 Open Access Article
Open Access ArticleKinetics of the photocatalysed reduction of oxygen by CdS probed using photoinduced absorption spectroscopy (PIAS)†
Aaron
McNeill
,
Christopher
O’Rourke
and
Andrew
Mills
 *
*
Queen's University Belfast, School of Chemistry and Chemical Engineering, David Keir Building, Stranmillis Road, Belfast, BT9 5AG, UK. E-mail: andrew.mills@qub.ac.uk
First published on 26th January 2021
Abstract
Photoinduced absorption spectroscopy, PIAS, is used to determine the order of reaction and so identify the rate determining step in the reduction of O2 by a sacrificial electron donor, photocatalysed by a nanoparticular film of CdS. This is the first report on the use of PIAS to probe the kinetics of photocatalysis exhibited by a non-oxide semiconductor.
Cadmium sulfide (CdS) is one of the most commonly employed, visible-light absorbing semiconductor photocatalysts (Ebg = 2.4 eV1) due to the highly reducing nature of its photogenerated conductance band electrons (ECB(e−) = −0.93 V vs. NHE at pH 72) and the highly oxidising nature of its photogenerated valence band holes (ECB(h+) = 1.47 V vs. NHE at pH 72). Unfortunately, CdS, like most visible-light absorbing photocatalysts, undergoes photoanodic corrosion, i.e.3
| 2h+ + CdS → Cd2+ + S↓ | (1) |
| e− + O2 → O2− | (2) |
| h+ + SED → SED+ | (3) |
 | (4) |
Recently, photoinduced absorption spectroscopy, PIAS, coupled with transient photocurrent (TC) measurements i.e., PIAS/TC, has been used to probe the kinetics of water oxidation exhibited by different semiconductor oxide photoanodes, such as Fe2O3, TiO2 and BiVO4.17–19 In these PIAS/TC studies the optical absorption, ΔAbsss, exhibited by photogenerated holes accumulated at the surface of the semiconductor photoanode and the photocurrent, Jss, are monitored simultaneously under ultra-bandgap, steady-state irradiation conditions and a large anodic bias. The latter ensures that the steady-state level of photogenerated electrons is so low that the reaction of the surface-accumulated photogenerated holes can be considered to be due to the oxidation of water exclusively. In all the PIAS/TC studies reported to date the values of ΔAbsss and Jss, are measured as a function of incident irradiance and the order of reaction of the photogenerated holes taken as the value of the gradient of the plot log(Jss) vs. log(ΔAbsss).
PIAS has never been used to probe a photocatalysed reduction reaction by an n-type semiconductor, nor probe a non-oxide photocatalyst. However, interestingly, it has been shown that nanoparticulate CdS exhibits a blue shift in its absorption edge upon electronic excitation. The effect increases with incident irradiance and decreasing particle size. This shift in absorption edge is usually interpreted as a Burstein shift i.e., an increase in the optical band gap due to the population of the conduction band with photogenerated electrons.20–24 Liu and Bard also found the same shift can be produced by an excess of holes.25
In studies of this effect21,23 using CdS colloids, the kinetics of decay of the resulting flash-induced change in absorption are complex and short-lived, i.e. <12 ns, but this lifetime is increased markedly when a SED is present. Thus, Albery et al.,24 in a microsecond flash photolysis study of a CdS colloid (17 nm particles) in the presence of a SED (0.02 M Na2S), reported a half-life of ca. 50 ms in the transient bleaching due to a blue shift in its absorption spectrum! These workers also found that, in the additional presence of methyl viologen, which acts as an electron acceptor, there exists a direct correlation between the kinetics of decay in the transient absorbance, ΔAbs, and those for the concomitant photogeneration of the reduced methyl radical,24 which suggests that ΔAbs is directly related to the concentration of the surface accumulated (i.e. trapped) conductance band electrons, [e−], photogenerated by the flash. This feature is exploited in this PIAS-based study of reaction (4), photocatalysed by CdS.
