 Open Access Article
Open Access ArticleHighly crystalline Na0.5Bi0.5TiO3 of a photocatalyst valence-band-controlled with Bi(III) for solar water splitting†
Kenta
Watanabe
 a,
Yoichi
Iikubo
a,
Yuichi
Yamaguchi
ab and
Akihiko
Kudo
a,
Yoichi
Iikubo
a,
Yuichi
Yamaguchi
ab and
Akihiko
Kudo
 *ab
*ab
aDepartment of Applied Chemistry, Faculty of Science, Tokyo University of Science, 1-3 Kagurazaka, Shinjuku-ku, Tokyo 162-8601, Japan. E-mail: a-kudo@rs.tus.ac.jp
bPhotocatalysis International Research Center, Research Institute for Science and Technology, Tokyo University of Science, 2641 Yamazaki Noda-Shi, Chiba-ken 278-8510, Japan
First published on 8th December 2020
Abstract
Na0.5Bi0.5TiO3 (BG 3.1 eV) with a valence band formed by Bi(III) was found as a new photocatalyst for solar water splitting. The water splitting activity of highly crystalline Na0.5Bi0.5TiO3 synthesized by a flux method was much higher than that of the samples synthesized by a solid-state reaction. The optimized RhCr2Ox(0.1 mol%)/Na0.5Bi0.5TiO3/CoOOH(0.02 mol%) gave a 5.1% apparent quantum yield at 350 nm and split water even under simulated sunlight irradiation with a 0.05% solar to hydrogen energy conversion efficiency. We have successfully achieved solar water splitting using a one-step photoexcitation type photocatalyst valence-band-controlled with Bi(III).
Hydrogen production by photocatalytic solar water splitting is attractive, and important science and technology from the viewpoint of resource, energy and environmental issues. Therefore, many researchers have developed various powder-based visible-light-driven photocatalysts working by a one-step photoexcitation and a Z-schematic two-step photoexcitation for water splitting.1–6 The one-step photoexcitation type photocatalyst especially possesses an advantage in practical use from the viewpoint of its simplicity. Domen and co-workers have reported that GaN–ZnO,7 ZnGeN2–ZnO,8 LaMg1/3Ta2/3O2N,9 TaON,10 CaTaO2N,11 Ta3N512 and Y2Ti2O5S213 of (oxy)nitride and oxysulfide photocatalysts show activities for one-step photoexcitation type water splitting under visible light irradiation. It is also reported that g-C3N4 of an organic semiconductor photocatalyst can split water under visible light irradiation.14 We have also reported that IrO2-loaded SrTiO3 co-doped with Rh and Sb of a metal oxide photocatalyst shows activity for water splitting under visible light irradiation.15 Although some of these visible-light-driven photocatalysts can split water under simulated sunlight irradiation, their solar to hydrogen energy conversion efficiencies (STH) are still low due to their low apparent quantum yields (AQYs). In recent years, Domen and co-workers have developed an Al-doped SrTiO3 (band gap (BG) 3.2 eV) photocatalyst loading Rh/Cr2O3 and CoOOH cocatalysts with almost 100% AQY, which was prepared by a flux treatment.16 Although the photocatalyst responds to only UV light, it shows the highest STH (0.65%) among one-step photoexcitation type photocatalysts.16 We have also reported that Rh0.5Cr1.5O3-loaded AgTaO3 (BG 3.4 eV) shows high AQY (38% at 340 nm) and STH (0.13%) for water splitting.17 The valence band maximum (VBM) of the Al-doped SrTiO3 is formed by O2p orbitals, while that of AgTaO3 is formed by Ag4d orbitals.18 A photocatalyst whose valence band (VB) is formed by orbitals of elements except oxygen like AgTaO3 is called a valence-band (VB)-controlled photocatalyst.3,17,19 The VB-controlled photocatalyst is very important from the viewpoint of extension of responsive wavelength. Although the STH of the Rh0.5Cr1.5O3-loaded AgTaO3 is the highest among the VB-controlled photocatalysts, the BG of the AgTaO3 is wider than that of the Al-doped SrTiO3. Therefore, it is important to achieve solar water splitting with high STH using a VB-controlled photocatalyst with a narrower BG than the Al-doped SrTiO3.
