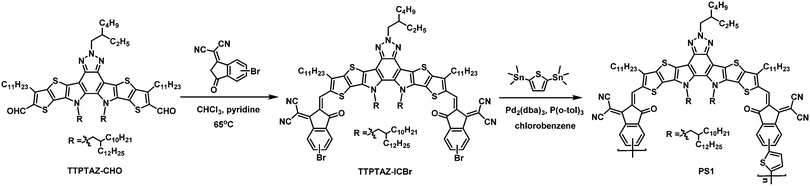
Constructing a new polymer acceptor enabled non-halogenated solvent-processed all-polymer solar cell with an efficiency of 13.8%†
Chunguang
Zhu
,
Zhenye
Li
,
Wenkai
Zhong
,
Feng
Peng
,
Zhaomiyi
Zeng
,
Lei
Ying
 *,
Fei
Huang
*,
Fei
Huang
 and
Yong
Cao
and
Yong
Cao
Institute of Polymer Optoelectronic Materials and Devices, State Key Laboratory of Luminescent Materials and Devices, South China University of Technology, Guangzhou, 510640, China. E-mail: msleiying@scut.edu.cn
First published on 16th December 2020
Abstract
A new polymer acceptor, PS1, was developed by connecting the non-fullerene acceptor building block of dithienothiophen[3,2-b]pyrrolobenzotriazole capped with 3-(dicyanomethylidene)-indan-1-one through a thiophene spacer. The solubilizing alkyl side groups in the central unit enabled PS1 to be readily dissolved in non-chlorinated solvents. By using 2-methyltetrahydrofuran as the processing solvent, the all-polymer solar cell (all-PSC) containing PS1 and a polymer donor PTzBI-oF in the light-harvesting layer exhibited an impressively high power conversion efficiency of 13.8%.
All-polymer solar cells (all-PSCs) present great potential for practical applications because of their remarkable thermal and photochemical stability, robust mechanical properties, and compatibility for large-scale roll-to-roll processing.1–6 The primary concern regarding the development of all-PSCs remains the photovoltaic performance, as the power conversion efficiency (PCE) lags behind those obtained from small-molecule non-fullerene acceptors (NFAs) that have achieved a remarkable value of 18%.7–12 In principle, the achievement of high photovoltaic performances for all-PSCs requires light-harvesting materials with efficient light absorption, high electron mobility, and excellent donor and acceptor miscibility to obtain a favorable film morphology.13–15 To realize high-performance all-PSCs, we developed a series of polymer donors based on an imide-functionalized benzotriazole (PTzBI) unit by fine-tuning the molecular structures.16–20 These copolymers could be easily dissolved in non-chlorinated solvents, for instance, 2-methyltetrahydrofuran (2-MeTHF), cyclopentyl methyl ether, d-limonene, and so forth.18–20 When combined with one of the most extensively used n-type polymer acceptors N2200, the resulting all-PSCs containing PTzBI derivatives presented a PCE exceeding 11% after delicately optimizing the device architecture and processing conditions.21 However, the low extinction coefficient of N2200 in the near-infrared region significantly limits the light-harvest of the bulk-heterojunction layer. In this regard, the short-circuit current densities (JSC) of all-PSCs utilizing the polymer acceptor of N2200 are typically below 20 mA cm−2.
An effective strategy for overcoming such limitations is the development of new polymer acceptors, which have high extinction coefficient in the NIR region. This can be achieved by copolymerizing the recently emerged building blocks of high-performance NFAs through a π-conjugated spacer.22–29 This strategy has been proven very effective, as a range of polymer acceptors containing fused rings with well-extended conjugated lengths have been developed and showed impressive PCEs of 13–15%.30–35 However, these all-PSCs are typically processed with chlorinated solvents (Table S1 in the ESI†) because of their large and coplanar structure. In pursuance of highly efficient all-PSCs that can be processed with non-chlorinated solvents to reduce the harmfulness to the environment,36–39 here we designed a narrow bandgap polymer acceptor, PS1 (Scheme 1). This copolymer contains a building block of dithienothiophen[3,2-b]pyrrolobenzotriazole end-capped with 3-(dicyanomethylidene)-indan-1-one. The long solubilizing side-chain in the central benzotriazole moiety imparts solubility in the non-chlorinated solvent of 2-MeTHF. Moreover, this copolymer presents a high absorption coefficient in the NIR region.
