Open Access Article
Open Access ArticleTriple-target stimuli-responsive anti-COVID-19 face mask with physiological virus-inactivating agents
Werner E. G.
Müller
 *ab,
Meik
Neufurth
*ab,
Meik
Neufurth
 a,
Ingo
Lieberwirth
a,
Ingo
Lieberwirth
 c,
Rafael
Muñoz-Espí
c,
Rafael
Muñoz-Espí
 d,
Shunfeng
Wang
d,
Shunfeng
Wang
 a,
Heinz C.
Schröder
ab and
Xiaohong
Wang
a,
Heinz C.
Schröder
ab and
Xiaohong
Wang
 *a
*a
aERC Advanced Investigator Grant Research Group at the Institute for Physiological Chemistry, University Medical Center of the Johannes Gutenberg University, Duesbergweg 6, 55128 Mainz, Germany. E-mail: wmueller@uni-mainz.de; wang013@uni-mainz.de
bNanotecMARIN GmbH, D-55128 Mainz, Germany
cMax Planck Institute for Polymer Research, Ackermannweg 10, D-55128 Mainz, Germany
dInstitute of Materials Science (ICMUV), Universitat de València, C/Catedràtic José Beltrán 2, 46980 Paterna, València, Spain
First published on 30th June 2021
Abstract
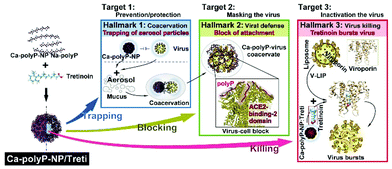
Conventional face masks to prevent SARS-CoV-2 transmission are mostly based on a passive filtration principle. Ideally, anti-COVID-19 masks should protect the carrier not only by size exclusion of virus aerosol particles, but also be able to capture and destroy or inactivate the virus. Here we present the proof-of-concept of a filter mat for such a mask, which actively attracts aerosol droplets and kills the virus. The electrospun mats are made of polycaprolactone (PCL) a hydrophilic, functionalizable and biodegradable polyester, into which inorganic polyphosphate (polyP) a physiological biocompatible, biodegradable and antivirally active polymer (chain length, ∼40 Pi units) has been integrated. A soluble Na-polyP as well as amorphous calcium polyP nanoparticles (Ca-polyP-NP) have been used. In this composition, the polyP component of the polyP-PCL mats is stable in aqueous protein-free environment, but capable of transforming into a gel-like coacervate upon contact with divalent cations and protein like mucin present in (virus containing) aerosol droplets. In addition, the Ca-polyP-NP are used as a carrier of tretinoin (all-trans retinoic acid) which blocks the function of the SARS-CoV-2 envelope (E) protein, an ion channel forming viroporin. The properties of this novel mask filter mats are as follows: First, to attract and to trap virus-like particles during the polyP coacervate formation induced in situ by aerosol droplets on the spun PCL fibers, as shown here by using SARS-CoV-2 mimicking fluorescent nanoparticles. Second, after disintegration the NP by the aerosol-mucus constituents, to release polyP that binds to and abolishes the function of the receptor binding domain of the viral spike protein. Third, to destroy the virus by releasing tretinoin, as shown by the disruption of virus-mimicking liposomes with the integrated recombinant viral viroporin. It is proposed that these properties, which are inducible (stimuli responsive), will allow the design of antiviral masks that are smart.
Introduction
With the outbreak of the COVID-19 disease, caused by SARS-CoV-2,1 in December 2019 the epidemic grew to a pandemic in March 2020, hitting (almost) all countries on the globe.2 This respiratory virus is transmitted through droplets of different sizes upon sneezing, coughing, and seldom by close contact breathing out.3 Based on the route of transmission most guidelines recommend masks to prevent droplet transmission and aerosol spread.4,5 These fine particles are spread through coughing but also talking, especially at increased sound level (Anfinrud 2020). The average size of the droplets has mostly a threshold at a minimum between 5 to 10 μm (ref. 5) and contains SARS-CoV-2 with a size 70 to 90 nm.6It is out of any doubt that wearing face masks, both cloth masks and surgical masks, is an efficient solution to control the pandemic.7 Many types of face masks have been fabricated and tested for compliance and effectiveness.8 Most of them are fabricated of polyurethane, polyester, polyester/nylon and polypropylene.9
It is the aim of this contribution to introduce a new generation of face masks, powered with a fleece/non-woven filter material that is distinguished from a conventional non-woven fleece. The novel fleece comprises the property to attract nanoparticles (mimicking SARS-CoV-2) and also to destroy the activity of the viral SARS-CoV-2/influenza A virus Matrix protein 2 (M2) envelope (E) protein required for virus envelope formation, assembly, budding, and pathogenesis.10,11 This viral E-protein has been grouped to the ion-guiding viroporins,10 which oligomerize to ion channels through which ions but also other small solutes can pass through.11 In SARS-CoV-2, the E protein assembles to a homopentameric cation channel that is crucially important for virus pathogenicity.12 This bipartite channel binds to potential antiviral drugs.13,14 Recently, based on virtual database screening, tretinoin (all-trans retinoic acid) was found to accumulate within the lumen of the channel.15
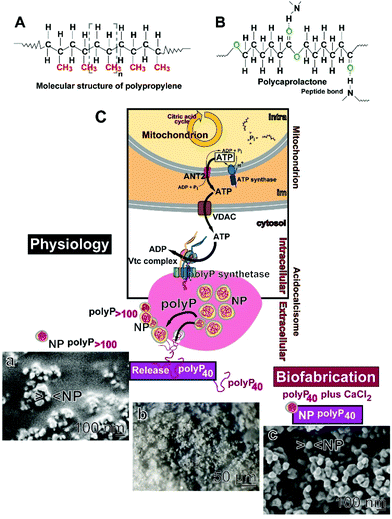
Polypropylene (Fig. 1A), one of the most commonly used polymers, is largely inert in the absence of any additive.16 In contrast to this material, polycaprolactone (PCL; poly[ε-caprolactone]) is a biodegradable but chemically resistant polyester, which is compatible with a number of other materials;17Fig. 1B. In nature, PCL is completely enzymatically/hydrolytically degraded after 16 weeks.18 PCL forms intermolecular hydrogen bonds with its carboxylic acid ester group and biomolecules, preferentially with peptide bonds.19 In addition, PCL can be functionalized, e.g. by cell-adhesive biomolecules,20 with hyaluronic acid or β-TCP.21 In addition and in contrast to polypropylene,22 PCL can be conveniently used as a polymer for electrospinning.23 During the electrospinning process nanoparticles, like amorphous polyphosphate (polyP) nanoparticles (NP), can be included into the material. Those particles can be loaded with bioactive molecules, like retinol24 or ascorbic acid as well as with dexamethasone.25
PolyP is a suitable polymer both for 3D printing26 or PCL-based electrospinning.23 This polymer is physiological and comprises the properties to be biocompatible, biodegradable and also regeneratively active.27 PolyP is synthesized in large amounts in the platelets and there in the acidocalcisomes.28–31 Intracellularly, polyP is produced from ATP which is generated in mitochondria;32,33 (Fig. 1C). Two polyP fractions are produced; short-chain (chain length below 100 Pi units) and long-chain polyP (above 100 Pi units). The short-chain polyP fraction remains soluble, while the long-chain fraction becomes encapsulated into polyP nanoparticles.34 Only the long-chain polyP fraction affects the blood clotting cascade, while the short-chain polyP does not have this property.35,36 The short-chain polymer even lowers the velocity of the cascade because it chelates Ca2+ and lowers thromboxane A2 during platelet aggregation.37
