Jones, J. S. Karp, J. Qi, W. W. Moses and R. D. Badawi, J. Nucl. Med., 2018, 59, 3–12 CrossRef CAS PubMed.
- A. Domling, W. Wang and K. Wang, Chem. Rev., 2012, 112, 3083–3135 CrossRef CAS PubMed.
- L. Li, M. N. Hopkinson, R. L. Yona, R. Bejot, A. D. Gee and V. Gouverneur, Chem. Sci., 2011, 2, 123–131 RSC.
- J. E. Blower, S. F. Cousin and A. D. Gee, EJNMMI Radiopharm. Chem., 2017, 2, 16 CrossRef PubMed.
- M. Y. Stevens, S. Y. Chow, S. Estrada, J. Eriksson, V. Asplund, A. Orlova, B. Mitran, G. Antoni, M. Larhed, O. Aberg and L. R. Odell, ChemistryOpen, 2016, 5, 566–573 CrossRef CAS PubMed.
- J. Xing, A. F. Brooks, D. Fink, H. Zhang, M. R. Piert, P. Scott and X. Shao, Synlett, 2017, 371–375 CAS.
- W. J. Elliott and C. V. S. Ram, J. Clin. Hypertens., 2011, 13, 687–689 CrossRef CAS PubMed.
- S. A. Khedkar and P. B. Auti, Mini-Rev. Med. Chem., 2014, 14, 282–290 CrossRef CAS PubMed.
- M. Holck, S. Thorens and G. Haeusler, Eur. J. Pharmacol., 1982, 85, 305–315 CrossRef CAS.
- U. Pleiss and P. Schmitt, J. Labelled Compd. Radiopharm., 1991, 29, 4 CrossRef.
- B. Duhm, W. Maul, H. Medenwald, K. Patzschke and L. A. Wegner, Arzneimittelforschung, 1972, 22, 42–53 CAS.
- S. S. Walkenstein, A. P. Intoccia, T. L. Flanagan, B. Hwang, D. Flint, J. Weinstock, A. J. Villani, D. Blackburn and H. Green, J. Pharm. Sci., 1973, 62, 580–584 CrossRef CAS PubMed.
- A. A. Wilson, R. F. Dannals, H. T. Ravert, H. D. Burns, S. Z. Lever and H. N. Wagner, J. Labelled Compd. Radiopharm., 1989, 27, 598 Search PubMed.
- M. Holschbach, W. Roden and W. Hamkens, J. Labelled Compd. Radiopharm., 1991, 29, 431–442 CrossRef CAS.
- F. Dollé, F. Hinnen, H. Valette, C. Fuseau, R. Duval, J. L. Péglion and C. Crouzel, Bioorg. Med. Chem., 1997, 5, 749–764 CrossRef.
- B. H. Rotstein, S. H. Liang, V. V. Belov, E. Livni, D. B. Levine, A. A. Bonab, M. I. Papisov, R. H. Perlis and N. Vasdev, Molecules, 2015, 20, 9550–9559 CrossRef CAS PubMed.
- D. R. Ferry and H. Glossmann, Naunyn-Schmiedeberg's Arch. Pharmacol., 1984, 325, 186–189 CrossRef CAS PubMed.
- N. M. Soldatov, Bioorg. Chem., 1989, 17, 141–158 CrossRef CAS.
- H. Sadeghpour, A. R. Jalilian, A. Shafiee, M. Akhlaghi, R. Miri and M. Mirzaei, Radiochim. Acta, 2008, 96, 849–853 CrossRef CAS.
- U. Pleiss, J. Labelled Compd. Radiopharm., 2007, 50, 13 CrossRef.
- N. Edraki, A. R. Mehdipour, M. Khoshneviszadeh and R. Miri, Drug Discovery Today, 2009, 14, 1058–1066 CrossRef CAS PubMed.
- J. Clayden, N. Greeves and S. Warren, Organic Chemistry, Oxford University Press, Oxford UK, 2 edn, 2001, vol. 30, pp. 763–764 Search PubMed.
- R. Ferrari, Eur. Heart J., 1997, 18, A56–A70 CrossRef CAS PubMed.
- I. De Proost, I. Brouns, I. Pintelon, J. Timmermans and D. Adriaensen, Histochem. Cell Biol., 2007, 128, 301–316 CrossRef PubMed.
- D. Lipscombe, T. D. Helton and W. Xu, J. Neurophysiol., 2004, 92, 2633–2641 CrossRef CAS PubMed.
- J. S. Grundy, L. A. Eliot and R. T. Foster, Biopharm. Drug Dispos., 1997, 18, 509–522 CrossRef CAS PubMed.
- K. D. Raemsch and J. Sommer, Hypertension, 1983, 5, II18–II24 CAS.
- J. Dokladalova, J. A. Tykal, S. J. Coco, P. E. Durkee, G. T. Quercia and J. J. Korst, J. Chromatogr., 1982, 231, 451–458 CrossRef CAS PubMed.
- H. Sadeghpour, A. R. Jalilian, M. Akhlaghi, A. Shafiee, M. Mirzaii, R. Miri and F. Saddadi, Nukleonika, 2008, 53, 4 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1cc00495f |
| This journal is © The Royal Society of Chemistry 2021 |