The synthesis of the target polymer acceptor PS1 was conducted through multiple steps, as shown in Scheme 1. By treating the dialdehyde compound TTPTAZ-CHO with a mono-brominated 2-(3-oxo-2,3-dihydro-1H-inden-1-ylidene) malononitrile, the dibromo monomer TTPTAZ-ICBr was obtained with a high yield of 80%. Synthesis of the target polymer PS1 was carried out via the palladium-catalyzed Stille polymerization of TTPTAZ-ICBr with 2,5-bis(trimethylstannyl)thiophene. The obtained copolymer PS1 was purified by Soxhlet extraction and extracted using chloroform (details are shown in the ESI†). The number-average molecular weight of PS1 was 10.9 kDa with a dispersity of 2.52, which was evaluated by high-temperature gel permeation chromatography, utilizing 1,2,4-trichlorobenzene as the eluent (measured at 150 °C, Fig. S1, ESI†).
The resulting polymer PS1 exhibited a broad absorption profile in the 600–1000 nm range, which was correlated with a strong charge-transfer effect and maximum absorption at approximately 810 nm (Fig. 1a). The absorption peak of PS1 in 2-MeTHF solution was 810 nm, which was bathochromically shifted by approximately 15 nm relative to that in chloroform (CF) solution (796 nm) (Fig. S4, ESI†). The PS1 thin film processed with 2-MeTHF had a high absorption coefficient of 7.0 × 104 cm−1, which was slightly higher than that of the film processed with chloroform (5.1 × 104 cm−1). The absorption profile of PS1 complemented the wide-bandgap conjugated donor polymer PTzBI-oF (Fig. 1a). The optical bandgap value of PS1 as estimated based on the onset of film absorption was 1.39 eV. Based on the cyclic voltammetry (CV) measurement (ferrocene/ferrocenium, Fc/Fc+ potential: 0.39 V versus Hg/Hg2Cl2 electrode), the highest occupied molecular orbital energy level (EHOMO) and the lowest unoccupied molecular orbital (ELUMO) energy level of PS1 were −5.56 eV and −3.70 eV, respectively (Fig. 1b), on the basis of the equation: EHOMO/ELUMO = −[e(Eox/Ered + 4.8 − EFc/Fc+)] (eV), where Eox/Ered is the onset oxidation/reduction potential versus the Hg/Hg2Cl2 electrode. We note that the bandgap calculated from the CV measurement is 1.86 eV, which is obviously higher than the optical bandgap. This can be understood as the optical bandgap correlating to the exciton of the electron–hole pair has a relatively high Coulomb force interaction (binding energy), while such binding energy is not involved in the CV measurement.
 | ||
| Fig. 1 (a) Normalized absorption spectra of PTzBI-oF and PS1 in thin films processed by 2-MeTHF. (b) Cyclic voltammetry curve of PS1. | ||
To evaluate the performance of PS1 as a polymer acceptor, all-PSCs were fabricated (structure: ITO/PEDOT:PSS/PTzBI-oF:PS1/PFN-Br/Ag), with the current density–voltage (J–V) curves shown in Fig. 2a. Here the PTzBI-oF:PS1 layer was processed with chloroform or non-chlorinated 2-MeTHF under the optimized conditions (Tables S3–S5, ESI†). Note that the device processed with chloroform or 2-MeTHF presented an identical open-circuit voltage (VOC) of 0.92 V. The device processed with 2-MeTHF delivered a higher PCE of 13.8% compared to 12.1% for the chloroform-processed device, which was primarily due to the concurrently increased short-circuit current density (JSC) of 22.47 mA cm−2 and a fill factor of 66.70% (Table 1), and it is among the highest values achieved so far for all-PSCs that were processed with non-chlorinated solvents (Table S8, ESI†). These JSC values were verified from the external quantum efficiency spectra (Fig. 2b). The obtained device presented good thermal stability, with the PCE remaining at about 95% with respect to the initial value after thermal annealing at 80 °C for 60 h (Fig. S9, ESI†). It is also worth pointing out that the large-area (1 cm2) device based on PTzBI-oF:PS1 presented an impressively high PCE of 12.4% (Fig. S9, ESI†), suggesting its great potential for fabricating module devices.