The CdS films on microscope glass used in this work were made from a CdS nanopowder (Sigma Aldrich; average particle size ca. 50 nm) using a modified version of a method reported by Ito et al. for making screen-printed TiO2 films26 details of which are given in S1 in the ESI,† file. The thickness of the dried film was ca. 1.1 μm, yellow and slightly opalescent in appearance, as illustrated by the photograph of the CdS film in Fig. 1(a). The absorption spectrum of the film, illustrated in Fig. 1(b), revealed an absorbance onset at ca. 534 nm, i.e. 2.3 eV, which corresponds closely with the known bandgap of 2.4 eV for CdS.1
In almost all previously reported PIAS studies, the semiconductor photocatalysis films were photoanodes and the steady-state photocurrent taken as a measure of the rate of the photocatalysed oxidation reaction. This approach allows the order of the photoelectrochemical oxidation to be determined, provided the faradaic efficiency is unity, which is not always the case.27 In contrast, in this work a quite different approach was employed in the application of PIAS; one in which, under steady-state irradiation conditions, the measured value of ΔAbsss due to the surface-accumulated photogenerated electrons, [e−]ss, was related to the actual, independently measured rate of the photocatalytic process, and not a photocurrent. In addition, the values of ΔAbsss and rates were not varied by altering the incident irradiance, ρ, as is the usual practice, but rather by changing the reactant concentration. In this work the photocatalytic reaction probed in this way was the reduction of oxygen by an SED, photocatalysed by CdS, i.e.Reaction (4), where SED = 0.1 M solution of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio sodium ascorbate and ascorbic acid (NaA/AA). The latter was selected as the SED, since a previous study reported it to be highly effective in preventing the photocorrosion of CdS in an air-saturated solution.15 Details of the PIAS system used in this work are given in S2 in the ESI,† which was used to determine the variation in the steady-state irradiation induced change in absorbance, ΔAbsss exhibited by a CdS nanoparticulate film in 20 mL of an Ar-purged, 0.1 M NaA/AA aqueous solution, with ρ (365 nm) = 29.3 mW cm−2, as a function of monitoring wavelength, λm. The results of this work are illustrated in Fig. 2 and reveal a very similar spectral profile to that reported by Albery et al. for a CdS colloid,24 although with an absorption maximum at 505, rather than 485 nm, and with a smaller shift in absorption spectrum (ca. 0.8 instead of 3 nm). The spectral differences are probably due to the differences in CdS particle size, i.e. 17 cf. 50 nm.
1 molar ratio sodium ascorbate and ascorbic acid (NaA/AA). The latter was selected as the SED, since a previous study reported it to be highly effective in preventing the photocorrosion of CdS in an air-saturated solution.15 Details of the PIAS system used in this work are given in S2 in the ESI,† which was used to determine the variation in the steady-state irradiation induced change in absorbance, ΔAbsss exhibited by a CdS nanoparticulate film in 20 mL of an Ar-purged, 0.1 M NaA/AA aqueous solution, with ρ (365 nm) = 29.3 mW cm−2, as a function of monitoring wavelength, λm. The results of this work are illustrated in Fig. 2 and reveal a very similar spectral profile to that reported by Albery et al. for a CdS colloid,24 although with an absorption maximum at 505, rather than 485 nm, and with a smaller shift in absorption spectrum (ca. 0.8 instead of 3 nm). The spectral differences are probably due to the differences in CdS particle size, i.e. 17 cf. 50 nm.