Na0.5Bi0.5TiO3 (BG 3.1 eV) with a perovskite structure is one of the candidates satisfying the conditions mentioned above, because Bi(III) is a suitable cation for the VB-controlled photocatalyst, as seen in BiVO4, Na0.5Bi0.5WO4 and Bi4Ti3O12.20–22 The VB of Na0.5Bi0.5TiO3 is formed by hybridized orbitals consisting of O2p and Bi6s, leading to the narrower BG than SrTiO3.23 Na0.5Bi0.5TiO3 showed activities for photocatalytic H2 and O2 evolution from aqueous solutions containing sacrificial reagents under UV irradiation as half reactions of water splitting.24,25 Na0.5Bi0.5TiO3 photocatalysts have also been used for degradation of dyes.26,27 However, water splitting into H2 and O2 at a stoichiometric ratio over the Na0.5Bi0.5TiO3 has not been achieved yet. The distortion of the perovskite framework in the crystal structure of Na0.5Bi0.5TiO3 is similar to that of AgTaO3 (Fig. S1 and Table S1, ESI†). Therefore, Na0.5Bi0.5TiO3 is expected to possess potential for photocatalytic water splitting. Moreover, the activity of Na0.5Bi0.5TiO3 will be improved by changing the synthesis method from a conventional solid state reaction to a flux method as well as that of SrTiO328 because Na0.5Bi0.5TiO3 is similar to SrTiO3 from the viewpoints of a perovskite structure consisting of TiO6 octahedra. A flux method is useful to control bulk properties of a material such as morphology and crystallinity.29–32 In the present study, we synthesized Na0.5Bi0.5TiO3 of a photocatalyst VB-controlled with Bi6s orbitals by a solid state reaction and a flux method, and investigated their photocatalytic water splitting with loading suitable cocatalysts.
Na0.5Bi0.5TiO3 was synthesized by a solid state reaction (SSR) and a flux method (FM) using Na2CO3 (Kanto Chemical; 99.8%), Bi2O3 (Kanto Chemical; 99.9%) and TiO2 (Kojundo; 99.99%) as starting materials. After the starting materials were mixed in an alumina mortar in a ratio of Na2CO3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Bi2O3
Bi2O3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TiO2 = 0.25
TiO2 = 0.25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.25
0.25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the mixture was calcined at 1173–1373 K for 5 h in air for the SSR. The obtained material was washed with distilled water. For the FM, 10 molar equivalence of NaCl (Kanto Chemical; 99.5%) to the objective was used as a flux reagent. The mixture of the starting materials and the NaCl-flux was heated at 1173–1473 K for 5 h in air. The obtained material was washed with distilled water to remove the NaCl-flux. Na0.5La0.5TiO3 and Na0.5La0.25Bi0.25TiO3 were also synthesized by FM at 1273 K for 5 h with the NaCl-flux in order to compare their band structures with Na0.5Bi0.5TiO3. Details of the experiment are described in the footnotes of the Figures and Tables, and the ESI.†
1, the mixture was calcined at 1173–1373 K for 5 h in air for the SSR. The obtained material was washed with distilled water. For the FM, 10 molar equivalence of NaCl (Kanto Chemical; 99.5%) to the objective was used as a flux reagent. The mixture of the starting materials and the NaCl-flux was heated at 1173–1473 K for 5 h in air. The obtained material was washed with distilled water to remove the NaCl-flux. Na0.5La0.5TiO3 and Na0.5La0.25Bi0.25TiO3 were also synthesized by FM at 1273 K for 5 h with the NaCl-flux in order to compare their band structures with Na0.5Bi0.5TiO3. Details of the experiment are described in the footnotes of the Figures and Tables, and the ESI.†
