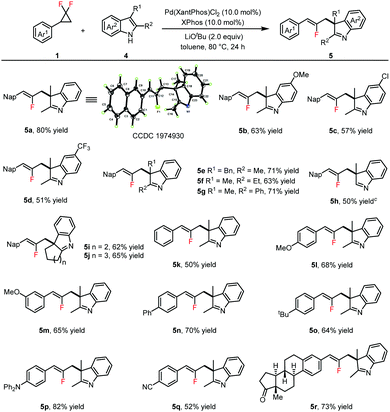
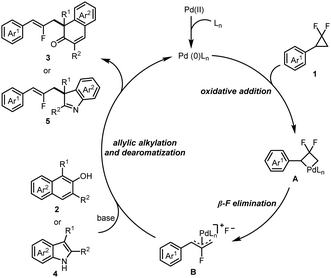
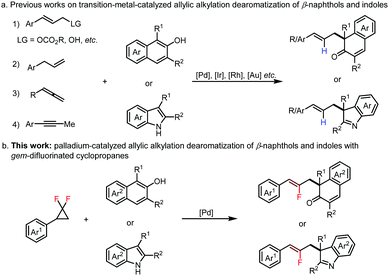
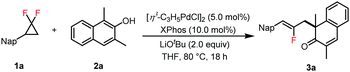Palladium-catalyzed allylic alkylation dearomatization of β-naphthols and indoles with gem-difluorinated cyclopropanes†
Zhiyuan
Fu‡
,
Jianping
Zhu‡
,
Songjin
Guo
* and
Aijun
Lin
 *
*
State Key Laboratory of Natural Medicines (SKLNM) and Department of Medicinal Chemistry, School of Pharmacy, China Pharmaceutical University, Nanjing 210009, P. R. China. E-mail: yd1029820332@163.com; ajlin@cpu.edu.cn
First published on 22nd December 2020
Abstract
A palladium-catalyzed allylic alkylation dearomatization of β-naphthols and indoles with gem-difluorinated cyclopropanes has been developed. This reaction provided an efficient route to access 2-fluoroallylic β-naphthalenones and indolenines bearing quaternary carbon centers in good yields with high Z-selectivity via C–C bond activation, C–F bond cleavage and the dearomatization process, benefiting from the wide substrate scope and good functional group tolerance. Moreover, 2-fluoroallylic furanoindoline and pyrroloindolines were achieved in good efficiency via cascade allylic alkylation, dearomatization and cyclization processes in the presence of Et3B.
Naphthols and indoles are abundant and readily available chemical feedstocks, which are widely used in organic synthesis chemistry.1 The dearomatization of β-naphthols and indoles shows great potential in rapidly accessing highly functionalized β-naphthalenones and indolenines bearing quaternary carbon centers,2 serving as pivotal scaffolds that are frequently found in biologically active natural products and pharmaceuticals.3 In the past decades, allylic esters and carbonates,4 allylic alcohols,5 allylbenzenes6 and allenes7 have been widely employed in transition-metal catalyzed allylic alkylation dearomatization of β-naphthols and indoles (Fig. 1a). Recently, our group described a redox-neutral palladium-catalyzed allylic alkylation dearomatization of β-naphthols and indoles with alkynes in high atom and step economy.8 Although impressive results were achieved in previous works, it is worth noting that the 2-position of the allyl framework in the products was short of functional groups. Thus, it is of great significance to explore novel allylating reagents, which could enable assembling some functional groups at the 2-position of the allyl fragment to expand the diversity and utility of β-naphthols and indoles.
 | ||
| Fig. 1 Strategies for transition-metal-catalyzed allylic alkylation dearomatization of β-naphthols or indoles. | ||
The incorporation of fluorine or fluorine-containing motifs into organic molecules brings about substantial improvement in bioactivity, and provides unique chemical and physical properties.9 Moreover, monofluoroalkenes have emerged as ideal peptide bond mimetics, which exhibit a similar steric and electronic profile to amides, and have been extensively applied in the fields of medicinal chemistry and drug-discovery.10 Recently, gem-difluorinated cyclopropanes as fluoro-alkenylating building blocks have attracted increasing attention in the construction of functional monofluoroalkenes.11 Herein, we describe a palladium-catalyzed allylic alkylation dearomatization of β-naphthols and indoles with gem-difluorinated cyclopropanes to the synthesis of β-naphthalenones and indolenines with 2-fluorinated allyl scaffolds in good efficiency for the first time (Fig. 1b).
