Biological sealing and integration of a fibrinogen-modified titanium alloy with soft and hard tissues in a rat model†
Abstract
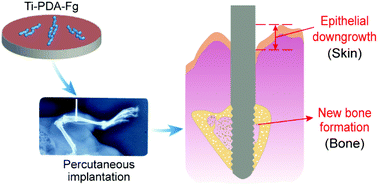
Percutaneous or transcutaneous devices are important and unique, and the corresponding biological sealing at the skin-implant interface is the key to their long-term success. Herein, we investigated the surface modification to enhance biological sealing, using a metal sheet and screw bonded by biomacromolecule fibrinogen mediated via pre-deposited synthetic macromolecule polydopamine (PDA) as a demonstration. We examined the effects of a Ti–6Al–4V titanium alloy modified with fibrinogen (Ti–Fg), PDA (Ti–PDA) or their combination (Ti–PDA–Fg) on the biological sealing and integration with skin and bone tissues. Human epidermal keratinocytes (HaCaT), human foreskin fibroblasts (HFF) and preosteoblasts (MC3T3-E1), which are closely related to percutaneous implants, exhibited better adhesion and spreading on all the three modified sheets compared with the unmodified alloy. After three-week subcutaneous implantation in Sprague-Dawley (SD) rats, the Ti–PDA–Fg sheets could significantly attenuate the soft tissue response and promote angiogenesis compared with other groups. Furthermore, in the model of percutaneous tibial implantation in SD rats, the Ti–PDA–Fg screws dramatically inhibited epithelial downgrowth and promoted new bone formation. Hence, the covalent immobilization of fibrinogen through the precoating of PDA is promising for enhanced biological sealing and osseointegration of metal implants with soft and hard tissues, which is critical for an orthopedic percutaneous medical device.


 Please wait while we load your content...
Please wait while we load your content...