 | ||
| Fig. 2 (a) Current density–voltage curves. (b) External quantum efficiency (EQE) and integrated Jsc curves for all-polymer solar cells based on PTzBI-oF:PS1 processed by chloroform (CF) or 2-MeTHF. | ||
The photoluminescence spectra of the pure PTzBI-oF films and PTzBI-oF:PS1 films processed with either chloroform or 2-MeTHF showed that the emission quenching of the 2-MeTHF-processed film was more pronounced than that of the chloroform-processed film (Fig. 3a). Furthermore, the device processed with 2-MeTHF presented hole and electron mobilities of 2.45 × 10−3 and 3.57 × 10−4 cm2 V−1 s−1, respectively, both of which were slightly higher than those of the device processed with chloroform (1.29 × 10−3 and 1.56 × 10−4 cm2 V−1 s−1, respectively), with the corresponding curves shown in Fig. S5 and Table S6 (ESI†). These observations were consistent with a slightly higher JSC of the 2-MeTHF-processed device.
To further elaborate the dependence of the photovoltaic performances of all-PSCs on the processing solvents, we recorded the photocurrent density (Jph)–effective voltage (Veff) characteristics (Fig. 3b). The Jph of the PTzBI-oF:PS1 devices processed with chloroform gradually approached saturated values (Jsat) of 23.04 mA cm−2, slightly lower than the 24.27 mA cm−2 for the 2-MeTHF processed device at Veff exceeding 1 V, respectively (Table S7, ESI†). On the basis of the ratio of Jph/Jsat, the charge dissociation probability parameter P(E,T) was estimated to be 90.7% and 93.9% for the devices processed with chloroform and 2-MeTHF, respectively. This finding implied that the exciton dissociation and charge collection efficiency of the 2-MeTHF-processed device is slightly more efficient than the counterpart device processed with chloroform, which is consistent with the higher EQE and JSC of the former. To explore the carrier recombination behaviors, we studied the correlation of JSC and the light intensity (Plight), following the characteristics of Jsc∝(Plight)S. The estimated exponential factor was 0.987 and 0.992 for the devices based on PTzBI-oF:PS1 processed with chloroform and 2-MeTHF, respectively, thereby suggesting a slightly lower bimolecular recombination in 2-MeTHF-processed devices. For the Plight–VOC characteristics, the devices processed with chloroform and 2-MeTHF presented slopes of 1.31 kT q−1 and 1.29 kT q−1, respectively (Fig. S6b, ESI†). These observations demonstrated that both bimolecular recombination and Shockley–Read–Hall recombination were negligible in both devices.