We started our studies with gem-difluorinated cyclopropane 1a and 1,3-dimethyl-2-naphthol 2a as the model substrates. After a systematic survey of the reaction conditions (see the ESI† for details), the desired product 3a was obtained in 92% yield with 5 mol% [η3-C3H5PdCl]2 as the catalyst, XPhos as the ligand and LiOtBu as the base in THF at 80 °C under an Ar atmosphere for 18 h (Table 1, entry 1). Other catalysts, such as Pd(OAc)2, Pd(OTFA)2, Pd2(dba)3 and Pd(PPh3)4, performed this reaction in 80–86% yields (Table 1, entries 2–5). Other ligands, such as Cy3P, SPhos, XantPhos, tBu-XPhos and DavePhos, offered 3a in less efficiency (Table 1, entries 6–10). Toluene and mesitylene offered 3a in 87% and 90% yields, while CH3CN and 1,4-dioxane gave 3a in 33% and 74% yields, respectively (Table 1, entries 11–14). No reaction occurred when this transformation was conducted without the Pd catalyst or the ligand (Table 1, entry 15).
| Entry | Deviation of standard conditionsa | Yieldb/(%) |
|---|---|---|
| a Standard reaction conditions: 1a (0.2 mmol), 2a (0.3 mmol), [η3-C3H5PdCl]2 (5.0 mol%), XPhos (10.0 mol%), LiOtBu (2.0 equiv.), THF (2.0 mL), at 80 °C under an Ar atmosphere for 18 h, sealed tube. b Isolated yield. n.r. = no reaction. Nap = 2-naphthyl. | ||
| 1 | None | 92 |
| 2 | Pd(OAc)2 instead of [η3-C3H5PdCl]2 | 83 |
| 3 | Pd(OTFA)2 instead of [η3-C3H5PdCl]2 | 86 |
| 4 | Pd2(dba)3 instead of [η3-C3H5PdCl]2 | 85 |
| 5 | Pd(PPh3)4 instead of [η3-C3H5PdCl]2 | 80 |
| 6 | Cy3P instead of XPhos | 46 |
| 7 | SPhos instead of XPhos | 58 |
| 8 | XantPhos instead of XPhos | 37 |
| 9 | t Bu-XPhos instead of XPhos | 15 |
| 10 | DavePhos instead of XPhos | 40 |
| 11 | Toluene instead of THF | 87 |
| 12 | Mesitylene instead of THF | 90 |
| 13 | CH3CN instead of THF | 33 |
| 14 | 1,4-Dioxane instead of THF | 74 |
| 15 | Without [η3-C3H5PdCl]2 or XPhos | n.r. |
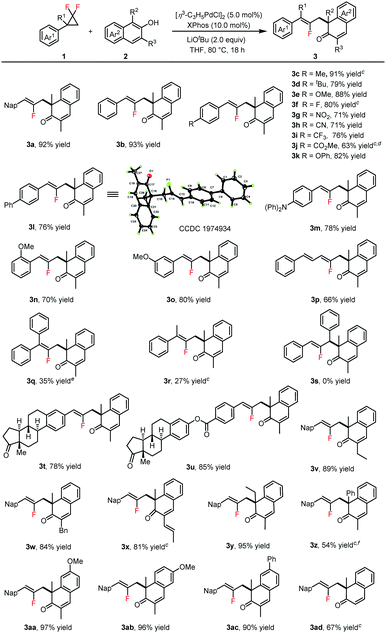
Having optimized the reaction conditions, we then focused on the substrate scope of this method (Table 2). Various gem-difluorinated cyclopropanes were all allowed to react with 2a well, and afforded the corresponding products 3a–3o in 63–93% yields. The structure of 3l was confirmed on the basis of a single-crystal X-ray crystallographic analysis (see the ESI,† for details). When 1p was employed as the substrate, the conjugated fluorodiene 3p was obtained in 66% yield. The 1,1-disubstituted gem-difluorinated cyclopropanes (1q and 1r) could be converted to products 3q and 3r in low yields (see the ESI,† for details), but 1,2-diphenyl substituted gem-difluorinated cyclopropane 1s could not offer the desired product 3s. To further demonstrate the potential utility of this reaction for the late-stage modification of