For the present experiments the polyP preparation with the short-chain length of ∼40 Pi units was used (Fig. 1C-b). Such a polyP material is that which is present in the circulating blood where it is functionally active.30 In addition, this polyP fraction was encapsulated into nanoparticles (NP) used as a depot form, which is likewise morphogenetically active and prone to hydrolysis by alkaline phosphatase (ALP).38 Those NP are more homogeneous (Fig. 1C-c) than those which are prepared from polyP with a chain-length of >100 Pi units (Fig. 1C-a). In the presence of ALP and of adenylate kinase (ADK), both enzymes exist in the mucus of the airway system,39 ADP and then ATP is formed.33,40
A distinguished feature of polyP is its property to undergo coacervation at physiological pH together with divalent cations.41 This phase, which is formed by liquid–liquid phase separation of nano-sized droplets, is a biocompatible state of polyP and allows an active as well as a passive import of particles and cells. During this process, in the presence of serum, the ζ potential drops and turns to values close to zero. Previously, this shift has been attributed to an interaction of the particles with peptides/proteins.42
In the present study three principles were used to eliminate SARS-CoV-2 present in aerosol droplets from the breathing air. First, the virus particles are trapped and cached onto the PCL spun fibers during the formation of a coacervate, second, the receptor-binding domain of the virus spike protein is inactivated by masking with antiviral polyP, and third, the virus particles are destroyed by exploiting the viroporin function of the viral envelope (E) protein.10 This envelope protein was integrated in liposomes under fabrication of virus-mimicking liposomes. In parallel, tretinoin was co-precipitated together with Na-polyP in the presence of Ca2+ under fabrication of nanoparticles.25
Both polyP and tretinoin are highly stable components at temperatures below 50 °C for a period of at least 6 months under dry conditions. No significant decomposition is measured.43–45
The experiments showed that addition of the nanoparticles, of Ca-polyP-NP with embedded tretinoin, to the virus-mimicking liposomes causes a destruction/fragmentation of the envelope protein supplemented liposomes.
Results and discussion
Fabrication/electrospinning of the facemask filters: the components
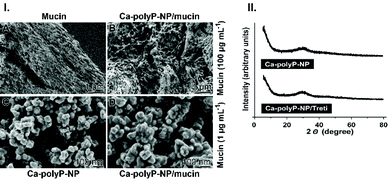
To prepare the filter mats two main components are required. First, the stabilizing scaffold and secondly, its functionalized surfaces with the antiviral properties.The Ca2+ salt, the amorphous nanoparticles, “Ca-polyP-NP”, is the depot form, the carrier of the soluble polyP cargo, which can be supplemented with other anionic components. For the fabrication of the “Ca-polyP-NP”, the introduced procedure was used.38 The NP were prepared at a superstoichiometric 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio between CaCl2 and Na-polyP at pH 10. The size of the particles is between 60 and 90 nm in diameter (Fig. 2.I.C). The particles are amorphous, as determined by XRD. This amorphous phase exists regardless of the addition of tretinoin to the particle starting material (Fig. 2.II).
1 molar ratio between CaCl2 and Na-polyP at pH 10. The size of the particles is between 60 and 90 nm in diameter (Fig. 2.I.C). The particles are amorphous, as determined by XRD. This amorphous phase exists regardless of the addition of tretinoin to the particle starting material (Fig. 2.II).
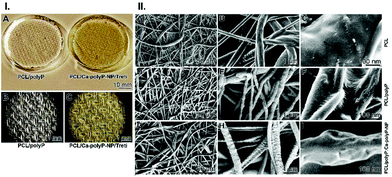
At electron microscopic magnification the fibrillar mat meshes can be assessed (Fig. 3.II-A). The plain PCL fibers, “PCL”, have a diameter between 500 nm and 1 μm (Fig. 3.II-A and B). The surface of the fibers is smooth (Fig. 3.II-C). In contrast, the surface of the PCL fibers, supplemented with Na-polyP, “PCL/polyP”, and measuring ∼300 nm in diameter (Fig. 3.II-D and E), is more ribbed (Fig. 3.II-F). Finally, the PCL fibers composed of Na-polyP and Ca-polyP NP, “PCL/polyP:Ca-polyP-NP”, have a pronounced granular surface (Fig. 3.II-G and H). In the close-up view it is seen that the ∼200 nm sized fibers have a surface with curved contours (Fig. 3.II-I).
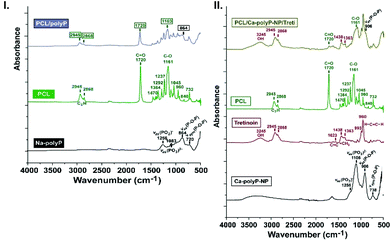
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), and 1161 cm−1 for (C–O). A mixture between the two components in “PCL/polyP” gave a spectrum in which the two constituents highlight; for PCL: 2945/2868 cm−1, 1720 cm−1 and 1163 cm−1 and for polyP: 864 cm−1. The other peaks of the individual spectra overlap with the corresponding other one.
O), and 1161 cm−1 for (C–O). A mixture between the two components in “PCL/polyP” gave a spectrum in which the two constituents highlight; for PCL: 2945/2868 cm−1, 1720 cm−1 and 1163 cm−1 and for polyP: 864 cm−1. The other peaks of the individual spectra overlap with the corresponding other one.
Coacervation process on the mats
We described previously that polyP, especially if the polymer is used as a Na salt, forms readily a coacervate phase. This process is accelerated if a peptide is present during the reaction41 and even more if polyP NP are present.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C at 1623 cm−1, with
C at 1623 cm−1, with ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 at 1438 cm−1 and 1363 cm−1 and with H–C
CH2 at 1438 cm−1 and 1363 cm−1 and with H–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H at 960 cm−1. In addition, the pattern for PCL is added in order to allow a mapping of the spun fibers, prepared from PCL, tretinoin and “Ca-polyP-NP”, the complex “PCL/Ca-polyP-NP/Treti” material. In the latter sample, the tretinoin signals appeared at 3245 cm−1 (OH), at 2945 cm−1 and 2868 cm−1 (C2H), and at 1438 cm−1 and 1363 cm−1 (
C–H at 960 cm−1. In addition, the pattern for PCL is added in order to allow a mapping of the spun fibers, prepared from PCL, tretinoin and “Ca-polyP-NP”, the complex “PCL/Ca-polyP-NP/Treti” material. In the latter sample, the tretinoin signals appeared at 3245 cm−1 (OH), at 2945 cm−1 and 2868 cm−1 (C2H), and at 1438 cm−1 and 1363 cm−1 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2). The PCL absorption is visible with the signal of 1161 cm−1 (C–O) and the polyP presence with the absorption at 906 cm−1 (for νas for P–O–P).
CH2). The PCL absorption is visible with the signal of 1161 cm−1 (C–O) and the polyP presence with the absorption at 906 cm−1 (for νas for P–O–P).
