Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Redox switching of an artificial transmembrane signal transduction system†
Lucia
Trevisan
 ,
Istvan
Kocsis
,
Istvan
Kocsis
 and
Christopher A.
Hunter
and
Christopher A.
Hunter
 *
*
Department of Chemistry, University of Cambridge, , Lensfield Road, Cambridge, CB2 1EW, UK. E-mail: herchelsmith.orgchem@ch.cam.ac.uk
First published on 22nd February 2021
Abstract
Transmission of chemical signals across lipid bilayer membranes can be achieved using membrane-anchored molecules, where molecular motion across the bilayer is controlled by switching the polarity of two different head groups. An external redox signal delivered by ascorbic acid was used to trigger membrane translocation in a synthetic transduction system.
Transmembrane signal transduction is a fundamentally important process in biology and forms the basis for cell–cell communication, sensory perception and information processing in the brain.1 Inspired by the mechanisms used by biological systems to achieve signal transduction across lipid bilayers,2,3 a range of synthetic systems that send chemical signals across vesicle membranes have been developed. The first examples involved transmembrane transport of cations by crown ethers and cryptands.4 Artificial channels based on molecular components that self-assemble to form tubular structures in lipid bilayers have also been described.5–8 However, synthetic systems that generate a chemical signal on the inside of a vesicle without physical transport are rare.9,10 We recently introduced a mechanism for artificial transmembrane signal transduction based on membrane translocation. A recognition event on the outside of a vesicle membrane releases a membrane-anchored transducer, which translocates to the internal compartment, where it generates an output signal by catalysing the hydrolysis of a substrate.11 We have shown that pH,11 metal–ligand binding12 and protein–ligand binding13 can be used as the input recognition event. Here we expand the range of input signals to redox activation of signal transduction.
We recently described a synthetic transmembrane signal transduction system that responds to metal–ligand binding.12 Addition of a ligand to the external solution of vesicles containing the metal complex of a synthetic transducer led to hydrolysis of an ester substrate on the inside the vesicles. Here we show that it is possible to activate a similar transmembrane signal transduction system using redox chemistry as the external input signal. The approach is illustrated in Fig. 1. Transducer 1 has three components: the phenanthroline recognition unit is a switchable head group, which is membrane permeable, but prefers to sit in an aqueous environment when bound to copper(II); the pyridine-oxime head group is a membrane permeable pro-catalyst, which is activated by binding cadmium(II); the steroid core is a membrane anchor, which is shorter than the thickness of the bilayer, so the location of the transducer in the membrane can be switched by varying the polarity of the two head groups. The values of log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K at pH 7 for the copper(II)·phenanthroline and cadmium(II)·2,6-diacetylpyridine dioxime complexes are 9.014 and 4.115 respectively. Thus formation of the copper(II) complex of 1 should maintain the system in an OFF state, with the recognition head group in the aqueous phase and the pro-catalytic head group inside the membrane. Reduction of copper(II) to copper(I) should reduce the affinity for the phenanthroline head group sufficiently to allow the transducer to dissociate and translocate across the bilayer.16 The pyridine-oxime head group can then pick up cadium(II) on the inside of the vesicles, and this complex catalyses the hydrolysis of ester substrate 2 to generate a fluorescent output signal 3.17 The net result is ester hydrolysis on the inside of the vesicles in response to a reduction event on the outside of the vesicles. The experiments below describe the use of sodium ascorbate as the external input signal used to promote redox-controlled transmembrane signalling.
K at pH 7 for the copper(II)·phenanthroline and cadmium(II)·2,6-diacetylpyridine dioxime complexes are 9.014 and 4.115 respectively. Thus formation of the copper(II) complex of 1 should maintain the system in an OFF state, with the recognition head group in the aqueous phase and the pro-catalytic head group inside the membrane. Reduction of copper(II) to copper(I) should reduce the affinity for the phenanthroline head group sufficiently to allow the transducer to dissociate and translocate across the bilayer.16 The pyridine-oxime head group can then pick up cadium(II) on the inside of the vesicles, and this complex catalyses the hydrolysis of ester substrate 2 to generate a fluorescent output signal 3.17 The net result is ester hydrolysis on the inside of the vesicles in response to a reduction event on the outside of the vesicles. The experiments below describe the use of sodium ascorbate as the external input signal used to promote redox-controlled transmembrane signalling.
Transducer 1 was synthesised as described previously.11 DOPC vesicles containing CdSO4 (250 μM) and the ester substrate 2 (250 μM) were prepared by extrusion followed by size exclusion chromatography. The copper(II) complex of the transducer, 1·Cu2+, was added to the vesicles, followed by various amounts of sodium ascorbate after a 15 min interval. Fig. 2 shows the time course of the fluorescence emission intensity due to 3, which is formed on hydrolysis of substrate 2. In the absence of sodium ascorbate, there is a small increase in fluorescence emission intensity with time, due to slow solvolysis of the substrate.18 Addition of the copper(II) complex of the transducer at time point (a) does not affect the rate of this reaction, confirming that the system is in the OFF state. When sodium ascorbate was added at time point (b), the fluorescence intensity increased significantly, and the rate of increase is directly related to the amount of sodium ascorbate added. Small amounts of sodium ascorbate (0.1 mM, orange data) have little effect on the transducer, because molecular oxygen dissolved in the water is reduced before copper(II).19 The solubility of oxygen in water at room temperature is 0.3 mM,20 so concentrations of sodium ascorbate higher than this value (e.g. 0.5 mM, green data) are required to switch the transducer into the ON state by reducing copper(II) to copper(I). These results suggest that reduction of copper(II) to copper(I) frees the transducer to translocate across the lipid bilayer to the inner leaflet, where the pyridine oxime head group can pick up cadmium(II) and catalyse hydrolysis of the ester substrate.