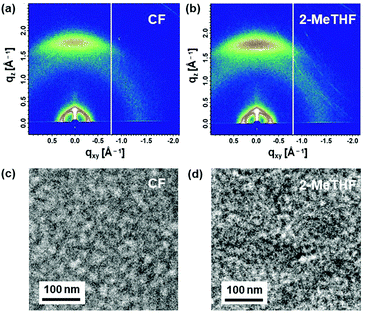
Two-dimensional grazing incidence wide-angle X-ray scattering was employed to reveal the molecular stacking inside the neat and blended films. The neat PS1 processed with chloroform and 2-MeTHF showed an intense π–π stacking peak at q of 1.68 Å−1 in the out-of-plane (OOP) direction, which was indicative of the preferential face-on polymer packing arrangement (Fig. S7, ESI†). The PTzBI-oF:PS1 blends processed with chloroform and 2-MeTHF displayed comparable scattering signals with a strong π–π stacking peak (q = 1.76 Å−1) in the OOP direction, associated with lamellar peaks (q = 0.35 Å−1) in both OOP and in-plane directions. The peaks generated by the 2-MeTHF-prepared blend showed enhanced intensities when compared with those from the chloroform-prepared blend (Fig. 4a and b). The mesoscale morphology of the PTzBI-oF:PS1 blend was also investigated through transmission electron microscopy (TEM). Delicate and inter-continuous bright and dark regions were visible in both blends in the TEM images, but these features were finer in the 2-MeTHF-processed blend (Fig. 4c and d). From the atomic force microscopy images, one can note that both blends presented uniform and smooth surfaces, with a relatively small root-mean-square roughness of approximately 1.5 nm (Fig. S8, ESI†). These observations demonstrated the improved crystallinity and reduced-size domains in the 2-MeTHF-processed blend, which can assist in charge transfer and transportation and eventually increase the JSC and fill factor of all-PSCs.
In summary, we have developed a new fused-aromatic-ring-constructed polymer acceptor, PS1, via a universal design strategy. The bulky and solubilizing alkyl chain on the key building block enabled the superior solubility of the polymer. PS1 possessed excellent light harvesting capabilities, suitable energy levels, and a more favorable film morphology when blended with an electron-donating copolymer PTzBI-oF. The photovoltaic performance of all-PSCs highly depends on the processing solvent, where the non-chlorinated solvent 2-MeTHF resulted in a more favorable film morphology than the halogenated solvent chloroform. The optimized all-PSCs based on 2-MeTHF-processed PTzBI-oF:PS1 delivered a high PCE of 13.8%. Our results suggest that this new polymer acceptor PS1 has great potential for use in high-performance and non-chlorinated solvent-processed organic photovoltaic devices in future practical applications.
This work was financially supported by the Basic and Applied Basic Research Major Program of Guangdong Province (No. 2019B030302007), the National Natural Science Foundation of China (21822505), and the National Key Research and Development Program of China (No. 2019YFA0705900) funded by MOST.
Conflicts of interest
There are no conflicts to declare.Notes and references
- G. Wang, F. S. Melkonyan, A. Facchetti and T. J. Marks, Angew. Chem., Int. Ed., 2019, 58, 4129 CrossRef CAS.
- C. Lee, S. Lee, G. U. Kim, W. Lee and B. J. Kim, Chem. Rev., 2019, 119, 8028 CrossRef CAS.
- S. Chen, S. Jung, H. J. Cho, N. H. Kim, S. Jung, J. Xu, J. Oh, Y. Cho, H. Kim, B. Lee, Y. An, C. Zhang, M. Xiao, H. Ki, Z.-G. Zhang, J. Y. Kim, Y. Li, H. Park and C. Yang, Angew. Chem., Int. Ed., 2018, 57, 13277 CrossRef CAS.
- Y. Min, C. Dou, H. Tian, Y. Geng, J. Liu and L. Wang, Angew. Chem., Int. Ed., 2018, 57, 2000 CrossRef CAS.
- N. B. Kolhe, D. K. Tran, H. Lee, D. Kuzuhara, N. Yoshimoto, T. Koganezawa and S. A. Jenekhe, ACS Energy Lett., 2019, 4, 1162 CrossRef CAS.
- Z. Luo, T. Liu, R. Ma, Y. Xiao, L. Zhan, G. Zhang, H. Sun, F. Ni, G. Chai, J. Wang, C. Zhong, Y. Zou, X. Guo, X. Lu, H. Chen, H. Yan and C. Yang, Adv. Mater., 2020, 32, 2005942 CrossRef CAS.
- J. Yuan, T. Huang, P. Cheng, Y. Zou, H. Zhang, J. L. Yang, S.-Y. Chang, Z. Zhang, W. Huang, R. Wang, D. Meng, F. Gao and Y. Yang, Nat. Commun., 2019, 10, 570 CrossRef CAS.