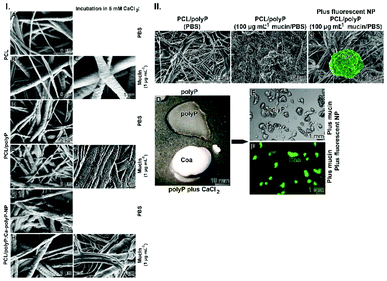
At first partially purified mucin was prepared (Fig. 2.I-A).The fibrous material with fibers of a diameter of ∼100 nm was suspended at a concentration of 100 μg mL−1 in PBS, which contained the “Ca-polyP-NP” particles (100 μg mL−1). After 8 h the organization of the mucin net was inspected by SEM. The material appears as clumpy to gel like aggregates (Fig. 2.I-B). The diameter of the ball-like spheres is around 100 nm, the size of the fabricated “Ca-polyP-NP” particles (Fig. 2.I-C). If a lower concentration of mucin (of 1 μg mL−1 in PBS) is used and added to the NP, the particles tend to stick together (Fig. 2.I-D).
The coacervate is present especially at the intersecting fibers. In contrast, for fibers dipped into PBS in the absence of mucin, no coacervate-like material is seen at the overcrossing fibers (Fig. 5.I-D).
The coacervate formation becomes more bulky if the PCL formed fibers contained, in addition to Na-polyP, “Ca-polyP-NP”, the “PCL/polyP:Ca-polyP-NP”. Again it is seen that the fibers immersed in PBS only, are not decorated with coacervate fragments and expose only ripples on their surfaces, originating from the NP (Fig. 5.I-G). In contrast, if they are submersed into the mucin solution the fibers become surrounded with coacervate and are glued together to larger bundles (Fig. 5.I-H and I).
First protection – trapping of aerosol particles onto the fiber mats
Based on a previous investigations49,50 the inclusion of virus particles is mimicked.In the parallel series Na-polyP (200 μg mL−1 PBS) was supplemented with CaCl2 (300 μg mL−1; at pH 7.4) in order to induce coacervate formation. The image in Fig. 5.II-D shows that the Na-polyP solution (top, without CaCl2) remains an almost clear fluid, while the sample reacted with CaCl2 turned to an induced coacervate phase (bottom). These two samples were transferred to a solution of mucin (1 μg mL−1) and then rapidly overlayed with 100 μl of a suspension with 100 nm sized fluorescent nanoparticles (0.1 mg mL−1). After washing the coacervate formation was initiated with CaCl2. Then, the samples were inspected under fluorescent light. Comparative images show that only the coacervate pieces incubated with the labeled nanoparticles light up in green (Fig. 5.II-F; Fig. 5.II-C[lighted up in false colors]). The Na-polyP material remains transparent (Fig. 5.II-E), while the coacervate fragment turns to a green fluorescent color (Fig. 5.II-F).
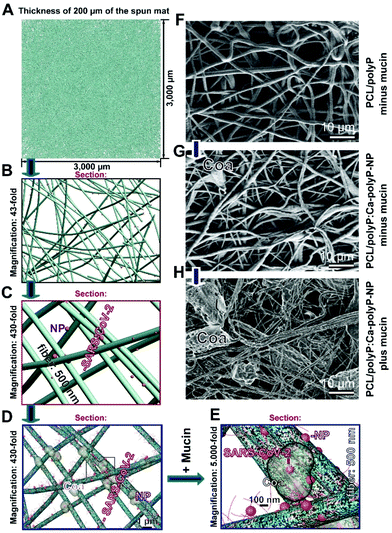
In the SEM images row (Fig. 6F–H), the aspects of “PCL/polyP” in the absence of a mucin environment [without any coacervate], of “PCL/polyP:Ca-polyP-NP” in the absence of mucin [small coacervate deposits], and of “PCL/polyP:Ca-polyP-NP” in the presence of mucin [bulky, large coacervate deposits] are depicted.
Second protection – antiviral activity of the polyP incorporated fiber mats
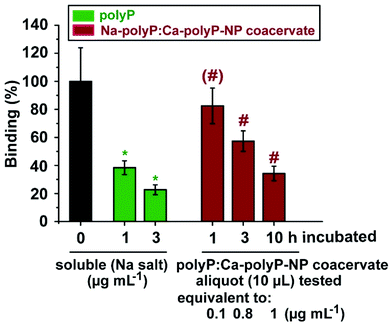
The effect of polyP to bind to the RBD of the SARS-CoV-2 S-protein and inhibit the association to the cell surface receptor ACE2 has been described recently.52,53 This blocking is not impaired in the presence of mucin.54The strength of inhibition by polyP is high. The physiological concentration of polyP in the circulating blood is ∼1 μg mL−1.30 This level is enough to block the interaction of the viral RBD with the ACE2 by 62% (Fig. 7). Even stronger is the effect of 3 μg mL−1 with 77%. In order to imitate the effect of polyP, polyP samples (100 mg of Na-polyP and 100 mg of “Ca-polyP-NP” in 1 mL PBS) were incubated in the presence of CaCl2 at physiological concentrations of 5 mM (ref. 55) and 100 μg mL−1 of mucin. Aliquots were taken from the particle-free supernatant and diluted by 100-fold with binding buffer in the RBD: ACE2 binding assay. The 10 μL aliquots caused a reduction by 19% after a 60 min incubation period, a value which is only slightly significant (p < 0.5). After a 3 h or 10 h incubation period the aliquots caused a significant reduction (p < 0.05) of the binding by 43% and 66%, respectively (Fig. 7).
Third protection – elimination of virus-mimicking particles
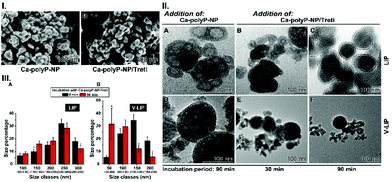
The strategy of the elimination is based on the interaction of tretinoin with the viral envelope protein E, a viroporin.56 Tretinoin has been described to interact with this channel and poison it.15,49 For mimicking the virus particles, liposomes were decorated with viroporin; they are termed virus-like liposomes V-LIP. The supply of tretinoin to them was reached with NP formed from polyP and tretinoin together with CaCl2; “Ca-polyP-NP/Treti”. As known from the literature, the liposomes prepared from 1-oleoyl-2-palmitoyl-sn-glycero-3-phosphocholine and 1-oleoyl-2-palmitoyl-sn-glycero-3-phosphoethanolamine are, under dry conditions, stable for more than 24 h.57The size of the liposomes was determined by dynamic light scattering (Fig. 8.III). The average size of the liposomes LIP was at 250 nm, and the one for the viroporin decorated particles, V-LIP, at 150 nm at time zero of an incubation period for 90 min.
A quantitative assessment of the changes of the liposome sizes was performed by dynamic light scattering (Fig. 8.III). The data show that no significant change can be seen for the LIP particles incubated with “Ca-polyP-NP/Treti”. In contrast, if the V-LIP particles are exposed to the “Ca-polyP-NP/Treti” particles, a distinct shift from ∼150 nm (time zero) to ∼50 nm, in the average, occurs after the 90 min incubation period.
Proposed interaction of viroporin with tretinoin
The structure of viroporin from SARS-CoV-2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 and the models for the potential interaction of tretinoin with viroporin15,49 have been published. Tretinoin binds to viroporin along the helix of the pore, especially to the moieties Val25, Ala22, Leu21, and Leu18 (Fig. 9.I-A). Viroporin as a pentamer forms a channel into which tretinoin is located and stabilized via hydrophobic amino acid moieties (Fig. 9.I-B).
