Histidine orientation in artificial peroxidase regioisomers as determined by paramagnetic NMR shifts†
Abstract
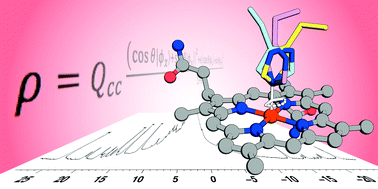
Fe-Mimochrome VI*a is a synthetic peroxidase and peroxygenase, featuring two different peptides that are covalently-linked to deuteroheme. To perform a systematic structure/function correlation, we purposely shortened the distance between the distal peptide and the heme, allowing for the separation and characterization of two regioisomers. They differ in both His axial-ligand orientation, as determined by paramagnetic NMR shifts, and activity. These findings highlight that synthetic metalloenzymes may provide an efficient tool for disentangling the role of axial ligand orientation over peroxidase activity.
- This article is part of the themed collection: Bioinspired metal complexes for chemical transformations and catalysis


 Please wait while we load your content...
Please wait while we load your content...