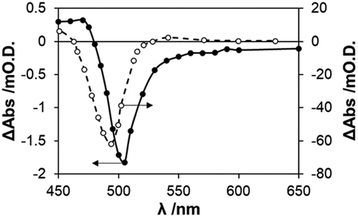 | ||
| Fig. 2 Transient bleaching in the difference absorption spectrum exhibited by CdS as (a) a colloid, no SED, 10 ms after flash24 (broken line) and (b) the CdS nanoparticle film on glass, measured using PIAS, with a NaA/AA SED (0.1 M) and ρ = 29.3 mW cm−2, 365 nm, solid line. Both solutions were O2 free. | ||
In a subsequent PIAS study of the CdS film carried out using λm = 505 nm in aqueous solution with 0.1 M NaA/AA, and ρ (420 nm) = 15.0 mW cm−2, the change in absorbance, i.e. ΔAbs, was recorded before during and after steady state irradiation in the presence of different saturation levels of dissolved O2 spanning the range 0 to 21%; the results of this work are illustrated in Fig. 3(a). The plot of the data generated from this work in the form of ΔAbsssvs. %O2, illustrated in Fig. 3(b), shows that the steady-state transient absorbance, ΔAbsss, due to photogenerated conductance band electrons, decreases in magnitude with increasing %O2. As illustrated by the solid line in Fig. 3(b), the variation in ΔAbsssvs. %O2 fits an equation with the following form,
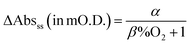 | (5) |
 | ||
| Fig. 3 (a) Plot of ΔAbs vs. time for a CdS film, recorded before during and after steady state irradiation ρ = 15.0 mW cm−2, 420 nm, with NaA/AA (0.1 M), and in the presence of dissolved O2 maintained at different saturation levels ranging from 0 to 21%; the %O2 saturation levels used were (from bottom to top): 0, 5, 10, 15 and 21%, respectively; (b) plot of ΔAbsssvs. %O2 constructed using data from (a), with a solid line of best fit based on eqn (5), with α and β equal to 2.9 and 0.035%O2−1, respectively. | ||
where α and β are fitting constants with best fit values of 2.9 and 0.035%O2−1, respectively. An explanation for this variation is provided by a kinetic analysis of the measured dark decays which are shown in greater detail in Fig. S3(a) in the ESI.† The value of ΔAbs(dark) at any time during the decay process is assumed to be a measure of the dark concentration of the surface-accumulated photogenerated conductance band electrons, [e−]. All of the ΔAbs(dark) vs. time decays exhibited first order kinetics, from which different first order rate constant, k1, values could be extracted. A plot of k1vs. %O2 illustrated in Fig. S3(b) in the ESI,† reveals a good straight line, which fits the following expression,
| k1 = kO2%O2 + k0 | (6) |
The value of k0, the first order rate constant for the decay of the photogenerated electrons in the absence of O2, is large, as indicated by the associated dark decay curve (red) illustrated in Fig. 3(a). This large value for k0, suggests that NaA/AA is not very effective hole scavenger in this system, so that either many of the photogenerated holes are trapped, e.g. as lattice bond S− radicals, or more likely, its oxidised form, dehydroascorbic acid, or intermediate, is able to efficiently back-react with surface accumulated photogenerated electrons. These results indicate that, despite the presence of an SED, electron–hole recombination is a dominant dark reaction, making k0 large, and reducing the reduction of O2 to the role of a relatively minor side reaction. Evidence that this is indeed the case for the CdS films comes from a simple study of the variation of ΔAbsss as a function of irradiance, the results for which are illustrated in Fig. 4 and reveal a direct dependence of ΔAbsss upon ρ0.5. The same dependence of ΔAbs upon ρ0.5 was found by Albery et al. in their flash photolysis study of a CdS colloid in the presence of 0.05 M cysteine as the SED and is taken there and here as indicative that electron–hole recombination dominates the fate of the photogenerated electron–hole pairs.24
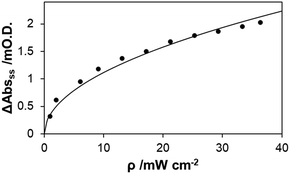 | ||
| Fig. 4 Plot of the measured variation in ΔAbsss for a CdS film as a function of incident irradiance, ρ, with all other conditions as in Fig. 2. The solid line of best fit has been calculated assuming ΔAbsss is ∝ ρ0.5. | ||