12 and the models for the potential interaction of tretinoin with viroporin15,49 have been published. Tretinoin binds to viroporin along the helix of the pore, especially to the moieties Val25, Ala22, Leu21, and Leu18 (Fig. 9.I-A). Viroporin as a pentamer forms a channel into which tretinoin is located and stabilized via hydrophobic amino acid moieties (Fig. 9.I-B).
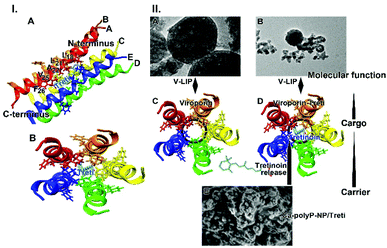 | ||
| Fig. 9 Binding of tretinoin to viroporin. (I) Interaction of viroporin, one helix is shown facing with its pore-facing residues, with the tretinoin molecule (models DB00755 and Q12321).12,58 (A) Some aa residues interacting with tretinoin, are labeled with the amino acids of: Phe(F)26; Val(V)25; Ala(A)22; Leu(L)21; Leu(L)18. All five helices are indicated. (B) Top view of the five pore-forming helices with the tretinoin in the center are sketched. (II) Proposed function of tretinoin on the integrity of the liposomes. (A and B) Liposomes with viroporin (V-LIP); SEM. (C) Schematic outline of the top view of viroporin without tretinoin; (D) top view with tretinoin. (E) NP, fabricated from Ca-polyP-NP and tretinoin, “Ca-polyP-NP/Treti”; SEM. It is proposed that during the incubation tretinoin is released from the NP and is switching to the viroporin, forming a tight interaction with the viroporin. | ||
It is shown here that tretinoin released as a functional cargo molecule from the bioactive and biodegradable carrier can bind to the virus protein with high affinity. The binding energy is high with −412.8 kJ mol−1.15 Surely the affinity between tretinoin, a compound with both hydrophilic and lipophilic potential,59 and the hydrophilic Ca-polyP-NP, like in “Ca-polyP-NP/Treti”, is lower, suggesting a comparably rapid release of tretinoin from the polymeric nanocarrier.
The data in this report show that co-incubation of liposomes decorated with viroporin together with “Ca-polyP-NP/Treti” leads in a rapid disintegration of the lipid particles (Fig. 9.II-A–E). This finding is strong evidence that the fragmentation is due to a lytic disruption of the liposomes.
Experimental
Materials
Na-polyphosphate (Na-polyP) with an average chain length of 40 Pi units (polyP40) was from Chemische Fabrik Budenheim (Budenheim; Germany). The following materials were purchased; mucin from bovine submaxillary glands (type I–S; prepared according to Tsuiki and Pigman,60 and subsequently enriched by Schömig et al.61) from Sigma (#M3895; Taufkirchen; Germany), and green fluorescent superparamagnetic, core–shell nanoparticles with a hydrodynamic diameter of 100 nm (screenMAG/G) from chemicell (Berlin; Germany).Preparation of the Ca-polyP nanoparticles
Amorphous Ca-polyP nanoparticles (Ca-polyP-NP) were prepared as described.38 The 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio between CaCl2 and Na-polyP (based on phosphate) was selected and the pH was adjusted to 10. For the process 1 g of Na-polyP was dissolved in 100 mL of distilled water and 2.8 g of CaCl2·2H2O (#T885.1; Roth, Karlsruhe, Germany) were dissolved in 100 mL. The CaCl2 solution was added dropwise to the polyP solution during a 60 min stirring period. During the process the pH was adjusted to 10 (with NaOH). After additional stirring for 12 h the nanoparticles (NP) were collected by filtration, washed at first twice with ethanol and then three-times with water. After drying at 50 °C the particles were collected; “Ca-polyP-NP”. In the method used the addition of CaCl2 to the polyP solution was shortened to 60 min in order to achieve a particle size of 60 to 90 nm.
1 molar ratio between CaCl2 and Na-polyP (based on phosphate) was selected and the pH was adjusted to 10. For the process 1 g of Na-polyP was dissolved in 100 mL of distilled water and 2.8 g of CaCl2·2H2O (#T885.1; Roth, Karlsruhe, Germany) were dissolved in 100 mL. The CaCl2 solution was added dropwise to the polyP solution during a 60 min stirring period. During the process the pH was adjusted to 10 (with NaOH). After additional stirring for 12 h the nanoparticles (NP) were collected by filtration, washed at first twice with ethanol and then three-times with water. After drying at 50 °C the particles were collected; “Ca-polyP-NP”. In the method used the addition of CaCl2 to the polyP solution was shortened to 60 min in order to achieve a particle size of 60 to 90 nm.
Where indicated the “Ca-polyP-NP” were loaded additionally with tretinoin (#PHR1187 [all-trans-retinoic acid]; Sigma-Merck, Taufkirchen; Germany) as previously described for the Ca-polyP particles.62 A tretinoin solution (50 mg in 100 mL ethanol) was prepared and added to the solutions of 1 g of Na-polyP and 2.8 g of CaCl2, dissolved in 100 mL of distilled water each. In order to prevent phase separation 2 g of poly(ethylene glycol) (#P5413; Sigma; average mol wt 8000) was added to the Na-polyP solution (100 mL). The emulsion was stirred for 6 h, then collected by filtration and washed three-times with water. The NP formed were dried at room temperature (overnight). The particles were termed “Ca-polyP-NP/Treti”.
The efficiency of tretinoin loading in “Ca-polyP-NP/Treti” was determined as follows. The particles were suspended in dehydrated ethanol followed by strong vortexing for 20 min. During this process tretinoin accumulates in the organic phase. There, the absorbance was determined at the characteristic wavelength of tretinoin at 347 nm. Then the concentration of tretinoin was calculated using a calibration curve. Using this approach a loading efficiency of the particles was determined with 22.7 ± 3.1%.
Spectroscopy
For X-Ray powder diffraction (XRD) spectral analysis dried powder samples were analyzed in a D8 Advance A25 diffractometer (Bruker, Billerica; MA) with a monochromatic Cu-Kα radiation. The Fourier transformed infrared spectroscopic (FTIR) analyses were performed after grinding with a micro-mill in an ATR (attenuated total reflectance)-FTIR spectroscope/Varian 660-IR spectrometer (Agilent, Santa Clara; CA), fitted with a Golden Gate ATR unit (Specac, Orpington; UK).Fabrication of polycaprolactone-loaded nanofibers
Polycaprolactone (PCL; #440752, Sigma; average Mw ∼14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) was dissolved (4 g of the polymer) in a mixture of 20 mL of acetone and 20 mL of dichloromethane. Where indicated the following supplements were added; either 7.5% [w/w] of Na-polyP of the polymer loading, or 7.5% of Ca-polyP-NP, or both components together. Then, the respective mixtures were stirred in airtight sealed bottles overnight (150 rpm). The samples were named: “PCL”, “PCL/polyP”, “PCL/Ca-polyP-NP”, or “PCL/polyP:Ca-polyP-NP”. In another series of experiments 7.5% “PCL/Ca-polyP-NP/Treti” was added to PCL and fabricated further.