natural products, the estrone derivatives (1t and 1u) were synthesized and tested, offering products 3t and 3u in good yields. Subsequently, the compatibility of β-naphthols 2 was examined, and all of them performed smoothly to deliver products 3v–3ad in good efficiency. Phenyl substituted β-naphthol gave 3z in 54% yield due to the increased steric bulk. Notably, 1-methylnaphthalen-2-ol converted to product 3ad in 67% yield with good regioselectivity.
| a Reaction conditions: 1 (0.2 mmol), 2 (0.3 mmol), [η3-C3H5PdCl]2 (5.0 mol%), XPhos (10.0 mol%), LiOtBu (2.0 equiv.), THF (2.0 mL), 80 °C, Ar atmosphere, 18 h, sealed tube. b Isolated yields. c Mesitylene (2.0 mL) as solvent. d 1j (0.3 mmol), 2a (0.2 mmol). e K3PO4 (2.0 equiv.) as base, mesitylene (2.0 mL) as solvent. f 100 °C. |
|---|
|
After checking the reactivity of β-naphthols, we then proceeded to explore the generality of indoles (Table 3). All 2,3-disubstituted indoles were found to be compatible with the reaction, and products 5a–5g were formed in moderate to good yields upon reaction with gem-difluorinated cyclopropane 1a employing Pd(XantPhos)Cl2 as the catalyst, XPhos as the ligand and LiOtBu as the base in toluene at 80 °C for 24 h (see the ESI,† for details on the optimization of reaction conditions). The structure of 5a was confirmed on the basis of a single-crystal X-ray crystallographic analysis (see the ESI,† for details). Cyclic olefin-fused indoles could deliver products 5i and 5j in 62% and 65% yields. Moreover, gem-difluorinated cyclopropanes were investigated upon reaction with 2,3-dimethylindole, offering products 5k–5r in moderate to good yields. Polycyclic indoline fragments are prevalent in natural products and biologically active molecules.12 Therefore, tryptophol and tryptamines were then subjected to the reaction with gem-difluorinated cyclopropane 1a. To our delight, furanoindoline 6a and pyrroloindolines 6b–6d were achieved in 80–90% yields via cascade allylic alkylation, dearomatization and cyclization processes in the presence of Et3B (Scheme 1).13
To demonstrate the synthetic utility of this method, the scale-up synthesis and further transformations of products were conducted as shown in Scheme 2. Product 3a (1.62 g) was achieved in 91% yield on the 5.0 mmol scale under the standard reaction conditions (Scheme 2a). The ketone group could be selectively reduced by NaBH4 to afford compound 7 in 90% yield, and the relative configuration was confirmed by the NOE spectra analysis (see the ESI† for details) (Scheme 2b). Product 5a reacted with AcCl to deliver compound 8 in 94% yield. Bisindole 9 bearing an ethylene bridge, a potent antitumor activity skeleton,14 could be synthesized by Aldol-type condensation (Scheme 2c).