- Y. Tong, Z. Xiao, X. Du, C. Zuo, Y. Li, M. Lv, Y. Yuan, C. Yi, F. Hao, Y. Hua, T. Lei, Q. Lin, K. Sun, D. Zhao, C. Duan, X. Shao, W. Li, H.-L. Yip, Z. Xiao, B. Zhang, Q. Bian, Y. Cheng, S. Liu, M. Cheng, Z. Jin, S. Yang and L. Ding, Sci. China: Chem., 2020, 63, 758 CrossRef CAS.
- Q. Liu, Y. Jiang, K. Jin, J. Qin, J. Xu, W. Li, J. Xiong, J. Liu, Z. Xiao, K. Sun, S. Yang, X. Zhang and L. Ding, Sci. Bull., 2020, 65, 272 CrossRef CAS.
- Q. Zheng, Y. Ma, D. Cai, S. Wan, P. Wang and J. Wang, Angew. Chem., Int. Ed., 2020, 59, 21627 CrossRef.
- C. Zhu, K. An, W. Zhong, Z. Li, Y. Qian, X. Su and L. Ying, Chem. Commun., 2020, 56, 4700 RSC.
- Y. Lin, Y. Firdaus, F. H. Isikgor, M. I. Nugraha, E. Yengel, G. T. Harrison, R. Hallani, A. El-Labban, H. Faber, C. Ma, X. Zheng, A. Subbiah, C. T. Howells, O. M. Bakr, I. McCulloch, S. D. Wolf, L. Tsetseris and T. D. Anthopoulos, ACS Energy Lett., 2020, 5, 2935 CrossRef CAS.
- P. Cheng and X. Zhan, Chem. Soc. Rev., 2016, 45, 2544 RSC.
- H.-H. Cho, S. Kim, T. Kim, V. G. Sree, S.-H. Jin, F. S. Kim and B. J. Kim, Adv. Energy Mater., 2018, 8, 1701436 CrossRef.
- J. Yang, B. Xiao, A. Tang, J. Li, X. Wang and E. Zhou, Adv. Mater., 2019, 31, 1804699 CrossRef CAS.
- B. Fan, Z. Zeng, W. Zhong, L. Ying, D. Zhang, M. Li, F. Peng, N. Li, F. Huang and Y. Cao, ACS Energy Lett., 2019, 4, 2466 CrossRef CAS.
- B. Fan, L. Ying, P. Zhu, F. Pan, F. Liu, J. Chen, F. Huang and Y. Cao, Adv. Mater., 2017, 29, 1703906 CrossRef.
- B. Fan, L. Ying, Z. Wang, B. He, X.-F. Jiang, F. Huang and Y. Cao, Energy Environ. Sci., 2017, 10, 1243 RSC.
- Z. Li, L. Ying, P. Zhu, W. Zhong, N. Li, F. Liu, F. Huang and Y. Cao, Energy Environ. Sci., 2019, 12, 157 RSC.
- M. L. Li, B. B. Fan, W. K. Zhong, Z. M. Y. Zeng, J. K. Xu and L. Ying, Chin. J. Polym. Sci., 2020, 38, 791 CrossRef CAS.
- L. Zhu, W. Zhong, C. Qiu, B. Lyu, Z. Zhou, M. Zhang, J. Song, J. Xu, J. Wang, J. Ali, W. Feng, Z. Shi, X. Gu, L. Ying, Y. Zhang and F. Liu, Adv. Mater., 2019, 31, 1902899 CrossRef CAS.
- Z.-G. Zhang, Y. Yang, J. Yao, L. Xue, S. Chen, X. Li, W. Morrison, C. Yang and Y. Li, Angew. Chem., Int. Ed., 2017, 56, 13503 CrossRef CAS.
- Y. Meng, J. Wu, X. Guo, W. Su, L. Zhu, J. Fang, Z.-G. Zhang, F. Liu, M. Zhang, T. P. Russell and Y. Li, Sci. China: Chem., 2019, 62, 845 CrossRef CAS.