000) was dissolved (4 g of the polymer) in a mixture of 20 mL of acetone and 20 mL of dichloromethane. Where indicated the following supplements were added; either 7.5% [w/w] of Na-polyP of the polymer loading, or 7.5% of Ca-polyP-NP, or both components together. Then, the respective mixtures were stirred in airtight sealed bottles overnight (150 rpm). The samples were named: “PCL”, “PCL/polyP”, “PCL/Ca-polyP-NP”, or “PCL/polyP:Ca-polyP-NP”. In another series of experiments 7.5% “PCL/Ca-polyP-NP/Treti” was added to PCL and fabricated further.
Electrospinning
For electrospinning the apparatus Spraybase 20 kV Electrospinning Rotating Drum (Avectas, Maynooth University, Kildare; Ireland) was used. The respective polymer solutions were filled into a syringe for electrospinning.23 The 5 mL plastic syringe, to which a metal blunt ended needle (spinning nozzle) was connected, was hooked to a pressure pump which allowed an injection velocity of 0.1 mL h−1 (Bio-Rad, Model EP-1 Econo Pump, Hertfordshire; UK). The distance of the needle tip to the grounded target plate was set to 15 cm. For the experiments described here a rotating metal cylinder was used as a collector. The process run with an electric field of 20–30 kV to adjust fibers at ∼500 nm in diameter. The thickness of the fibrous mats was 280 to 330 μm. Spinning was performed at room temperature using a positive output lead of a high voltage power supply (PNC3p, 30000-2; Heinzinger Electronic, Rosenheim; Germany). The negative pole was attached to the platform.After the electrospinning process the mats were washed by immersion in ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (70/30 [v/v]) at pH ∼7 for 60 min, followed by two washing cycles in phosphate buffered saline (PBS; pH 7.4). Then, the electrospun mats were dried at 40 °C for 24 h. Prior to use the mats were sterilized by immersing into 75% (v/v) ethanol aqueous solution for 30 min. Finally, the mats were exposed to ultraviolet radiation (280–315 nm UVB; 20 mJ cm−2) for 1 h. If not mentioned otherwise the mats were termed “PCL” mats (without addition), “PCL/polyP” mats (addition of “Na-polyP”), “PCL/Ca-polyP-NP” mats (with “Ca-polyP-NP”) or “PCL/polyP:Ca-polyP-NP” mats (containing “Na-polyP” and “Ca-polyP-NP”). In separate experiments the PCL-based mats were spun with NP, supplemented with “Ca-polyP-NP/Treti” and termed “PCL/Ca-polyP-NP/Treti” mats.
water (70/30 [v/v]) at pH ∼7 for 60 min, followed by two washing cycles in phosphate buffered saline (PBS; pH 7.4). Then, the electrospun mats were dried at 40 °C for 24 h. Prior to use the mats were sterilized by immersing into 75% (v/v) ethanol aqueous solution for 30 min. Finally, the mats were exposed to ultraviolet radiation (280–315 nm UVB; 20 mJ cm−2) for 1 h. If not mentioned otherwise the mats were termed “PCL” mats (without addition), “PCL/polyP” mats (addition of “Na-polyP”), “PCL/Ca-polyP-NP” mats (with “Ca-polyP-NP”) or “PCL/polyP:Ca-polyP-NP” mats (containing “Na-polyP” and “Ca-polyP-NP”). In separate experiments the PCL-based mats were spun with NP, supplemented with “Ca-polyP-NP/Treti” and termed “PCL/Ca-polyP-NP/Treti” mats.
Generation of aerosol particles
The aerosol particles were generated by using the commercial collision nebulizer (BGI, Inc., Waltham, MA) as described.63 The particles were characterized by using the laser diffraction-based particle sizer Spraytec (Malvern Instruments, Malvern; UK) as described.64Preparation of virus-mimicking liposomes
The method applied for the fabrication of the liposomes based on the described protocols.65 The formulation contained 1-oleoyl-2-palmitoyl-sn-glycero-3-phosphocholine (POPCP; #4142; Sigma, Taufkirchen; Germany), 1-oleoyl-2-palmitoyl-sn-glycero-3-phosphoethanolamine (POPE; #01991, Sigma), sphingomyelin (#85615; Sigma) and cholesterol (#C8667; Sigma) in the ratio 37.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 34.2
34.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.7
5.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 22.8, which is matching the membrane lipids of mammalian cells.66 The samples were prepared in chloroform. After mixing this solvent was evaporated under nitrogen gas and then kept in a desiccator under vacuum (overnight). The dried lipid film was suspended in PBS reaching a lipid concentration of ∼2 mg mL−1. During this procedure the SARS-CoV-2 envelope (E) protein (recombinant SARS-CoV-2 envelope protein; #32-190021-100; Bio-Trend, Köln; Germany) or Influenza A virus matrix protein 2 (M2) (recombinant protein; MBS7019655; MyBioSource, San Diego; CA) was added at a concentration of 10 μg mL−1.67 Finally, the assay was extruded through a polycarbonate filter (Whatman Nucleopore; Sigma; #111103N pore size of 50 nm). In the supernatant the protein content was detected by SDS-PAGE (Na-dodecyl sulfate polyacrylamide gel electrophoresis) with Coomassie blue detecting, as the 12 kDa E-protein. By calculation it was determined that between 70 and 80% of the applied protein had been incorporated into the liposomes. Liposomes without the viral protein were termed LIP, and those liposomes, mimicking the viral envelope and containing the E-protein, V-LIP.
22.8, which is matching the membrane lipids of mammalian cells.66 The samples were prepared in chloroform. After mixing this solvent was evaporated under nitrogen gas and then kept in a desiccator under vacuum (overnight). The dried lipid film was suspended in PBS reaching a lipid concentration of ∼2 mg mL−1. During this procedure the SARS-CoV-2 envelope (E) protein (recombinant SARS-CoV-2 envelope protein; #32-190021-100; Bio-Trend, Köln; Germany) or Influenza A virus matrix protein 2 (M2) (recombinant protein; MBS7019655; MyBioSource, San Diego; CA) was added at a concentration of 10 μg mL−1.67 Finally, the assay was extruded through a polycarbonate filter (Whatman Nucleopore; Sigma; #111103N pore size of 50 nm). In the supernatant the protein content was detected by SDS-PAGE (Na-dodecyl sulfate polyacrylamide gel electrophoresis) with Coomassie blue detecting, as the 12 kDa E-protein. By calculation it was determined that between 70 and 80% of the applied protein had been incorporated into the liposomes. Liposomes without the viral protein were termed LIP, and those liposomes, mimicking the viral envelope and containing the E-protein, V-LIP.
