Open Access Article
Open Access Article18F-Labeled magnetic nanovectors for bimodal cellular imaging†
Markus B.
Schütz
a,
Alexander M.
Renner
a,
Shaista
Ilyas
 a,
Khan
Lê
a,
Mehrab
Guliyev
b,
Philipp
Krapf
b,
Bernd
Neumaier
a,
Khan
Lê
a,
Mehrab
Guliyev
b,
Philipp
Krapf
b,
Bernd
Neumaier
 b and
Sanjay
Mathur
b and
Sanjay
Mathur
 *a
*a
aInstitute of Inorganic Chemistry, University of Cologne, D-50939 Cologne, Germany. E-mail: sanjay.mathur@uni-koeln.de; Tel: +49 221 470 5627
bInstitute of Neuroscience and Medicine-Nuclear Chemistry (INM-5), Forschungszentrum Jülich, D-52428 Jülich, Germany
First published on 25th May 2021
Abstract
Surface modification of nanocarriers enables selective attachment to specific molecular targets within a complex biological environment. Besides the enhanced uptake due to specific interactions, the surface ligands can be utilized for radiolabeling applications for bimodal imaging ensured by positron emission topography (PET) and magnetic resonance imaging (MRI) functions in one source. Herein, we describe the surface functionalization of magnetite (Fe3O4) with folic acid as a target vector. Additionally, the magnetic nanocarriers were conjugated with appropriate ligands for subsequent copper-catalyzed azide–alkyne cycloaddition or carbodiimide coupling reactions to successfully achieve radiolabeling with the PET-emitter 18F. The phase composition (XRD) and size analysis (TEM) confirmed the formation of Fe3O4 nanoparticles (6.82 nm ± 0.52 nm). The quantification of various surface functionalities was performed by Fourier-transform infrared spectroscopy (FT-IR) and ultraviolet-visible microscopy (UV-Vis). An innovative magnetic-HPLC method was developed in this work for the determination of the radiochemical yield of the 18F-labeled NPs. The as-prepared Fe3O4 particles demonstrated high radiochemical yields and showed high cellular uptake in a folate receptor overexpressing MCF-7 cell line, validating bimodal imaging chemical design and a magnetic HPLC system. This novel approach, combining folic acid-capped Fe3O4 nanocarriers as a targeting vector with 18F labeling, is promising to apply this probe for bimodal PET/MR-studies.
1. Introduction
Nanoparticles (NPs) of various compositions (e.g. metals, oxides, and lanthanide-doped) have gained significant attention in the field of biomedical imaging.1–10 They are mainly used as contrast agents11–16 following a significant function in numerous emerging applications such as photodynamic therapy, hyperthermia based cancer treatments or magnetic resonance imaging (MRI).17 Magnetic NPs are favored as MRI contrast agents18 due to their low cytotoxicity in the human body as validated by various clinical studies.19–21 To this end, multimodal imaging and simultaneous therapy can provide complementary information for precise diagnosis and imaging-guided focused tumor therapy, which points out the need for dual-action probes with integrated imaging and therapeutic functions. Positron emission tomography22–25 (PET) using positron emitters such as 11C, 13N, 18F or 68Ga is widely used in clinical practice for tumor detection or the elucidation of neurological disorders.23,24,26–33 Fluor-18 is the most frequently applied radionuclide in diagnosis due to its favorable decay properties with a half-life of 109.8 min and low β+-energy and should also be suitable for the labeling of NPs.30,34–41The common strategies for producing radioactively labeled nanoparticles include either the labeling of the particle core or of the particle shell. For example, the core of iron oxide nanoparticles can be radioactively labeled by the nuclear reaction of 58Fe(n,γ)59Fe; however, due to the natural isotopic distribution of iron (91.72% 56Fe, 2.2% 57Fe and 0.28% 58Fe), the labeling yield is very low, and the irradiation times are very long.42 A promising alternative involves the co-precipitation of radioactive 59Fe salts for synthesis, where the advantage lies in the high half-life time of 59Fe (t1/2 = 45d).43 A more versatile method is the radioactive labeling of the organic periphery through surface-attached biomolecules, antibodies and other target ligands. Devaraj et al. reported on a synthetic route for the radioactive labeling of magnetic iron oxide nanoparticles with [18F]Fluoride in which cross-linked dextran superparamagnetic iron oxide nanoparticles were modified with an 18F-PEG3 radiotracer.30 However, the combination of magnetic iron oxide nanoparticles and the [18F]Fluoride radiotracer remains elusive due to the orthogonality of functional characteristics and prerequisites of the biomedical imaging protocols.