X-ray diffraction (XRD) patterns revealed that Na0.5Bi0.5TiO3 was successfully synthesized by SSR and FM (Fig. S2, ESI†). The FWHMs of the (012) peaks of the sample synthesized by FM at 1273–1473 K were similar to each other and smaller than those of the other samples. This result indicates that these samples synthesized by FM at 1273–1473 K possessed higher crystallinity than the other samples. The change in width of the peak split between (104) and (110) depending on the calcination temperature suggests that distortion of the perovskite framework slightly changed. The effect of synthesis method on the morphology of Na0.5Bi0.5TiO3 particles was investigated by scanning electron microscopy (SEM) measurements, as shown in Fig. 1. The particle surfaces of the samples synthesized by FM were more faceted than those of the samples synthesized by SSR. Particles prepared by FM were small comparing with SSR at the same calcination temperature. The surface area (S.A.) of the samples synthesized by FM was larger than those of the samples synthesized by SSR. All absorption edges of diffuse reflectance spectra (DRS) of the Na0.5Bi0.5TiO3 were located at around 400–420 nm, as shown in Fig. 2, indicating that the BG of Na0.5Bi0.5TiO3 was 3.0–3.1 eV. The BG of Na0.5La0.5TiO3 in which the VB is formed by O2p orbitals was estimated to be 3.4 eV from its DRS. Therefore, the BG of Na0.5Bi0.5TiO3 was narrowed about 0.3 eV due to the contribution of Bi6s orbitals to its VB. The absorption edge of Na0.5La0.25Bi0.25TiO3 was located between those of the Na0.5La0.5TiO3 and the Na0.5Bi0.5TiO3 (Fig. S3, ESI†). XPS spectra at the VB regions of Na0.5Bi0.5TiO3 and Na0.5La0.5TiO3 synthesized by FM at 1273 K were measured (Fig. S4, ESI†). The peak of the Na0.5Bi0.5TiO3 was wider than that of the Na0.5La0.5TiO3. The onset binding energy of the peak of the Na0.5Bi0.5TiO3 shifted to a lower energy of about 0.3 eV than that of the Na0.5La0.5TiO3. These results also suggest that Bi6s orbitals contributed to the valence band maximum (VBM) of the Na0.5Bi0.5TiO3. DFT calculations also revealed that Bi(III) contributed to the VB of the Na0.5Bi0.5TiO3.23 The VBM formed by O2p orbitals of materials consisting of MO6 (M = metal cation) such as Na0.5La0.5TiO3 are located around +3.0 eV vs. RHE.33 Thus, the conduction band minimum (CBM) of Na0.5La0.5TiO3 was calculated to be −0.4 eV vs. RHE from its BG. CBM depends on the distortion of M–O–M bond angles.34 Therefore, the CBM of Na0.5Bi0.5TiO3 was also −0.4 eV vs. RHE because the Ti–O–Ti bond angle of the Na0.5Bi0.5TiO3 was similar to that of the Na0.5La0.5TiO3 (Table S1, ESI†). The CBM of Na0.5Bi0.5TiO3 has an advantage in a driving force for reduction of water to hydrogen compared with that of SrTiO3 (−0.2 V vs. RHE). The BG of the Na0.5Bi0.5TiO3 was narrowed 0.3 eV by the contribution of Bi6s to its VBM compared with Na0.5La0.5TiO3, as mentioned above. The VBM of the Na0.5Bi0.5TiO3 was calculated to be +2.7 eV from its BG and CBM. The small shift in the absorption edges seemed to be due to the difference in the distortion of the crystal structure and the particle size depending on the synthesis conditions. Additionally, the molar ratios of Bi/Ti for the samples synthesized by FM at 1173–1373 K were slightly smaller than those for the other samples (Table S2, ESI†). The smaller molar ratios of Bi/Ti for the samples synthesized by FM at 1173–1373 K also seemed to affect the difference in the absorption edges because Bi6s orbitals contributed to the VBM of Na0.5Bi0.5TiO3.