The size of the liposomes was determined by dynamic light scattering (Zetasizer Nano ZS90; Malvern Instruments; Malvern; UK) as described.64
Incubation of virus-mimicking liposomes with tretinoin loaded “Ca-polyP-NP”
The content of lipid in the liposome sample was determined gravimetrically.68 After extraction with chloroform/methanol and addition of a 0.5% NaCl solution, an aliquot was taken from the chloroform (lower) layer and transferred into a small beaker. After evaporation the samples were weighted. The liposomes not loaded with viroporin, LIP, or those supplemented with viroporin, V-LIP, at a concentration of ∼1 mg mL−1 were suspended in PBS, containing 5 mM CaCl2 and 100 μg mL−1 of mucin in PBS (pH 7), and incubated for up to 90 min at room temperature. The samples were exposed to 50 μg mL−1 of either “Ca-polyP-NP” or “Ca-polyP-NP/Treti”. Then the integrity of the liposomes was assessed by TEM.Electron microscopy
Modelling of the fibrous mats
The organization of the fibers within the spun mats was modelled by using the 3DS Max Software. The fibers were read in by choosing a cube with an edge length of 3000 μm. Based on the diameter of 0.5 μm, 1000 fibers were arranged randomly in one fiber layer. Then 400 layers were stockpiled to reach a thickness of 200 μm. For the determination of the area through which the exhalation air is released under imaginary conditions an opening of 100 mm × 50 mm was chosen. From this 3D image file, a 430-fold template was calculated as a start. It was used for the pictures evaluated further under “Results”.Binding assay for the interaction of the viral RBD to the ACE2
A Screening Assay Kit (BPS Bioscience/Tebu-bio, Offenbach; Germany) was used as described.52,53 In this system the recombinant ACE2 receptor (50 ng per well) was bound to the bottom of the 96 well plate which subsequently interacted with the RBD/S1-protein (100 ng per well), labeled with biotin. Prior to the assay the Arg residues, present in the RBD, were modified with 1,2-cyclohexanedione [CHD] (#W345806; Sigma) as described.69 The reaction was run in a 0.25 M Na-borate buffer at pH 9.0 for 2 h.53Then 20 μL with the RBD (100 ng) were pre-incubated with the 10 μL of polyP solution (binding buffer) and added to ACE2 in a final volume of 50 μL (consisting of 20 μL RBD, 10 μL polyP solution, and 20 μL binding buffer). The extent of binding of the RBD to ACE2 was determined after reacting for 60 min (23 °C) and detection with streptavidin–horseradish peroxidase (HRP) and the HRP substrate. After subsequent washing with 10 mM HEPES buffer (pH 7) the chemiluminescence was quantitated with a PerkinElmer-Wallac victor 3 V multi-label microplate reader (PerkinElmer, Waltham, MA, USA). The values for the blank (immuno buffers and loosely bound components) were subtracted from the readings. The values obtained for the samples without inhibitor served as reference and were set to 100%.
The polyP, released from the polyP formulation, a mixture of 100 mg of Na-polyP and 100 mg of “Ca-polyP-NP” was prepared in 1 mL PBS (pH 7) containing 5 mM CaCl2 and 100 μg mL−1 of mucin. The coacervation process was finished after 20 min. Then the material was shortly washed and continued to be incubated in PBS, supplemented with 1 mM CaCl2. After standing for 1 h, 3 h, or 10 h, aliquots of 10 μL were taken from the supernatant and tested in the RBD - ACE2 Screening Assay Kit. The samples were diluted 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 prior to the addition to the assay.
10 prior to the addition to the assay.
Statistical analysis
For the quantitative results the average ± standard deviations (σ) are given. The Student's t-test was applied to assess the significance level between two groups using the GraphPad Prism 7.0 software (GraphPad Software, La Jolla; CA). Values of p < 0.05 were considered as statistically significant (*).Conclusions
The hitherto introduced anti-COVID-19 masks are composed of passive sieving filters with the hope to eliminate SARS-CoV-2 from inhalant and exhalent respiratory air. At present, it does not seem to be feasible to fabricate filters with a pore size around 100 nm; they are very uncomfortable and perhaps even dangerous to wear. In the present proof-of-concept study, it is demonstrated that it is straightforwardly possible to fabricate spun mats of PCL with the active ingredients polyP and Ca-polyP-NP, which potently act against COVID-19 due to the distinguished property of this polymer to form a coacervate (target 1 in Fig. 10) and to bind to and mask the virus spike protein (target 2). In addition to the virus-attracting property of the polyP scaffold an active killing function has been included. During this step (target 3) virus-like particles, with integrated viroporin channels, are disintegrated through tretinoin. This retinoid integrates into the channel and causes the virus to be eliminated. With this innovative concept two members of the natural innate immunity, polyP and tretinoin, are coherently implemented into the fibrous scaffold of the spun mats. Next, an application is projected in operational environment.Author contributions
Conceptualization: W.E.G.M. and X.H.W.; data curation: W.E.G.M., M.N., I.L., R.M.E., S.W., H.C.S. and X.H.W.; formal analysis: W.E.G.M., M.N., I.L., R.M.E., S.W., H.C.S. and X.H.W.; funding acquisition: W.E.G.M. and X.H.W.; investigation: M.N., I.L., R.M.E. and S.W.; methodology: M.N., I.L., R.M.E. and S.W.; project administration: W.E.G.M. and X.H.W.; resources: W.E.G.M. and X.H.W.; supervision: W.E.G.M. and X.H.W.; validation: M.N., I.L., R.M.E. and S.W.; visualization: W.E.G.M., M.N., I.L., R.M.E., S.W., H.C.S. and X.H.W.; writing – original draft: W.E.G.M. and X.H.W.; writing – review & editing: W.E.G.M., M.N., I.L., R.M.E., S.W., H.C.S. and X.H.W.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank very much for the expert help in the fields of electron microscopy. Ms Dr Maria Kokkinopoulou (TEM; Max Planck Institute for Polymer Research, Mainz) and Mr Gunnar Glasser (SEM; Max Planck Institute for Polymer Research, Mainz). This work was supported by the European Research Council (W.E.G.M. is the Investigator of an Advanced Grant and three related Proof of Concept Grants) (grant numbers 268476, 324564, 662486, and 767234). In addition, this work was supported by the grants from the European Commission (grant numbers 604036 and 311848), the International Human Frontier Science Program and the BiomaTiCS research initiative of the University Medical Center, Mainz. Further support came from the BMBF (grant number 13GW0403B) and the BMWi (grant number: ZF4294002AP9).References
- Y. Orooji, H. Sohrabi, N. Hemmat, F. Oroojalian, B. Baradaran, A. Mokhtarzadeh, M. Mohaghegh and H. Karimi-Maleh, An overview on SARS-CoV-2 (COVID-19) and other human coronaviruses and their detection capability via amplification assay, chemical sensing, biosensing, immunosensing, and clinical Assays, Nanomicro Lett., 2021, 13, 18 Search PubMed.
- Coronavirus Outbreak, DAX infiziert - Nackte Panik an den Börsen, https://www.worldometers.info/coronavirus/, (accessed January 2020).
- S. Khan, J. Liu and M. Xue, Transmission of SARS-CoV-2, Required Developments in Research and Associated Public Health Concerns, Front. Med., 2020, 7, 310 CrossRef.
- NIH Safety Guidance, Return to Physical Workplace Safety Guide – ORS, 2021, OMSmonitoringprogram@mail.nih.gov.
- J. Howard, A. Huang, Z. Li, Z. Tufekci, V. Zdimal, H. van der Westhuizen, A. von Delft, A. Price, L. Fridman, L. Tang, V. Tang, G. L. Watson, C. E. Bax, R. Shaikh, F. Questier, D. Hernandez, L. F. Chu, C. M. Ramirez and A. W. Rimoin, Face masks against COVID-19: An evidence review, Preprints, 2020 DOI:10.20944/preprints202004.0203.v1.
- S. Kumar and H. P. Lee, The perspective of fluid flow behavior of respiratory droplets and aerosols through the facemasks in context of SARS-CoV-2, Phys. Fluids, 2020, 32, 111301 CrossRef CAS PubMed.