Folate receptors (glycophosphatidylinositol, FRs) are recognized as a useful therapeutic site due to their overexpression in many tumor sites including those in lung, colon, breast and ovarian cancers.44 This membrane protein binds to folic acid with high affinity and facilitates its intracellular transport via the endocytic process. Consequently, folic acid has been considered as a potential target ligand for the directed delivery of their payloads to cancer cells.44,45 We report here on alkyne-functionalized magnetic nanoparticles for subsequent “click” conjugation46 for radiolabeling exhibiting promising labeling yields and site-specific cellular uptake. The carbodiimide coupling reaction was performed on the surface of magnetic carriers to attach folic acid for directing the dual-action labels to the site of interest. In addition, we also report for the first time an innovative purification strategy that efficiently separates nanoparticles from starting materials and unbound radioactive molecules to visualize radioactively labeled particles. The dual-mode labels can be used for magnetic resonance imaging (MRI) and positron emission tomography (PET) studies to demonstrate their potential in selective targeting and bimodal imaging.
2. Results and discussion
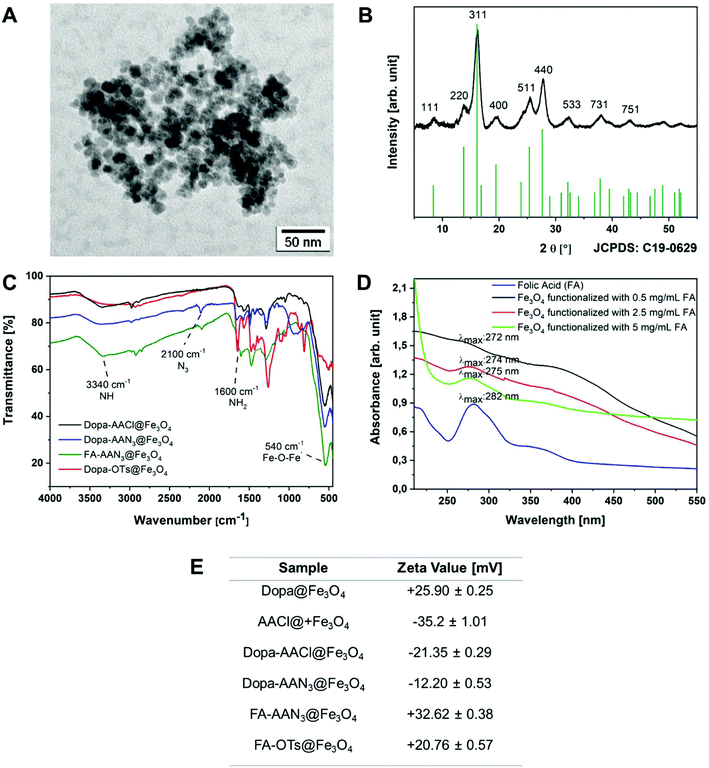
Magnetite (Fe3O4) nanoparticles with different surface functionalities were synthesized by the hydrothermal decomposition of an iron(III) salt in the presence of sodium ascorbate as the surfactant and reducing agent, 4-(chloroacetyl)catechol (AACl) and dopamine (Fig. 1, step 1). The in situ reduction of the Fe(III) species (to Fe(II)) was controlled by adding a stoichiometric amount of the reducing agent. The catechol function of the ligand molecules is essential for the functionalization since it has a strong binding affinity to iron oxides that enabled a stable surface attachment.47 Folic acid was coupled to dopamine amino groups exploiting carbodiimide chemistry (Fig. 1, step 3) as a targeting molecule for cancer cells with overexpressed folate receptors. The chloride of the AACl was substituted by an azide group (Fig. 1, step 2) for the subsequent azide–alkyne cycloaddition reaction. Two different pathways were explored for the radiolabelling. In the first approach, the tosylate (OTs) leaving group needed for subsequent labeling with 18F (Fig. 1, step 5) was conjugated by coupling pent-4-ynyl tosylate to the introduced azide moiety by a copper-catalyzed azide–alkyne cycloaddition (Fig. 1, steps 4 + 5) and the radiolabelling was conducted afterwards. For the other pathway, the radiolabelling building block (18F-pent-4-ynyl) was pre-synthesized separately and then coupled to the azide bound to the nanoparticles (Fig. 1, step 2) using the same copper-catalyzed cycloaddition as before.The as-obtained nanoparticles displayed a spherical shape that was verified by transmission electron microscopy showing an average size of 6.82 nm ± 0.52 nm. The observed agglomeration in the coated iron oxide nanoparticles is possibly due to their ultra-small size and the inherent magnetic properties of Fe3O4 (Fig. 2A). The powder X-ray diffraction data confirmed the formation of phase pure Fe3O4 particles (Fig. 2B). The NPs showed enhanced colloidal stability after surface conjugation with dopamine and 4-(chloroacetyl) catechol ligands displaying an average hydrodynamic radius of 171.10 nm ± 2.51 nm as determined by DLS measurements. The infrared spectra displayed the (Fig. 2C) vibrational bands corresponding to both surface ligands. The Fe3O4 NPs coated with only dopamine possessed a ζ-potential in a positive range (25.90 mV ± 0.25 mV) due to the presence of protonated amino groups on the surface, whereas after modification with AACl, the surface ζ-potential shifted to negative values (−35.2 mV ± 1.01 mV). Consequently, the surface modification of NPs with both dopamine and AACl ligands led to a less negative ζ-potential of −21.35 mV ± 0.29 mV (Fig. 2E).