 | ||
| Fig. 1 SEM images and surface areas of Na0.5Bi0.5TiO3 synthesized by SSR and FM at various temperatures. | ||
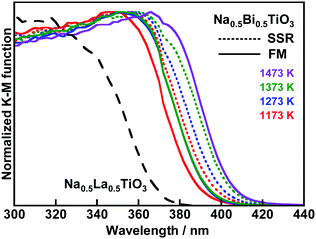 | ||
| Fig. 2 Diffuse reflectance spectra of Na0.5Bi0.5TiO3 synthesized by SSR and FM at various temperatures and Na0.5La0.5TiO3 synthesized by FM at 1273 K. | ||
Photocatalytic water splitting over Na0.5Bi0.5TiO3 under UV irradiation (λ >300 nm) was examined, as shown in Table 1. RhCr2Ox is an excellent cocatalyst for water splitting.35,36 We applied the cocatalyst to the present Na0.5Bi0.5TiO3. All samples produced H2 and O2 in a stoichiometric ratio. The samples synthesized by FM at 1273 and 1373 K especially showed high photocatalytic activities, because these samples possessed good crystallinity as seen in XRD (Fig. S2, ESI†). Photogenerated carriers easily migrated to the surface of such well-crystallized particles, resulting in high activities. Although the sample synthesized by FM at 1473 K possessed similar crystallinity to the samples synthesized by FM at 1273 and 1373 K, it showed a quite low activity because of its very small S.A. (<0.1 m2 g−1). Non-loaded Na0.5Bi0.5TiO3 synthesized by FM at 1273 K hardly showed photocatalytic activity for water splitting under UV irradiation (Table S3, ESI†). 0.1 mol% was the optimum loading amount of the RhCr2Ox cocatalyst. Coloading of the RhCr2Ox cocatalyst with a CoOOH of an O2-evolving cocatalyst is effective for water splitting over the Al-doped SrTiO3 photocatalyst.16,37 The water splitting activity of the RhCr2Ox(0.1 mol%)-loaded Na0.5Bi0.5TiO3 was further improved by loading of the CoOOH cocatalyst. RhCr2Ox(0.1 mol%) and CoOOH(0.02 mol%)-coloaded Na0.5Bi0.5TiO3 showed the highest activity for photocatalytic water splitting (Table S3 and Fig. S5, ESI†). The binding energy of the Rh3d5/2 peak in the XPS spectrum of the RhCr2Ox(0.1 mol%)/Na0.5Bi0.5TiO3/CoOOH(0.02 mol%) (308.9 eV) was similar to not that of Rh2O3 (308.3 eV) but those of Rh0.5Cr1.5O3(0.2 mol%)/Na0.5Bi0.5TiO3 (308.9 eV) loaded by an impregnation method and previously reported Rh2−xCryO3/(Ga1−xZnx)(N1−xOx) (309.0 eV) loaded by a simultaneous PD method (Fig. S6, ESI†).35,36 Therefore, the RhCr2Ox loaded on the Na0.5Bi0.5TiO3 was suggested to be mainly a Rh2−yCryO3 of a mixed-oxide. The optimum Na0.5Bi0.5TiO3 also showed much higher photocatalytic activity for water splitting than Na0.5La0.5TiO3 and Na0.5La0.25Bi0.25TiO3 (Table S3, ESI†). This might be because photogenerated holes in the Na0.5Bi0.5TiO3 could easily migrate to the particle surfaces because of the contribution of Bi(III) to the VB.
Fig. 3(A) shows an action spectrum of photocatalytic water splitting over the RhCr2Ox(0.1 mol%)/Na0.5Bi0.5TiO3/CoOOH(0.02 mol%). The onset wavelength of the activity agreed with that of the absorption spectrum of Na0.5Bi0.5TiO3. This agreement indicates that water splitting proceeded with photoexcitation from the VB formed by Bi6s orbitals to the CB formed by Ti3d, as shown in Fig. 3(B). The AQY was 5.1% at 350 nm. As far as we know, among metal oxide photocatalysts with VB formed by Bi6s orbitals, the AQY is the highest for one-step photoexcitation type water splitting. Additionally, the AQY was slightly higher than that of RhCrOx-loaded GaN–ZnO which shows the highest AQY among a visible-light-driven photocatalyst for water splitting.7 Moreover, RhCr2Ox(0.1 mol%)/Na0.5Bi0.5TiO3/CoOOH(0.02 mol%) responded to light up to 420 nm.
| Synthesis condition | FWHM of (012) peak/° | S. A./m2 g−1 | Activity/μmol h−1 | ||
|---|---|---|---|---|---|
| Method | Temp./K | H2 | O2 | ||
| Photocatalyst: 0.3 g, reactant solution: distilled water (120 mL), cell: top-irradiation cell with a Pyrex window, light source: 300 W Xe-arc lamp (λ >300 nm). A RhCr2Ox(0.2 mol%) cocatalyst was loaded in situ by a photodeposition (PD) method. | |||||
| SSR | 1173 | 0.11 | 1.3 | 30 | 14 |
| SSR | 1273 | 0.10 | 0.7 | 33 | 16 |
| SSR | 1373 | 0.10 | 0.6 | 10 | 4.9 |
| FM | 1173 | 0.12 | 2.6 | 9 | 4 |
| FM | 1273 | 0.09 | 0.9 | 102 | 50 |
| FM | 1373 | 0.09 | 0.9 | 58 | 27 |
| FM | 1473 | 0.09 | <0.1 | 1 | 0.4 |
Water splitting proceeded over the RhCr2Ox(0.1 mol%)/Na0.5Bi0.5TiO3/CoOOH(0.02 mol%) even under simulated sunlight irradiation, as shown in Fig. 4. H2 and O2 steadily evolved for a long time at the rates of 203 mL h−1 m−2 and 91 mL h−1 m−2, respectively. The STH was estimated to be 0.05%. Thus, we successfully achieved one-step photoexcitation type solar water splitting using RhCr2Ox(0.1 mol%)/Na0.5Bi0.5TiO3/CoOOH(0.02 mol%) as a photocatalyst VB-controlled with Bi(III) possessing a narrower BG than SrTiO3.