- L. Peeples, What the data say about wearing face masks, Nature, 2020, 586, 186–189 CrossRef CAS PubMed.
- D. Wang, Y. You, X. Zhou, Z. Zong, H. Huang, H. Zhang, X. Yong, Y. Cheng, L. Yang, Q. Guo, Y. Long, Y. Liu, J. Huang and L. Du, Selection of homemade mask materials for preventing transmission of COVID-19: A laboratory study, PLoS One, 2020, 15, e0240285 CrossRef CAS PubMed.
- A. Gilbert, M. Graham and J. Young, in Meshes: Benefits and Risks, ed. V. Schumpelick and L. M. Nyhus, Springer, Berlin, Heidelberg, 2004, pp. 101–104 Search PubMed.
- A. Kolpe, B. Schepens, W. Fiers and X. Saelens, M2-based influenza vaccines: recent advances and clinical potential, Expert Rev. Vaccines, 2017, 16, 123–136 CrossRef CAS PubMed.
- T. R. Ruch and C. E. Machamer, The coronavirus E protein: assembly and beyond, Viruses, 2012, 4, 363–382 CrossRef CAS PubMed.
- V. S. Mandala, M. J. McKay, A. A. Shcherbakov, A. J. Dregni, A. Kolocouris and M. Hong, Structure and drug binding of the SARS-CoV-2 envelope protein in phospholipid bilayers, Nat. Struct. Mol. Biol., 2020, 27, 1202–1208 CrossRef PubMed.
- P. G. Rytik, V. F. Eremin, Z. B. Kvacheva, N. N. Poleschuk, A. S. Popov, H. C. Schröder, B. E. Weiler, M. Bachmann and W. E. G. Müller, Susceptibility of human astrocytes to human immunodeficiency virus infection in vitro; Anti-HIV activity of memantine, AIDS Res. Hum. Retrovir., 1991, 7, 89–95 CrossRef CAS PubMed.
- W. E. G. Müller, H. C. Schröder, H. Ushijima, J. Dapper and J. Bormann, Gp120 of HIV-1 induces apoptosis in rat cortical cell cultures: Prevention by memantine, Eur. J. Pharmacol., 1992, 226, 209–214 CrossRef.
- D. Dey, S. Borkotoky and M. Banerjee, In silico identification of Tretinoin as a SARS-CoV-2 envelope (E) protein ion channel inhibitor, Comput. Biol. Med., 2020, 127, 104063 CrossRef CAS PubMed.
- J. P. Pires, A. S. Ramos, G. M. Miranda, G. L. de Souza, F. Fraga, C. M. N. Azevedo, R. A. Ligabue, J. E. A. de Lima and R. V. Lourega, Natural freshwater degradation of polypropylene blends with additives of a distinct nature, Polym. Bull., 2021, 78, 2025–2042 CrossRef CAS.
- C. L. Salgado, E. M. Sanchez, C. A. Zavaglia and P. L. Granja, Biocompatibility and biodegradation of polycaprolactone-sebacic acid blended gels, J. Biomed. Mater. Res., Part A, 2012, 100, 243–251 CrossRef PubMed.
- K. Krasowska, A. Heimowska and M. Morawska, Environmental degradability of polycaprolactone under natural conditions, E3S Web Conf., 2016, 10, 00048 CrossRef.
- M. Srisa-Ard, Y. Baimark and Y. Srisuwan, Conformation transition and thermal properties study of silk fibroin and poly (ε-caprolactone) blends, J. Appl. Sci., 2008, 8, 3518–3522 CrossRef CAS.
- F. J. Xu, Z. H. Wang and W. T. Yang, Surface functionalization of polycaprolactone films via surface-initiated atom transfer radical polymerization for covalently coupling cell-adhesive biomolecules, Biomaterials, 2010, 31, 3139–3147 CrossRef CAS PubMed.
- J. Jensen, D. C. Kraft, H. Lysdahl, C. B. Foldager, M. Chen, A. A. Kristiansen, J. H. Rölfing and C. E. Bünger, Functionalization of polycaprolactone scaffolds with hyaluronic acid and β-TCP facilitates migration and osteogenic differentiation of human dental pulp stem cells in vitro, Tissue Eng., Part A, 2015, 21, 729–739 CrossRef CAS PubMed.
- J. Daenicke, M. Lämmlein, F. Steinhübl and D. W. Schubert, Revealing key parameters to minimize the diameter of polypropylene fibers produced in the melt electrospinning process, e-Polym., 2019, 19, 330–340 CAS.
- W. E. G. Müller, E. Tolba, H. C. Schröder, B. Diehl-Seifert, T. Link and X. H. Wang, Biosilica-loaded poly(ε-caprolactone) nanofibers mats provide a morphogenetically active surface scaffold for the growth and mineralization of the osteoclast-related SaOS-2 cells, Biotechnol. J., 2014, 9, 1312–1321 CrossRef PubMed.
- W. E. G. Müller, E. Tolba, B. Dorweiler, H. C. Schröder, B. Diehl-Seifert and X. H. Wang, Electrospun bioactive mats enriched with Ca-polyphosphate/retinol nanospheres as potential wound dressing, Biochem. Biophys. Rep., 2015, 3, 150–160 Search PubMed.
- W. E. G. Müller, E. Tolba, S. Wang, M. Neufurth, I. Lieberwirth, M. Ackermann, H. C. Schröder and X. H. Wang, Nanoparticle-directed and ionically forced polyphosphate coacervation: A versatile and reversible core-shell system for drug delivery, Sci. Rep., 2020, 10, 17147 CrossRef PubMed.
- M. Neufurth, X. H. Wang, S. F. Wang, R. Steffen, M. Ackermann, N. D. Haep, H. C. Schröder and W. E. G. Müller, 3D printing of hybrid biomaterials for bone tissue engineering: Calcium-polyphosphate microparticles encapsulated by polycaprolactone, Acta Biomater., 2017, 64, 377–388 CrossRef CAS PubMed.
- X. H. Wang, H. C. Schröder and W. E. G. Müller, Amorphous polyphosphate, a smart bioinspired nano-/bio-material for bone and cartilage regeneration: Towards a new paradigm in tissue engineering, J. Mater. Chem. B, 2018, 6, 2385–2412 RSC.
- N. N. Rao, M. R. Gómez-García and A. Kornberg, Inorganic polyphosphate: essential for growth and survival, Annu. Rev. Biochem., 2009, 78, 605–647 CrossRef CAS PubMed.
- R. Docampo and S. N. Moreno, Acidocalcisomes, Cell Calcium, 2011, 50, 113–119 CrossRef CAS PubMed.
- J. H. Morrissey, S. H. Choi and S. A. Smith, Polyphosphate: an ancient molecule that links platelets, coagulation, and inflammation, Blood, 2012, 119, 5972–5979 CrossRef CAS PubMed.
- H. C. Schröder and W. E. G. Müller, Inorganic Polyphosphates - Biochemistry, Biology, Biotechnology, Springer, Heidelberg, 1999 Search PubMed.
- P. R. Angelova, A. Y. Baev, A. V. Berezhnov and A. Y. Abramov, Role of inorganic polyphosphate in mammalian cells: from signal transduction and mitochondrial metabolism to cell death, Biochem. Soc. Trans., 2016, 44, 40–45 CrossRef CAS PubMed.