The terminal chloride groups of the AAC molecules attached on the surface were replaced by azide groups in a SN2 nucleophilic substitution reaction. The substitution reaction was confirmed by the observation of the characteristic azide vibration band at a wavenumber of 2100 cm−1 (Fig. 2C). Additionally, the ζ-potential change from −21.35 mV ± 0.29 mV to −12.20 mV ± 0.53 mV also suggested a change in the surface chemistry upon individual functionalization steps (Fig. 2E). The presence of surface-terminating azide groups was necessary to conjugate alkynated-18F for the radiolabeling study following the click-chemistry protocol. Furthermore, the amino functionality was used to perform a carbodiimide coupling reaction for the attachment of folic acid which is a UV active targeting ligand. In the consequent steps, the NPs were tested for different concentrations of folic acid (0.25 mg mL−1, 0.5 mg mL−1, 2.5 mg mL−1, 5 mg mL−1) by performing conjugation reactions under similar reaction conditions. Different amounts of folic acid units immobilized on the surface of the nanoparticles were verified by absorption spectra and through the changes in the UV/Vis maxima as a function of the initial quantity of the ligand, as is evident in Fig. 2D. The intensity of λmax in the wavelength range of 272 nm to 282 nm showed a significant decrease depending upon the additional folic acid in the reaction. The ζ-potential changed from −12.20 mV ± 0.53 mV to 32.62 mV ± 0.38 mV indicating a significant alteration of the chemical topography of the particles. The folic acid molecule has several –NH2 groups that can be protonated in aqueous solution that accounts for the significant change in the ζ-potential.
In order to perform radiolabeling experiments, azide groups of the modified Fe3O4 particles were used following two different methods. In the first approach, pent-4-ynyl tosylate (tosylate leaving group)48 was coupled to the surface of the nanoparticles using the “click” reaction followed by direct labeling with the radioactive 18F label. The outcome of the reaction was confirmed by ζ-potential and IR measurements. In the IR spectra, the observable decrease of the azide signal at a wavenumber of 2100 cm−1 was visible, indicating the coupling of the azide moiety with alkyne groups of pent-4-ynyl tosylate. The specific bands for the triazole group with a wavenumber between 1290 and 825 cm−1 could not be clearly detected due to an overlap with other bands in the spectra. The ζ-potential shifted from 32.62 mV ± 0.38 mV to 20.76 mV ± 0.57 mV. The magnitude of the ζ-potential indicated the potential high stability of colloidal nanoconjugates. Additionally, the colloidal stability was characterised and was in a range of 60 min to 420 min. The FA-AAN3@Fe3O4 and AACl@Fe3O4 particles showed the highest stability in water.
In the second approach, the magnetic particles were separated from the reaction chamber after the conjugation of 18F labeled pent-4-ynyl tosylate (OTs) followed by the click reaction. The success of the radiolabeling reaction between azide modified particles and alkynated radioactive ligands was controlled using radio-HPLC. The results confirmed that after the purification step by distillation only the radioactive product was present in the reaction chamber that validated the efficacy of the magnetic separation approach.
In comparison with radioactive labeled proteins or small molecules, nanoparticles have solid surfaces and they tend to agglomerate in different solvents due to interparticle interactions. The appropriate use of a conventional HPLC system with a suitable separation column is not effective because a controlled passage through the column cannot be guaranteed for particle agglomerates that poses a high risk of blocking the pores of the whole system. Therefore, in this work an innovative set-up was developed for cleaning magnetic nanoparticles, which could be successfully used for radioactive labeled particles as well. For this purpose, instead of the column, a permanent magnet was added to the HPLC system, while the other parts remained the same as shown in Fig. 3. A tube wrapped around the magnet leads to the accumulation of the magnetic particles next to the magnet. All nonmagnetic starting materials or side products were washed out continuously. After removing the magnet, the collected magnetic particles were washed out from the tube and detected with a scintillation detector. This cleaning setup was evaluated with two other HPLC systems with different magnetic nanoparticles that confirmed its versatility for the separation of magnetic NPs.