Based on previous works,11 a plausible catalytic cycle of this reaction is shown in Scheme 3. Initially, the activated Pd(0) catalyst reacts with the gem-difluorinated cyclopropanes 1 through oxidative addition to generate the four-membered palladacycle intermediate A, which undergoes β-F elimination to form the π-allylpalladium species B. Subsequent nucleophilic attack of β-naphthols 2 or indoles 4 at the sterically less hindered carbon atom of intermediate B affords the products 2-fluoroallylic β-naphthalenones 3 or indolenines 5, and regenerates Pd(0) catalysts for the next catalytic cycle.
In conclusion, we have developed a palladium-catalyzed allylic alkylation dearomatization of β-naphthols and indoles with gem-difluorinated cyclopropanes for the first time. This reaction provided an efficient approach to prepare 2-fluoroallylic β-naphthalenones and indolenines in good to excellent yields. In addition, functional furanoindoline and pyrroloindolines could also be synthesized via cascade allylic alkylation, dearomatization and cyclization processes from tryptophol and tryptamines. Further study on the asymmetrical process of this reaction is currently underway in our laboratory (see the ESI† for details).
The authors acknowledge generous financial support from the National Natural Science Foundation of China (NSFC22071267), the Foundation of the Open Project of State Key Laboratory of Natural Medicines (SKLNMZZ202023), the Innovation Team of “the Double-First Class” Disciplines (CPU2018GY35 and CPU2018GY04), and the National Science & Technology Major Project “Key New Drug Creation and Manufacturing Program”, China (2020ZX09201015).
Conflicts of interest
There are no conflicts to declare.Notes and references
- For a book, see: (a) M. Weber, M. Weber and M. Kleine-Boymann, “Phenol” in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2004. For selected reviews, see Search PubMed; (b) G. R. Humphrey and J. T. Kuethe, Chem. Rev., 2006, 106, 2875 CrossRef CAS; (c) A. J. Kochanowska-Karamyan and M. T. Hamann, Chem. Rev., 2010, 110, 4489 CrossRef CAS; (d) K. Nagaraju and D. Ma, Chem. Soc. Rev., 2018, 47, 8018 RSC; (e) A. Palmieri and M. Petrini, Nat. Prod. Rep., 2019, 36, 490 RSC.
- For selected reviews, see: (a) C.-X. Zhuo, C. Zheng and S.-L. You, Acc. Chem. Res., 2014, 47, 2558 CrossRef CAS; (b) W.-T. Wu, L. Zhang and S.-L. You, Chem. Soc. Rev., 2016, 45, 1570 RSC; (c) Q. Ding, X. Zhou and R. Fan, Org. Biomol. Chem., 2014, 12, 4807 RSC; (d) C.-X. Zhuo, W. Zhang and S.-L. You, Angew. Chem., Int. Ed., 2012, 51, 12662 CrossRef; (e) Z.-L. Xia, Q.-F. Xu-Xu, C. Zheng and S.-L. You, Chem. Soc. Rev., 2020, 49, 286 RSC.
- For selected reviews, see: (a) S. P. Roche and J. A. Porco Jr, Angew. Chem., Int. Ed., 2011, 50, 4068 CrossRef CAS; (b) W. Sun, G. Li, L. Hong and R. Wang, Org. Biomol. Chem., 2016, 14, 2164 RSC . For selected examples, see: ; (c) S. Amand, M. Vallet, L. Guedon, G. Genta-Jouve, F. Wien, S. Mann, J. Dupont, S. Prado and B. Nay, Org. Lett., 2017, 19, 4038 CrossRef CAS; (d) X. Zhang, H. Zhu, S. Zhang, Q. Yu and L. Xuan, Nat. Prod., 2007, 70, 1526 CrossRef CAS.