As illustrated by Fig. S3(b) in the ESI,† and eqn (6), the above study also shows that there is a direct dependence of k1 upon %O2, which indicates that, although reaction (4) is a minor process, the rate-determining step, rds, in the reduction of O2 by photogenerated electrons on the CdS film particles is first order with respect to ΔAbs (i.e. [e−]), which strongly suggests that it is reaction (2), the initial production of superoxide that is the rds. This finding and conclusion are consistent with those made by others studying the reduction of O2 by photogenerated conductance band electrons on TiO2.28
In the CdS film/SED system, given the above, it follows that under steady-state irradiation conditions the rate of photogeneration of surface accumulated conductance band electrons, which is proportional to ρ0.5, will be matched by the rate of their loss, so that,
| δρ0.5 = (kO2%O2 + k0)ΔAbsss(or[e−]ss) | (7) |
If reaction (2) is the rate determining step in the photocatalysed reduction of O2, then the rate of reduction of O2, r, will be equal to kO2%O2[e−]ss. If the latter term is combined with eqn (7) the following expression can be derived,
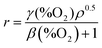 | (8) |
 | (9) |
In order to test the validity of eqn (8), derived from the PIAS study of the CdS film in 0.1 M NaA/AA, the variation of the rate of reduction of O2, r, was measured as a function of %O2, using a set up that was very similar to that used in the PIAS study, with the exception that the system was sealed and the dissolved level of O2 was measured using an O2xyDot® (OxySense31). Further details of the steady-state irradiation system are given in S4 in the ESI.† The results of this work are illustrated in Fig. 5 with a solid line of best fit based on eqn (8) with γ.%O2ρ0.5 = 1.42%O2 min−1 and β = 0.038%O2−1. Reassuringly, the latter value (β = 0.038%O2−1) is near identical to the value of β = 0.035%O2−1 reported earlier, see Fig. 3(b), and eqn (5), despite the fact that they were derived by two very different methods, namely from ΔAbsss (PIAS) and initial rate (r) measurements. The strong similarity in the two values for β provides further support that the rate of reduction of O2 in reaction (4) by the CdS films used in this work, with the SED = 0.1 M NaA/AA, is first order with respect to the steady-state, concentration of surface-accumulated photogenerated electrons, [e−]ss, making it likely that reaction (2) is the rate determining step.
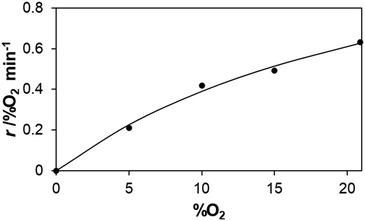 | ||
| Fig. 5 Measured initial rate of the reduction of O2 by 0.1 M NaA/AA, photocatalysed by a CdS film, irradiated with 420 nm LED (30 mW cm−2), as a function of the %O2 used to initially saturate the solution. The solid line fit to the data is based on eqn (8) with γ.%O2ρ0.5 = 1.42%O2 min−1 and β = 0.038%O2−1. | ||
In conclusion, PIAS, combined with steady-state reaction rate studies, can be used to probe the reduction of O2 by a SED, photocatalysed by a film comprised of CdS nanoparticles. This is the first report of the use of PIAS to study the kinetics of photocatalysis of a non-oxide semiconductor and the first use of PIAS to study a photocatalysed reduction reaction. This work suggests that PIAS can be used more widely to provide invaluable mechanistic information for many different photocatalyst materials, including non-oxides, in powder form.
Conflicts of interest
There are no conflicts to declare.Notes and references
- F. Boakye and D. Nusenu, Solid State Commun., 1997, 102, 323–326 CrossRef CAS.
- A. Mills, N. Wells and C. O'Rourke, J. Photochem. Photobiol., A, 2017, 338, 123–133 CrossRef CAS.
- D. Meissner, R. Memming, B. Kastening and D. Bahnemann, Chem. Phys. Lett., 1986, 127, 419–423 CrossRef CAS.
- G. Mattioli, F. Filippone and A. A. Bonapasta, J. Am. Chem. Soc., 2006, 128, 13772–13780 CrossRef CAS.
- F. Filippone, G. Mattioli and A. A. Bonapasta, Catal. Today, 2007, 129, 169–176 CrossRef CAS.
- E. T. Wahyuni and N. H. Aprilita, Photoreduction Processes over TiO2 Photocatalyst, 2018, 129–145 Search PubMed.
- M. Berr, A. Vaneski, A. S. Susha, J. Rodríguez-Fernández, M. Döblinger, F. Jäckel, A. L. Rogach and J. Feldmann, Appl. Phys. Lett., 2010, 97, 093108 CrossRef.
- D. W. Wakerley, M. F. Kuehnel, K. L. Orchard, K. H. Ly, T. E. Rosser and E. Reisner, Nat. Energy, 2017, 2, 17021 CrossRef CAS.