 | ||
| Fig. 3 Schematic construction of the HPLC system with a new component for the separation of magnetic nanoparticles. | ||
To evaluate the radioactive yield of the folic acid and 18F labeled nanoparticles, different reaction parameters were developed. It was possible to increase the radiochemical yield up to 15% with acetonitrile as the solvent during the radioactive labeling. A number of other common solvents that are typically used for nanoparticle dispersions were also analyzed to define the most suitable solvent system. The utilization of MeOH (0.3%), DMF (2.4%), DMA (1.7%), diethylether (0.13%), DMSO (0.43%), tert-butyl alcohol (0.7%), EtOH (0.13%), water (0%), ACN/water (7.47%) and ACN/EtOH (3.47%) led to a lower radiochemical yield (Fig. 4).
 | ||
| Fig. 4 Graphical evaluation of the radiochemical yield of 18F labeled magnetite NPs in various solvents. | ||
It is necessary to select an appropriate number of input parameters such as reaction temperature and time to evaluate the effectiveness of the labeling yield. The collective assessment of these parameters showed (Fig. 5) an increase in the labeling efficiency from 0 min to 30 min at a temperature of 80 °C. An increase in the reaction time up to 60 min was found to provide 1.09% higher yield. The increase in the temperature from 80 °C to 110 °C did not affect the yield, which shows that surface conjugation is quantitatively achieved already at 80 °C. In conclusion the radioactive labeling gave the best results in acetonitrile at 80 °C with a reaction time of 30 min as shown in Fig. 5.
 | ||
| Fig. 5 Optimization of the (A) reaction time and (B) reaction temperature for the radioactive labeling of magnetite nanoparticles with 18F in acetonitrile. | ||
After establishing the optimal reaction conditions, the nanoparticles with different concentrations of folic acid surface-ligands were labeled in a one-step approach. The particles with the lowest amount of folic acid (0.50 mg mL−1) showed the weakest radioactive yield (2.5%) after a reaction at 80 °C for 30 min. The results showed that the radioactive yield increases in relation to the amount of folic acid attached on the surface of the nanoparticles (2.50 mg mL−1: 6% RCY, 5.00 mg mL−1: 13% RCY). Therefore, the steric hindrance of folic acid is unlikely to have a significant impact in the examined system, as a reduced amount of folic acid did not increase the radioactive yield.
The one-step labeling offers several benefits in comparison with the multistep labeling approach. Firstly, the RCY is higher (15% compared to 7%) and the overall reaction time is shorter enabling more efficient use of 18F which is a crucial parameter due to the short half-life of the radionuclide. Secondly, the two-step reaction requires an additional cleaning step after the synthesis of the radiolabeled ligand, resulting in higher losses of particles and the decay of 18F.
The versatility of the surface conjugation and magnetic separation approaches developed in this work was demonstrated for another nanoparticle type by using the same surface chemistry and labeling protocols. The γ-Fe2O3 nanoparticles with pronounced magnetic properties and an average size of 250 nm ± 2 nm were synthesized by solution processing to obtain a crystalline material that was confirmed by powder XRD data, which showed the presence of a minor phase that could not be unambiguously detected (Fig. 6A & B). The particles were tested for a click reaction between 4-(azidoacetyl)catechol and pent-4-ynyl tosylate. After the functionalization, IR-measurements showed the introduced catechol ligand with a stretching frequency at a wavenumber of 2100 cm−1 indicating the presence of the azide function (Fig. 6C). After the click reaction this vibration band disappeared due to the transformation of the azide moiety into a triazole group. The signals for the catechol ligand in an area between 1500 and 1750 cm−1 appeared as somewhat blurred bands. The ζ-potential of the nanoparticles shifted from −11.80 mV ± 0.49 mV to −19.50 mV ± 0.51 mV after functionalization with 4-(azidoacetyl)catechol that changed to −28.30 mV ± 1.76 mV after the completion of the click reaction (Fig. 6D).
After radiolabeling with 18F under conditions optimized for magnetite particles, the NPs demonstrated a radiochemical yield of 37.79% ± 2.78%. The higher yield in comparison with that with the folic acid-labeled particles is possibly due to the larger size of the particles and the additional higher amount of azide groups on the surface. In comparison, there are no additional extra dopamine groups on the surface which leads to more OTs leaving groups which can react with the free 18F. The attractive labelling yield for these particles shows the practicability of this method that will be useful for nanomedicine and can be adapted for the functionalization and radiolabelling of several other magnetic particles with different shapes and surface properties.