In conclusion, Na0.5Bi0.5TiO3, which is a photocatalyst VB-controlled with Bi(III), synthesized by a flux method at 1273 K has arisen as a new photocatalyst for solar water splitting in a suspension system when RhCr2Ox(0.1 mol%) and CoOOH(0.02 mol%) were coloaded. Na0.5Bi0.5TiO3 synthesized by FM was more highly crystalized and faceted than the samples synthesized by SSR. XPS measurements revealed that the VBM of Na0.5Bi0.5TiO3 was formed by Bi6s orbitals. The highly crystalline Na0.5Bi0.5TiO3 with facets obtained by FM showed higher water splitting activity under UV irradiation than Na0.5Bi0.5TiO3 obtained by SSR. This seemed to be because photogenerated carriers were able to easily migrate to the particle surfaces due to its high crystallinity. Optimized RhCr2Ox(0.1 mol%)/Na0.5Bi0.5TiO3/CoOOH(0.02 mol%) responded to light up to 420 nm. This photocatalyst gave 5.1% AQY at 350 nm and 0.05% STH. The AQY and STH were the highest among photocatalysts VB-controlled with Bi(III). Thus, we have demonstrated solar water splitting via one-step type photoexcitation using a VB-controlled photocatalyst consisting of Bi(III) with a narrower BG than SrTiO3.
This work was supported by JSPS KAKENHI Grant Numbers 17H06433 and 17H06440 in Scientific Research on Innovative Areas “Innovations for Light-Energy Conversion (I4LEC)”, and 17H01217.
Conflicts of interest
There are no conflicts to declare.References
- F. E. Osterloh, Chem. Mater., 2008, 20, 35–54 CrossRef CAS.
- Y. Inoue, Energy Environ. Sci., 2009, 2, 364–386 RSC.
- A. Kudo and Y. Miseki, Chem. Soc. Rev., 2009, 38, 253–278 RSC.
- R. Abe, J. Photochem. Photobiol., C, 2010, 11, 179–209 CrossRef CAS.
- T. Hisatomi, J. Kubota and K. Domen, Chem. Soc. Rev., 2014, 43, 7520–7535 RSC.
- K. Maeda and K. Domen, Bull. Chem. Soc. Jpn., 2016, 89, 627–648 CrossRef CAS.
- K. Maeda, K. Teramura, D. Lu, T. Takata, N. Saito, Y. Inoue and K. Domen, Nature, 2006, 440, 295 CrossRef CAS PubMed.
- Y. Lee, H. Terashima, Y. Shimodaira, K. Teramura, M. Hara, H. Kobayashi, K. Domen and M. Yashima, J. Phys. Chem. C, 2007, 111, 1042–1048 CrossRef CAS.
- C. Pan, T. Takata, M. Nakabayashi, T. Matsumoto, N. Shibata, Y. Ikuhara and K. Domen, Angew. Chem., Int. Ed., 2015, 54, 2955–2959 CrossRef CAS.
- K. Maeda, D. Lu and K. Domen, Chem. – Eur. J., 2013, 19, 4986–4991 CrossRef CAS.
- J. Xu, C. Pan, T. Takata and K. Domen, Chem. Commun., 2015, 51, 7191–7194 RSC.
- Z. Wang, Y. Inoue, T. Hisatomi, R. Ishikawa, Q. Wang, T. Takata, S. Chen, N. Shibata, Y. Ikuhara and K. Domen, Nat. Catal., 2018, 1, 756–763 CrossRef CAS.
