Base-enabled access to diastereoselective spirofuran oxindoles and γ-functionalized allenoates†
Abstract
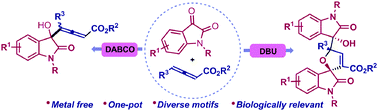
Base assisted divergent reactivity of isatins and allenoates has been achieved, which afforded diastereoselective spirofuran oxindoles and γ-functionalized allenoates. The DBU mediated Morita–Baylis–Hillman (MBH) reaction followed by the cascade annulation through the stabilized β-ammonium enolate intermediate led to the spiro-framework, wherein DABCO furnished the γ-functionalized allenoates. The protocol offers access to biologically relevant functionalized oxindole scaffolds with an excellent substrate scope under mild reaction conditions.
- This article is part of the themed collection: 2021 Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...