- (a) C.-X. Zhuo and S.-L. You, Angew. Chem., Int. Ed., 2013, 52, 10056 CrossRef CAS; (b) C.-X. Zhuo and S.-L. You, Adv. Synth. Catal., 2014, 356, 2020 CrossRef CAS; (c) Q. Cheng, Y. Wang and S.-L. You, Angew. Chem., Int. Ed., 2016, 55, 3496 CrossRef CAS; (d) X. Zhang, W.-B. Liu, H.-F. Tu and S.-L. You, Chem. Sci., 2015, 6, 4525 RSC; (e) J. Chen and M. J. Cook, Org. Lett., 2013, 15, 1088 CrossRef; (f) Q.-F. Wu, C. Zheng and S.-L. You, Angew. Chem., Int. Ed., 2012, 51, 1680 CrossRef CAS; (g) Q.-F. Wu, H. He, W.-B. Liu and S.-L. You, J. Am. Chem. Soc., 2010, 132, 11418 CrossRef CAS; (h) Y. Wang, C. Zheng and S.-L. You, Angew. Chem., Int. Ed., 2017, 56, 15093 CrossRef CAS; (i) R.-D. Gao, L. Ding, C. Zheng, L.-X. Dai and S.-L. You, Org. Lett., 2018, 20, 748 CrossRef CAS; (j) C.-X. Zhuo, Y. Zhou, Q. Cheng, L. Huang and S.-L. You, Angew. Chem., Int. Ed., 2015, 54, 14146 CrossRef CAS; (k) T. M. Kaiser and J. Yang, Eur. J. Org. Chem., 2013, 3983 CrossRef CAS; (l) Y. Liu and H. Du, Org. Lett., 2013, 15, 740 CrossRef CAS; (m) T. D. Montgomery, Y. Zhu, N. Kagawa and V. H. Rawal, Org. Lett., 2013, 15, 1140 CrossRef CAS; (n) Q.-L. Xu, L.-X. Dai and S.-L. You, Chem. Sci., 2013, 4, 97 RSC; (o) X. Zhang, W.-B. Liu, Q.-F. Wu and S.-L. You, Org. Lett., 2013, 15, 3746 CrossRef CAS.
- (a) H.-F. Tu, C. Zheng, R.-Q. Xu, X.-J. Liu and S.-L. You, Angew. Chem., Int. Ed., 2017, 56, 3237 CrossRef CAS; (b) S.-B. Tang, H.-F. Tu, X. Zhang and S.-L. You, Org. Lett., 2019, 21, 6130 CrossRef CAS; (c) D. Shen, Q. Chen, P. Yan, X. Zeng and G. Zhong, Angew. Chem., Int. Ed., 2017, 56, 3242 CrossRef CAS; (d) S.-Z. Jiang, X.-Y. Zeng, X. Liang, T. Lei, K. Wei and Y.-R. Yang, Angew. Chem., Int. Ed., 2016, 55, 4044 CrossRef CAS; (e) H.-J. Zhang, Q. Gu and S.-L. You, Org. Lett., 2019, 21, 9420 CrossRef CAS; (f) X. Zhang, Z.-P. Yang, C. Liu and S.-L. You, Chem. Sci., 2013, 4, 3239 RSC; (g) M. Kimura, M. Futamata, R. Mukai and Y. Tamaru, J. Am. Chem. Soc., 2005, 127, 4592 CrossRef CAS.
- (a) H. Zhang, R.-B. Hu, N. Liu, S.-X. Li and S.-D. Yang, Org. Lett., 2016, 18, 28 CrossRef CAS; (b) M. Jin, W. Ren, D.-W. Qian and S.-D. Yang, Org. Lett., 2018, 20, 7015 CrossRef CAS.
- (a) C. Romano, M. Jia, M. Monari, E. Manoni and M. Bandini, Angew. Chem., Int. Ed., 2014, 53, 13854 CrossRef CAS; (b) J. An, L. Lombardi, S. Grilli and M. Bandini, Org. Lett., 2018, 20, 7380 CrossRef CAS; (c) B. Yang, X. Zhai, S. Feng, D. Hu, Y. Deng and Z. Shao, Org. Lett., 2019, 21, 330 CrossRef CAS.
- (a) S. Gao, Z. Wu, X. Fang, A. Lin and H. Yao, Org. Lett., 2016, 18, 3906 CrossRef CAS; (b) X. Fang, Y. Zeng, Q. Li, Z. Wu, H. Yao and A. Lin, Org. Lett., 2018, 20, 2530 CrossRef CAS.