The cellular uptake of radiolabeled nanoparticles was carried out in triplicate (n = 3) with MCF-7 cancer cells (Fig. 7). The 18F-labeled and folic acid modified Fe3O4 nanoparticles showed a 35.15% ± 0.56% efficiency after one hour and a 26.00% ± 1.46% efficiency after two hours of application. For the control sample 18F-labelled Fe3O4 nanoparticles without folic acid functionalization were used and a lower uptake (2 h: 19.3% ± 0.99%, 1 h: 16.4% ± 4.03%) was observed. The higher cellular uptake for the folic acid functionalized nanoparticles is possibly due to the overexpression of folate receptors in the MCF-7 cells and is proof for the successful functionalization.49 These observations suggest that the cellular uptake of nanoparticles followed receptor mediated endocytosis.
In comparison with the standard tracer [18F]FET which is used especially for brain tumors, the nanoparticle described here showed a higher cellular uptake than the results known for the standard tracer (2 h: 2.73% ± 0.35%, 1 h: 2.28% ± 0.03%) probes, which are evidently much weaker. This demonstrates the potential of radioactive-labelled nanoparticles for cellular imaging and can be tested for the in vivo cellular imaging of cancer cells.
3. Conclusions
Hydrothermally synthesized magnetic nanoparticles (Fe3O4 and γ-Fe2O3) were successfully labelled with 18F-radioactive nuclides and conjugated with folic acid as target ligands following click chemistry protocols. Precisely, a chemical conjugation approach provided a facile pathway to immobilize tosylate groups on the outer surface of carrier nanoparticles for grafting radiolabeled ligands. The 18F-labelled particles were separated and purified in an improved HPLC system developed in this work. Comprehensive characterization of surface-attached functional groups and solution behavior by IR, ζ-potential, and DLS analyses confirmed the presence of amino- and azido-units that could be selectively activated by carbodiimide coupling and cycloaddition reactions to obtain novel dual-action magnetic probes suited for simultaneous MRI and PET imaging. The attachment of different amounts of folic acid units on the surface of nanoparticles by carbodiimide coupling showed that steric hinderance does not play any predominant role in the radiolabeling of nanoparticles and the complementarity of the reaction partners is decisive for obtaining dual-action radioactive magnetic carriers. The challenge of separating the bimodal PET-MRI tracer from the unlabeled nanoparticles and excess ligands was addressed by developing a novel, efficient and economical modification in the HPLC set-up that demonstrated the separation efficiency and detection of purified radiolabeled nanoparticles from the starting materials. This component can be useful for the differentiation of nanoparticles suspended in various organic solvents for radioactive labeling protocols. The study of radiolabeling efficiency in various solvent systems showed acetonitrile to be most promising with the highest radiolabeling yield (>15%) at 80 °C after 30 min. Additionally, the radioactive and folic acid labeled nanoparticles showed higher receptor-mediated endocytosis of particles in comparison with the particles without folic acid used as the reference. Finally, it was demonstrated that co-conjugated nanoparticles bearing target ligands and a PET source have extraordinary potential for cellular imaging as compared to standard radiotracers. The results reported here demonstrate the translational potential of magnetic nanocarriers as bimodal tracer systems that can be further improved by attachment of multiple radiotracers.4. Experimental section
Instrumentation
(1) Dionex Ultimate 3000, Thermo Fisher Scientific with an integrated UV-detector in combination with a radio detector HERM LB 500 (high energy radio monitor) (Berthold Technologies, Bad Wildbad, Germany).
(2) Knauer pump, a Knauer K-2500 UV/VIS detector (Knauer, Berlin, Germany) a manual Rheodyne injector (1 ml loop) and a NaI(Tl) well-type scintillation detector (EG&G Ortec; modul 276 Photomultiplier Base) with an ACE Mate Amplifier and BIAS supply (all from Ortec Ametek, Meerbusch, Germany). Data acquisition and interpretation were performed using Gina software (Raytest).
Methods
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 30 min and redispersed in H2O/EtOH. The Fe3O4 nanospheres were analysed by XRD, IR, DLS/Zeta measurements and images were recorded using SEM/TEM. The particles were used for surface modification with NaN3, folic acid and pent-4-ynyl tosylate and subsequently radiolabelled.
000 rpm for 30 min and redispersed in H2O/EtOH. The Fe3O4 nanospheres were analysed by XRD, IR, DLS/Zeta measurements and images were recorded using SEM/TEM. The particles were used for surface modification with NaN3, folic acid and pent-4-ynyl tosylate and subsequently radiolabelled.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 30 min and redispersed in H2O/EtOH. The Fe3O4 nanospheres were analysed by XRD, IR, DLS/Zeta measurements and images were recorded using SEM/TEM. The particles were used for surface modification with 4-(azidoacetyl)catechol and subsequent radiolabeling.