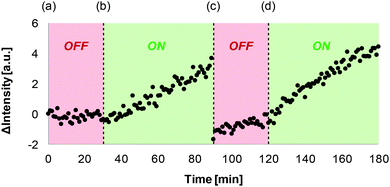
Fig. 3 shows that this signal transduction system can be reversibly switched between ON and OFF states by successive additions of sodium ascorbate and copper(II) chloride to the vesicle suspension. At time point (a), the OFF state was generated by adding the 1·Cu2+ complex to vesicles containing CdSO4 and the ester substrate 2. At time point (b), sodium ascorbate was added to reduce Cu(II) to Cu(I), generating the ON state, and an increase in fluorescence emission intensity was observed. Addition of CuCl2 at time point (c) switched the system back to the OFF state, and addition of sodium ascorbate at time point (d) turned it back ON again. There is a drop in fluorescence emission intensity at time point (c), because addition of high concentrations of copper ions leads to some quenching.21
This work expands the range of different input signals that can be used to control transmembrane signal transduction in artificial systems. We have shown previously that signal transduction using 1 could be triggered by adding EDTA to remove copper(II).11 However under the conditions used in the experiments described in this paper, EDTA failed to trigger signal transduction, because it has to compete against a high background concentration of metal ions (see Fig. S4, ESI†). Redox signalling does not suffer from this limitation, providing a useful orthogonal alternative input signal and expanding the range of conditions in which these artificial systems operate. The essential role played by ascorbic acid (vitamin C) in Nature suggests that the development of this compound as a redox trigger could open the way to interface signalling in synthetic systems with biological processes.
We thank AstraZeneca for a PhD studentship for LT and EPSRC (EP/R005397/1) for funding.
Conflicts of interest
There are no conflicts to declare.Notes and references
- J. Singer, Science, 1992, 255, 1671–1677 CrossRef.
- M. I. Simon, M. P. Strathmann and N. Gautam, Science, 1991, 252, 802–808 CrossRef CAS.
- J. Schlessinger, Cell, 2000, 103, 211–225 CrossRef CAS.
- J.-M. Lehn, Science, 1985, 227, 849–856 CrossRef CAS.
- J. Montenegro, M. R. Ghadiri and J. R. Granja, Acc. Chem. Res., 2013, 46, 1–21 CrossRef.
- R. García-Fandiño, M. Amorín, L. Castedo and J. R. Granja, Chem. Sci., 2012, 3, 3280–3285 RSC.
- E. Licsandru, I. Kocsis, Y. Shen, S. Murail, Y. Legrand, A. van der Lee, D. Tsai, M. Baaden, M. Kumar and M. Barboiu, J. Am. Chem. Soc., 2016, 138, 5403–5409 CrossRef CAS.
- D. P. August, S. Borsley, S. L. Cockroft, F. della Sala, D. A. Leigh and S. J. Webb, J. Am. Chem. Soc., 2020, 142, 18859–18865 CrossRef CAS.
- P. Barton, C. A. Hunter, T. J. Potter, S. J. Webb and N. H. Williams, Angew. Chem., Int. Ed., 2002, 41, 3878–3881 CrossRef CAS.
- K. Bernitzki, M. Maue and T. Schrader, Chem. – Eur. J., 2012, 18, 13412–13417 CrossRef CAS.
- M. J. Langton, F. Keymeulen, M. Ciaccia, N. H. Williams and C. A. Hunter, Nat. Chem., 2017, 9, 426–430 CrossRef CAS.
- M. J. Langton, N. H. Williams and C. A. Hunter, J. Am. Chem. Soc., 2017, 139, 6461–6466 CrossRef CAS.
- Y. Ding, N. H. Williams and C. A. Hunter, J. Am. Chem. Soc., 2019, 141, 17847–17853 CrossRef CAS.
- H. Irving and D. H. Mellor, J. Chem. Soc., 1962, 0, 5222–5237 RSC.
- A. K. Yatsimirsky, P. Gómez-Tagle, S. Escalante-Tovar and L. Ruiz-Ramírez, Inorg. Chim. Acta, 1998, 273, 167–174 CrossRef CAS.
- R. J. P. Williams, J. Chem. Soc., 1955, 137–145 RSC.
- R. Biswas, N. Maillard, J. Kofoed and J. Reymond, Chem. Commun., 2010, 46, 8746–8748 RSC.
- O. S. Wolfbeis and E. Koller, Anal. Biochem., 1983, 129, 365–370 CrossRef CAS.
- E. V. Shtamm, A. P. Purmal and Y. I. Skurlatov, Int. J. Chem. Kinet., 1979, 11, 461–494 CrossRef CAS.
- R. Battino, T. R. Rettich and T. Tominaga, J. Phys. Chem. Ref. Data, 1983, 12, 163–178 CrossRef CAS.
- S. Biswas, S. C. Bhattacharya and S. P. Moulik, J. Colloid Interface Sci., 2004, 271, 157–162 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: General procedures for vesicle preparation and fluorescence experiments, additional control experiments. See DOI: 10.1039/d0cc08322d |
| This journal is © The Royal Society of Chemistry 2021 |