- For selected reviews, see: (a) S. Purser, P. R. Moore, S. Swallow and V. Gouverneur, Chem. Soc. Rev., 2008, 37, 320 RSC; (b) M. Cametti, B. Crousse, P. Metrangolo, R. Milani and G. Resnati, Chem. Soc. Rev., 2012, 41, 31 RSC; (c) K. Müller, C. Faeh and F. Diederich, Science, 2007, 317, 1881 CrossRef; (d) T. Liang, C. N. Neumann and T. Ritter, Angew. Chem., Int. Ed., 2013, 52, 8214 CrossRef CAS; (e) D. O’Hagan and H. Deng, Chem. Rev., 2014, 115, 634 CrossRef.
- (a) S. Couve-Bonnaire, D. Cahard and X. Pannecoucke, Org. Biomol. Chem., 2007, 5, 1151 RSC; (b) S. Oishi, H. Kamitani, Y. Kodera, K. Watanabe, K. Kobayashi, T. Narumi, K. Tomita, H. Ohno, T. Naito, E. Kodama, M. Matsuokab and N. Fujii, Org. Biomol. Chem., 2009, 7, 2872 RSC; (c) Q. Shao and Y. Huang, Chem. Commun., 2015, 51, 6584 RSC; (d) M. Drouin, J. L. Arenas and J.-F. Paquin, ChemBioChem, 2019, 20, 1817 CAS.
- For selected reviews, see: (a) X. Song, C. Xu and M. Wang, Tetrahedron Lett., 2017, 58, 1806 CrossRef CAS; (b) W. R. Dolbier and M. A. Battiste, Chem. Rev., 2003, 103, 1071 CrossRef CAS . For selected examples, see: ; (c) J. Xu, E.-A. Ahmed, B. Xiao, Q.-Q. Lu, Y.-L. Wang, C.-G. Yu and Y. Fu, Angew. Chem., Int. Ed., 2015, 54, 8231 CrossRef CAS; (d) J. Wenz, C. A. Rettenmeier, H. Wadepohl and L. H. Gade, Chem. Commun., 2016, 52, 202 RSC; (e) J. Ni, B. Nishonov, A. Pardaev and A. Zhang, J. Org. Chem., 2019, 84, 13646 CrossRef CAS; (f) E.-A. M. A. Ahmed, A. M. Y. Suliman, T.-J. Gong and Y. Fu, Org. Lett., 2019, 21, 5645 CrossRef CAS; (g) X. Song, C. Xu, D. Du, Z. Zhao, D. Zhu and M. Wang, Org. Lett., 2017, 19, 6542 CrossRef CAS; (h) E.-A. M. A. Ahmed, A. M. Y. Suliman, T.-J. Gong and Y. Fu, Org. Lett., 2020, 22, 1414 CrossRef CAS.
- (a) R. P. Loach, O. S. Fenton and M. Movassaghi, J. Am. Chem. Soc., 2016, 138, 1057 CrossRef CAS; (b) A. Steven and L. E. Overman, Angew. Chem., Int. Ed., 2007, 46, 5488 CrossRef CAS; (c) C. Zheng and S.-L. You, Nat. Prod. Rep., 2019, 36, 1589 RSC; (d) X. Zhang, L. Han and S.-L. You, Chem. Sci., 2014, 5, 1059 RSC.
- A. Lin, J. Yang and M. Hashim, Org. Lett., 2013, 15, 1950 CrossRef CAS.
- (a) J. Zheng, L. Deng, M. Chen, X. Xiao, S. Xiao, C. Guo, G. Xiao, L. Bai, W. Ye, D. Zhang and H. Chen, Eur. J. Med. Chem., 2013, 65, 158 CrossRef CAS; (b) X. Fang, Q. Li, R. Shi, H. Yao and A. Lin, Org. Lett., 2018, 20, 6084 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 1974930 and 1974934. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0cc07529a |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2021 |