000 rpm for 30 min and redispersed in H2O/EtOH. The Fe3O4 nanospheres were analysed by XRD, IR, DLS/Zeta measurements and images were recorded using SEM/TEM. The particles were used for surface modification with 4-(azidoacetyl)catechol and subsequent radiolabeling.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 30 min, redispersing the particles in H2O/EtOH after each centrifugation. The surface modified particles were analyzed by XRD, DLS/Zeta and IR spectroscopy. They were used for surface modification with folic acid and pent-4-ynyl tosylate and subsequently radiolabelled.
000 rpm for 30 min, redispersing the particles in H2O/EtOH after each centrifugation. The surface modified particles were analyzed by XRD, DLS/Zeta and IR spectroscopy. They were used for surface modification with folic acid and pent-4-ynyl tosylate and subsequently radiolabelled.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 30 min and redispersed in H2O/EtOH after each step. In the next steps they were used for surface modification with pent-4-ynyl tosylate and subsequently radiolabelled. The particles were analyzed by XRD, DLS/Zeta, IR spectroscopy and UV/vis measurements.
000 rpm for 30 min and redispersed in H2O/EtOH after each step. In the next steps they were used for surface modification with pent-4-ynyl tosylate and subsequently radiolabelled. The particles were analyzed by XRD, DLS/Zeta, IR spectroscopy and UV/vis measurements.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to obtain the product as a pale-yellow oil with a yield of 2.24 g (9.40 mmol, 47%). The product was analyzed by NMR spectroscopy and was used for radiolabeling.
1) to obtain the product as a pale-yellow oil with a yield of 2.24 g (9.40 mmol, 47%). The product was analyzed by NMR spectroscopy and was used for radiolabeling.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 30 min and redispersed in H2O/EtOH after each step. In the next step they were used for radiolabeling with 18F−. The particles were analyzed by XRD, DLS/Zeta and IR spectroscopy.
000 rpm for 30 min and redispersed in H2O/EtOH after each step. In the next step they were used for radiolabeling with 18F−. The particles were analyzed by XRD, DLS/Zeta and IR spectroscopy.
Radiosynthesis
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to acknowledge the financial support and infrastructure provided by the University of Cologne in the frame of the Excellence Strategy and for supporting the UoC-Forum “Transformative Nanocarriers for RNA Transport and Tracking”.References
- L. M. Nieves, J. C. Hsu, K. C. Lau, A. D. A. Maidment and D. P. Cormode, Nanoscale, 2021, 13, 163–174 RSC.
- S. Ilyas, M. Ilyas, R. van der Hoorn and S. Mathur, ACS Nano, 2013, 7, 9655–9663 CrossRef CAS PubMed.
- B. Klębowski, J. Depciuch, M. Parlińska-Wojtan and J. Baran, Int. J. Mol. Sci., 2018, 19, 4031 CrossRef PubMed.
- M. D. Mauricio, S. Guerra-Ojeda, P. Marchio, S. L. Valles, M. Aldasoro, I. Escribano-Lopez, J. R. Herance, M. Rocha, J. M. Vila and V. M. Victor, Oxid. Med. Cell. Longev., 2018, 2018, 6231482 CAS.
- A. Jurewicz, S. Ilyas, J. Uppal, I. Ivandic, S. Korsching and S. Mathur, ACS Appl. Nano Mater., 2020, 3, 1621–1629 CrossRef CAS.
- L. Labrador-Páez, E. C. Ximendes, P. Rodríguez-Sevilla, D. H. Ortgies, U. Rocha, C. Jacinto, E. Martín Rodríguez, P. Haro-González and D. Jaque, Nanoscale, 2018, 10, 12935–12956 RSC.
- A. M. Renner, S. Ilyas, K. Wennhold, A. Szymura, S. Roitsch, H. A. Schlößer and S. Mathur, Langmuir, 2020, 36, 14819–14828 CrossRef CAS PubMed.
- M. B. Schütz, S. Ilyas, K. Lê, M. Valldor and S. Mathur, ACS Appl. Nano Mater., 2020, 3, 5936–5943 CrossRef.
- R. Haldavnekar, K. Venkatakrishnan and B. Tan, Nat. Commun., 2018, 9, 3065 CrossRef PubMed.
- B. Lin, J. Wu, Y. Wang, S. Sun, Y. Yuan, X. Tao and R. Lv, Biomater. Sci., 2021, 9, 1000–1007 RSC.
- S. M. Siribbal, J. Schläfer, S. Ilyas, Z. Hu, K. Uvdal, M. Valldor and S. Mathur, Cryst. Growth Des., 2018, 18, 633–641 CrossRef CAS.