- Q. Fan, W. Su, S. Chen, T. Liu, W. Zhuang, R. Ma, X. Wen, Z. Yin, Z. Luo, X. Guo, L. Hou, K. Moth-Poulsen, Y. Li, Z. Zhang, C. Yang, D. Yu, H. Yan, M. Zhang and E. Wang, Angew. Chem., Int. Ed., 2020, 59, 1 CrossRef.
- H. Yao, L. K. Ma, H. Yu, J. Yu, P. C. Y. Chow, W. Xue, X. Zou, Y. Chen, J. Liang, L. Arunagiri, F. Gao, H. Sun, G. Zhang, W. Ma and H. Yan, Adv. Energy Mater., 2020, 10, 2001408 CrossRef CAS.
- J. Du, K. Hu, L. Meng, I. Angunawela, J. Zhang, S. Qin, A. Liebman-Pelaez, C. Zhu, Z. Zhang, H. Ade and Y. Li, Angew. Chem., Int. Ed., 2020, 59, 15181 CrossRef CAS.
- J. Wu, Y. Meng, X. Guo, L. Zhu, F. Liu and M. Zhang, J. Mater. Chem. A, 2019, 7, 16190 RSC.
- A. Tang, J. Li, B. Zhang, J. Peng and E. Zhou, ACS Macro Lett., 2020, 9, 706 CrossRef CAS.
- Z. Yin, Y. Wang, Q. Guo, L. Zhu, H. Liu, J. Fang, X. Guo, F. Liu, Z. Tang, M. Zhang and Y. Li, J. Mater. Chem. C, 2020, 10.1039/D0TC03303K.
- T. Jia, J. Zhang, W. Zhong, Y. Liang, K. Zhang, S. Dong, L. Ying, F. Liu, X. Wang, F. Huang and Y. Cao, Nano Energy, 2020, 72, 104718 CrossRef CAS.
- W. Wang, Q. Wu, R. Sun, J. Guo, Y. Wu, M. Shi, W. Yang, H. Li and J. Min, Joule, 2020, 4, 1070 CrossRef CAS.
- H. Sun, H. Yu, Y. Shi, J. Yu, Z. Peng, X. Zhang, B. Liu, J. Wang, R. Singh, J. Lee, Y. Li, Z. Wei, Q. Liao, Z. Kan, L. Ye, H. Yan, F. Gao and X. Guo, Adv. Mater., 2020, 32, 2004183 CrossRef CAS.
- Y. Wu, Q. Wu, W. Wang, R. Sun and J. Min, Sol. RRL, 2020, 4, 2000409 CrossRef CAS.
- Q. Fan, Q. An, Y. Lin, Y. Xia, Q. Li, M. Zhang, W. Su, W. Peng, C. Zhang, F. Liu, L. Hou, W. Zhu, D. Yu, M. Xiao, E. Moons, F. Zhang, T. D. Anthopoulos, O. Inganäs and E. Wang, Energy Environ. Sci., 2020, 13, 5017 RSC.
- F. Peng, K. An, W. Zhong, Z. Li, L. Ying, N. Li, Z. Huang, C. Zhu, B. Fan, F. Huang and Y. Cao, ACS Energy Lett., 2020, 5, 3702 CrossRef CAS.
- Z. Ma, B. Zhao, Y. Gong, J. Deng and Z. A. Tan, J. Mater. Chem. A, 2019, 7, 22826 RSC.
- X. Liao, L. Zhang, X. Hu, L. Chen, W. Ma and Y. Chen, Nano Energy, 2017, 41, 27 CrossRef CAS.
- S. Li, H. Zhang, W. Zhao, L. Ye, H. Yao, B. Yang, S. Zhang and J. Hou, Adv. Energy Mater., 2016, 6, 1501991 CrossRef.
- H. Jung, A. R. Jung, S.-M. Jin, S. Kim, H. Heo, H. V. T. Nguyen, M. J. Kim, P. Ahn, M. H. Kim, Y. Lee, K.-K. Lee, J. H. Cho, E. Lee and B. Kim, Nano Energy, 2020, 77, 105106 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0cc07213c |
| This journal is © The Royal Society of Chemistry 2021 |