- Q. Wang, M. Nakabayashi, T. Hisatomi, S. Sun, S. Akiyama, Z. Wang, Z. Pan, X. Xiao, T. Watanabe, T. Yamada, N. Shibata, T. Takata and K. Domen, Nat. Mater., 2019, 18, 827–832 CrossRef CAS.
- G. Zhang, Z.-A. Lan, L. Lin, S. Lin and X. Wang, Chem. Sci., 2016, 7, 3062–3066 RSC.
- R. Asai, H. Nemoto, Q. Jia, K. Saito, A. Iwase and A. Kudo, Chem. Commun., 2014, 50, 2543–2546 RSC.
- T. Takata, J. Jiang, Y. Sakata, M. Nakabayashi, N. Shibata, V. Nandal, K. Seki, T. Hisatomi and K. Domen, Nature, 2020, 581, 411–414 CrossRef CAS.
- K. Watanabe, A. Iwase and A. Kudo, Chem. Sci., 2020, 11, 2330–2334 RSC.
- H. Kato, H. Kobayashi and A. Kudo, J. Phys. Chem. B, 2002, 106, 12441–12447 CrossRef CAS.
- A. Kudo, H. Kato and I. Tsuji, Chem. Lett., 2004, 33, 1534–1539 CrossRef CAS.
- A. Kudo, K. Omori and H. Kato, J. Am. Chem. Soc., 1999, 121, 11459–11467 CrossRef CAS.
- H. Kato, N. Matsudo and A. Kudo, Chem. Lett., 2004, 33, 1216–1217 CrossRef CAS.
- A. Kudo and S. Hijii, Chem. Lett., 1999, 1103–1104 CrossRef CAS.
- M. Zeng, S. W. Or and H. L. W. Chan, J. Appl. Phys., 2010, 107, 043513 CrossRef.
- Y. Iikubo, H. Kato and A. Kudo, Abstract of 94th CATSJ Meeting, 2004, P038; Y. Iikubo, MPhil thesis, Tokyo University of Science, 2005 Search PubMed.
- L. Wang and W. Wang, Int. J. Hydrogen Energy, 2012, 37, 3041–3047 CrossRef CAS.
- Z. Ai, G. Lu and S. Lee, J. Alloys Compd., 2014, 613, 260–266 CrossRef CAS.
- R. Zhang, X. Wu, Y. Li, W. Shao, Y. Zhang, Z. Liu, J. Nie, J. Tan and W. Ye, RSC Adv., 2020, 10, 7443–7451 RSC.
- Y. Ham, T. Hisatomi, Y. Goto, Y. Moriya, Y. Sakata, A. Yamakata, J. Kubota and K. Domen, J. Mater. Chem. A, 2016, 4, 3027–3033 RSC.
- X. Liu, N. Fechler and M. Antonietti, Chem. Soc. Rev., 2013, 42, 8237–8265 RSC.
- Q. Wang, Q. Guo, L. Wang and B. Li, Dalton Trans., 2016, 45, 17748–17758 RSC.
- Q. Wang, B. Zhang, X. Lu, X. Zhang, H. Zhu and B. Li, Catal. Sci. Technol., 2018, 8, 6180–6195 RSC.
- J. Boltersdorf, N. King and P. A. Maggard, CrystEngComm, 2015, 17, 2225–2241 RSC.
- D. E. Scaife, Sol. Energy, 1980, 25, 41–54 CrossRef CAS.
- H. Kato and A. Kudo, J. Phys. Chem. B, 2001, 105, 4285–4292 CrossRef CAS.
- K. Maeda, D. Lu, K. Teramura and K. Domen, J. Mater. Chem., 2008, 18, 3539–3542 RSC.
- K. Maeda, D. Lu, K. Teramura and K. Domen, Energy Environ. Sci., 2010, 3, 470–477 RSC.
- H. Lyu, T. Hisatomi, Y. Goto, M. Yoshida, T. Higashi, M. Katayama, T. Takata, T. Minegishi, H. Nishiyama, T. Yamada, Y. Sakata, K. Asakura and K. Domen, Chem. Sci., 2019, 10, 3196–3201 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental detail, crystal structure, XRD, XRF, XPS, DRS and a time course of an activity. See DOI: 10.1039/d0cc07371g |
| This journal is © The Royal Society of Chemistry 2021 |