- D. P. Cormode, P. C. Naha and Z. A. Fayad, Contrast Media Mol. Imaging, 2014, 9, 37–52 CrossRef CAS PubMed.
- A. Dash, B. Blasiak, B. Tomanek, A. Banerjee, S. Trudel, P. Latta and F. C. J. M. van Veggel, ACS Appl. Nano Mater., 2021, 4, 1235–1242 CrossRef CAS.
- J. Kim, P. Chhour, J. Hsu, H. I. Litt, V. A. Ferrari, R. Popovtzer and D. P. Cormode, Bioconjugate Chem., 2017, 28, 1581–1597 CrossRef CAS PubMed.
- A. L. Bernstein, A. Dhanantwari, M. Jurcova, R. Cheheltani, P. C. Naha, T. Ivanc, E. Shefer and D. P. Cormode, Sci. Rep., 2016, 6, 26177 CrossRef CAS PubMed.
- J. Wang, Y. Jia, Q. Wang, Z. Liang, G. Han, Z. Wang, J. Lee, M. Zhao, F. Li, R. Bai and D. Ling, Adv. Mater., 2021, 33, 2004917 CrossRef CAS PubMed.
- J. Jeevanandam, A. Barhoum, Y. S. Chan, A. Dufresne and M. K. Danquah, Beilstein J. Nanotechnol., 2018, 9, 1050–1074 CrossRef CAS PubMed.
- R. A. Revia and M. Zhang, Mater. Today, 2016, 19, 157–168 CrossRef CAS PubMed.
- G. Jarockyte, E. Daugelaite, M. Stasys, U. Statkute, V. Poderys, T.-C. Tseng, S.-H. Hsu, V. Karabanovas and R. Rotomskis, Int. J. Mol. Sci., 2016, 17, 1193 CrossRef PubMed.
- Q. Feng, Y. Liu, J. Huang, K. Chen, J. Huang and K. Xiao, Sci. Rep., 2018, 8, 2082 CrossRef PubMed.
- M. A. Abakumov, A. S. Semkina, A. S. Skorikov, D. A. Vishnevskiy, A. V. Ivanova, E. Mironova, G. A. Davydova, A. G. Majouga and V. P. Chekhonin, J. Biochem. Mol. Toxicol., 2018, 32, e22225 CrossRef PubMed.
- S. Berke, A.-L. Kampmann, M. Wuest, J. J. Bailey, B. Glowacki, F. Wuest, K. Jurkschat, R. Weberskirch and R. Schirrmacher, Bioconjugate Chem., 2018, 29, 89–95 CrossRef CAS PubMed.
- B. D. Zlatopolskiy, J. Zischler, D. Schäfer, E. A. Urusova, M. Guliyev, O. Bannykh, H. Endepols and B. Neumaier, J. Med. Chem., 2018, 61, 189–206 CrossRef CAS PubMed.
- L. Feni, M. A. Omrane, M. Fischer, B. D. Zlatopolskiy, B. Neumaier and I. Neundorf, Pharmaceuticals, 2017, 10, 99 CrossRef PubMed.
- E. J. Keliher, T. Reiner, G. M. Thurber, R. Upadhyay and R. Weissleder, ChemistryOpen, 2012, 1, 177–183 CrossRef CAS PubMed.
- A. R. Jalilian and J. Osso Jr., Iran. J. Nucl. Med., 2017, 25, 1–10 CAS.
- C. J. Anderson and R. Ferdani, Cancer Biother. Radiopharm., 2009, 24, 379–393 CrossRef CAS PubMed.
- A. Sasikumar, A. Joy, M. R. A. Pillai, R. Nanabala and B. Thomas, Clin. Nucl. Med., 2017, 42, e126–e127 CrossRef PubMed.
- E. A. Aalbersberg, B. J. de Wit-van der Veen, M. W. J. Versleijen, L. J. Saveur, G. D. Valk, M. E. T. Tesselaar and M. P. M. Stokkel, Eur. J. Nucl. Med. Mol. Imaging, 2019, 46, 696–703 CrossRef CAS PubMed.
- N. K. Devaraj, E. J. Keliher, G. M. Thurber, M. Nahrendorf and R. Weissleder, Bioconjugate Chem., 2009, 20, 397–401 CrossRef CAS PubMed.
- L.-L. Zhang, W.-C. Li, Z. Xu, N. Jiang, S.-M. Zang, L.-W. Xu, W.-B. Huang, F. Wang and H.-B. Sun, Eur. J. Nucl. Med. Mol., 2021, 48, 483–492 CrossRef CAS PubMed.