- T. Uekert, M. F. Kuehnel, D. W. Wakerley and E. Reisner, Energy Environ. Sci., 2018, 11, 2853–2857 RSC.
- Y. Shiraishi, M. Katayama, M. Hashimoto and T. Hirai, Chem. Commun., 2018, 54, 452–455 RSC.
- A. E. Raevskaya, A. L. Stroyuk and S. Y. Kuchmii, J. Nanopart. Res., 2004, 6, 149–158 CrossRef CAS.
- Y. Zhang, Y. Zhao, Z. Xu, H. Su, X. Bian, S. Zhang, X. Dong, L. Zeng, T. Zeng, M. Feng, L. Li and V. K. Sharma, Appl. Catal., B, 2020, 262, 118306 CrossRef CAS.
- A. Mills and A. Green, J. Photochem. Photobiol., A, 1991, 59, 199–208 CrossRef CAS.
- J. R. Darwent and G. Porter, J. Chem. Soc., Chem. Commun., 1981, 4, 145–146 RSC.
- A. McNeill and A. Mills, J. Phys. Energy, 2020, 2, 044003 CrossRef CAS.
- A. J. Nozik, Appl. Phys. Lett., 1977, 30, 567–569 CrossRef CAS.
- F. Le Formal, E. Pastor, S. D. Tilley, C. A. Mesa, S. R. Pendlebury, M. Grätzel and J. R. Durrant, J. Am. Chem. Soc., 2015, 137, 6629–6637 CrossRef CAS.
- Y. Ma, C. A. Mesa, E. Pastor, A. Kafizas, L. Francàs, F. Le Formal, S. R. Pendlebury and J. R. Durrant, ACS Energy Lett., 2016, 1, 618–623 CrossRef CAS.
- A. Kafizas, Y. Ma, E. Pastor, S. R. Pendlebury, C. Mesa, L. Francàs, F. Le Formal, N. Noor, M. Ling, C. Sotelo-Vazquez, C. J. Carmalt, I. P. Parkin and J. R. Durrant, ACS Catal., 2017, 7, 4896–4903 CrossRef CAS.
- E. Burstein, Phys. Rev., 1954, 93, 632–633 CrossRef CAS.
- P. V. Kamat, N. M. Dimitrijevic and A. J. Nozik, J. Phys. Chem., 1989, 93, 2873–2875 CrossRef CAS.
- C.-Y. Liu and A. J. Bard, J. Phys. Chem., 1989, 93, 3232–3237 CrossRef CAS.
- P. V. Kamat, T. W. Ebbesen, N. M. Dimitrijevic and A. J. Nozik, Chem. Phys. Lett., 1989, 157, 384–389 CrossRef CAS.
- W. J. Albery, G. T. Brown, J. R. Darwent and E. Saievar-Iranizad, J. Chem. Soc., Faraday Trans. 1, 1985, 81, 1999–2007 RSC.
- C.-Y. Liu and A. J. Bard, J. Phys. Chem., 1989, 93, 7749–7750 CrossRef CAS.
- S. Ito, P. Chen, P. Comte, M. K. Nazeeruddin, P. Liska, P. Péchy and M. Grätzel, Prog. Photovoltaics, 2007, 15, 603–612 CAS.
- C. A. Mesa, L. Francàs, K. R. Yang, P. Garrido-Barros, E. Pastor, Y. Ma, A. Kafizas, T. E. Rosser, M. T. Mayer, E. Reisner, M. Grätzel, V. S. Batista and J. R. Durrant, Nat. Chem., 2020, 12, 82–89 CrossRef CAS.
- H. H. Mohamed, R. Dillert and D. W. Bahnemann, J. Photochem. Photobiol., A, 2011, 217, 271–274 CrossRef CAS.
- A. Mills, C. O'Rourke and K. Moore, J. Photochem. Photobiol., A, 2015, 310, 66–105 CrossRef CAS.
- A. Mills and J. Wang, Z. Phys. Chem., 1999, 213, 49–58 CrossRef CAS.
- OxySense, https://oxysense.com, accessed December 2020.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0cc08208b |
| This journal is © The Royal Society of Chemistry 2021 |