- R. J. Hicks, P. Jackson, G. Kong, R. E. Ware, M. S. Hofman, D. A. Pattison, T. Akhurst, E. Drummond, P. Roselt, J. Callahan, R. Price, C. Jeffery, E. Hong, W. Noonan, A. Herschtal, L. J. Hicks, M. Harris, A. Hedt, B. M. Paterson and P. Donnelly, J. Nucl. Med., 2019, 60, 777–785 CrossRef CAS PubMed.
- H. D. Zacho, R. F. Fonager, J. B. Nielsen, C. Haarmark, H. W. Hendel, M. B. Johansen, J. C. Mortensen and L. J. Petersen, J. Nucl. Med., 2020, 61, 344–349 CrossRef CAS PubMed.
- C. Bouter and Y. Bouter, Front. Med., 2019, 6, 71 CrossRef.
- P. K. Garg, S. J. Lokitz, L. Truong, B. Putegnat, C. Reynolds, L. Rodriguez, R. Nazih, J. Nedrelow, M. de la Guardia, J. K. Uffman, S. Garg and P. S. Thornton, PLoS One, 2017, 12, e0186340 CrossRef PubMed.
- J. W. Kiser, J. R. Crowley, D. A. Wyatt and R. K. Lattanze, Front. Med., 2018, 5, 143 CrossRef PubMed.
- G. Luurtsema, H. H. Boersma, M. Schepers, A. M. T. de Vries, B. Maas, R. Zijlma, E. F. J. de Vries and P. H. Elsinga, EJNMMI Radiopharm. Chem., 2016, 1, 7 CrossRef PubMed.
- M. Pretze, C. Wängler and B. Wängler, Biomed Res. Int., 2014, 2014, 674063 CrossRef CAS PubMed.
- T. H. Ribeiro, R. S. Filho, A. C. G. Castro, E. Paulino and M. Mamede, Rev. Assoc. Med. Bras., 2017, 63, 109–111 CrossRef PubMed.
- Z. Sun, K. Cheng, F. Wu, H. Liu, X. Ma, X. Su, Y. Liu, L. Xia and Z. Cheng, Nanoscale, 2016, 8, 19644–19653 RSC.
- M. Takeuchi, T. Nihashi, A. Gafter-Gvili, F. J. García-Gómez, E. Andres, D. Blockmans, M. Iwata and T. Terasawa, Medicine, 2018, 97, e12909 CrossRef PubMed.
- M.-T. Zhu, W.-Y. Feng, Y. Wang, B. Wang, M. Wang, H. Ouyang, Y.-L. Zhao and Z.-F. Chai, Toxicol. Sci., 2009, 107, 342–351 CrossRef CAS PubMed.
- R. Weissleder, D. D. Stark, B. L. Engelstad, B. R. Bacon, C. C. Compton, D. L. White, P. Jacobs and J. Lewis, Am. J. Roentgenol., 1989, 152, 167–173 CrossRef CAS PubMed.
- S. Ilyas, N. K. Ullah, M. Ilyas, K. Wennhold, M. Iqbal, H. A. Schlößer, M. S. Hussain and S. Mathur, ACS Biomater. Sci. Eng., 2020, 6, 6138–6147 CrossRef CAS PubMed.
- L. Wortmann, S. Ilyas, D. Niznansky, M. Valldor, K. Arroub, N. Berger, K. R. J. Holmes and S. Mathur, ACS Appl. Mater. Interfaces, 2014, 6, 16631–16642 CrossRef CAS PubMed.
- A. Szymura, S. Ilyas, M. Horn, I. Neundorf and S. Mathur, J. Mol. Liq., 2020, 317, 114002 CrossRef CAS.
- K. V. Korpany, D. D. Majewski, C. T. Chiu, S. N. Cross and A. S. Blum, Langmuir, 2017, 33, 3000–3013 CrossRef CAS PubMed.
- M. Pretze, D. Pietzsch and C. Mamat, Molecules, 2013, 18, 8618–8665 CrossRef CAS PubMed.
- J. P. Marshalek, P. S. Sheeran, P. Ingram, P. A. Dayton, R. S. Witte and T. O. Matsunaga, J. Controlled Release, 2016, 243, 69–77 CrossRef CAS PubMed.
- P. Patra, S. Mitra, N. Debnath, P. Pramanik and A. Goswami, Bull. Mater. Sci., 2014, 37, 199–206 CrossRef CAS.
- A. M. Klester and C. Ganter, Helv. Chim. Acta, 1983, 66, 1200–1209 CrossRef CAS.
- K. Hamacher and H. H. Coenen, Appl. Radiat. Isot., 2002, 57, 853–856 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1bm00616a |
| This journal is © The Royal Society of Chemistry 2021 |