- W. E. G. Müller, H. C. Schröder and X. H. Wang, Inorganic polyphosphates as storage for and generator of metabolic energy in the extracellular matrix, Chem. Rev., 2019, 119, 12337–12374 CrossRef PubMed.
- J. J. Verhoef, A. D. Barendrecht, K. F. Nickel, K. Dijkxhoorn, E. Kenne, L. Labberton, O. J. McCarty, R. Schiffelers, H. F. Heijnen, A. P. Hendrickx, H. Schellekens, M. H. Fens, S. de Maat, T. Renné and C. Maas, Polyphosphate nanoparticles on the platelet surface trigger contact system activation, Blood, 2017, 129, 1707–1717 CrossRef CAS PubMed.
- L. Faxälv, N. Boknäs, J. O. Ström, P. Tengvall, E. Theodorsson, S. Ramström and T. L. Lindahl, Putting polyphosphates to the test: evidence against platelet-induced activation of factor XII, Blood, 2013, 122, 3818–3824 CrossRef PubMed.
- T. L. Lindahl, S. Ramström, N. Boknäs and L. Faxälv, Caveats in studies of the physiological role of polyphosphates in coagulation, Biochem. Soc. Trans., 2016, 44, 35–39 CrossRef CAS PubMed.
- X. Yang, M. Wan, T. Liang, M. Peng and F. Chen, Synthetic polyphosphate inhibits endogenous coagulation and platelet aggregation in vitro, Biomed. Rep., 2017, 6, 57–62 CrossRef CAS PubMed.
- W. E. G. Müller, E. Tolba, H. C. Schröder, S. Wang, G. Glaßer, R. Muñoz-Espí, T. Link and X. H. Wang, A new polyphosphate calcium material with morphogenetic activity, Mater. Lett., 2015, 148, 163–166 CrossRef.
- M. Picher and R. C. Boucher, Human airway ecto-adenylate kinase. A mechanism to propagate ATP signaling on airway surfaces, J. Biol. Chem., 2003, 278, 11256–11264 CrossRef CAS PubMed.
- W. E. G. Müller, E. Tolba, Q. Feng, H. C. Schröder, J. S. Markl, M. Kokkinopoulou and X. H. Wang, Amorphous Ca2+ polyphosphate nanoparticles regulate ATP level in bone-like SaOS-2 cells, J. Cell Sci., 2015, 128, 2202–2207 CrossRef PubMed.
- W. E. G. Müller, S. Wang, E. Tolba, M. Neufurth, M. Ackermann, R. Muñoz-Espí, I. Lieberwirth, G. Glasser, H. C. Schröder and X. H. Wang, Transformation of amorphous polyphosphate nanoparticles into coacervate complexes: an approach for the encapsulation of mesenchymal stem cells, Small, 2018, 14, e1801170 CrossRef PubMed.
- I. Ostolska and M. Wiśniewska, Application of the zeta potential measurements to explanation of colloidal Cr2O3 stability mechanism in the presence of the ionic polyamino acids, Colloid Polym. Sci., 2014, 292, 2453–2464 CrossRef CAS PubMed.
- K. S. Lim, S. T. Bee, L. T. Sin, T. T. Tee, C. T. Ratnam, D. Hui and A. R. Rahmat, A review of application of ammonium polyphosphate as intumescent flame retardant in thermoplastic composites, Composites, Part B, 2016, 84, 155–174 CrossRef CAS.
- Ž. T. Rakuša, P. Škufca, A. Kristl and R. Roškar, Retinoid stability and degradation kinetics in commercial cosmetic products, J. Cosmet. Dermatol., 2020 DOI:10.1111/jocd.13852.
- M. Brisaert, M. Gabriëls and J. Plaizier-Vercammen, Investigation of the chemical stability of an erythromycin-tretinoin lotion by the use of an optimization system, Int. J. Pharm., 2000, 197, 153–160 CrossRef CAS PubMed.
- X. H. Wang, M. Ackermann, E. Tolba, M. Neufurth, F. Wurm, Q. Feng, S. Wang, H. C. Schröder and W. E. G. Müller, Artificial cartilage bio-matrix formed of hyaluronic acid and Mg2+-polyphosphate, Eur. Cells Mater., 2016, 32, 271–283 CrossRef CAS PubMed.
- H. Kim, Y. Hu, D. Jeong, B. H. Jun, E. Cho and S. Jung, Synthesis, characterization, and retinol stabilization of fatty amide-β-cyclodextrin conjugates, Molecules, 2016, 21, 963 CrossRef PubMed.
- Y. Liu, H. H. Winter and S. L. Perry, Linear viscoelasticity of complex coacervates, Adv. Colloid Interface Sci., 2017, 239, 46–60 CrossRef CAS PubMed.
- D. Dey, S. I. Siddiqui, P. Mamidi, S. Ghosh, C. S. Kumar, S. Chattopadhyay, S. Ghosh and M. Banerjee, The effect of amantadine on an ion channel protein from Chikungunya virus, PLoS Neglected Trop. Dis., 2019, 13, e0007548 CrossRef CAS PubMed.
- A. Shukla, D. Dey, K. Banerjee, A. Nain and M. Banerjee, The C-terminal region of the non-structural protein 2B from Hepatitis A Virus demonstrates lipid-specific viroporin-like activity, Sci. Rep., 2015, 5, 15884 CrossRef CAS PubMed.
- J. Leal, H. D. C. Smyth and D. Ghosh, Physicochemical properties of mucus and their impact on transmucosal drug delivery, Int. J. Pharm., 2017, 532, 555–572 CrossRef CAS PubMed.
- M. Neufurth, X. H. Wang, E. Tolba, I. Lieberwirth, S. Wang, H. C. Schröder and W. E. G. Müller, The inorganic polymer, polyphosphate, blocks binding of SARS-CoV-2 spike protein to ACE2 receptor at physiological concentrations, Biochem. Pharmacol., 2020, 182, 114215 CrossRef CAS PubMed.
- W. E. G. Müller, M. Neufurth, H. Schepler, S. Wang, E. Tolba, H. C. Schröder and X. H. Wang, The biomaterial polyphosphate blocks stoichiometrically binding of the SARS-CoV-2 S-protein to the cellular ACE2 receptor, Biomater. Sci., 2020, 8, 6603–6610 RSC.
- W. E. G. Müller, M. Neufurth, S. F. Wang, R. W. Tan, H. C. Schröder and X. H. Wang, Morphogenetic (mucin expression) as well as potential anti-corona viral activity of the marine secondary metabolite polyphosphate on A549 cells, Mar. Drugs, 2020, 18, 639 CrossRef PubMed.
- J. F. Forstner and G. G. Forstner, Effects of calcium on intestinal mucin: implications for cystic fibrosis, Pediatr. Res., 1976, 10, 609–613 CrossRef CAS PubMed.
- J. L. Nieva, V. Madan and L. Carrasco, Viroporins: structure and biological functions, Nat. Rev. Microbiol., 2012, 10, 563–574 CrossRef CAS PubMed.
- M. Grit and D. J. Crommelin, Chemical stability of liposomes: implications for their physical stability, Chem. Phys. Lipids, 1993, 64, 3–18 CrossRef CAS PubMed.
- U. Pieper, B. M. Webb, G. Q. Dong, D. Schneidman-Duhovny, H. Fan, S. J. Kim, N. Khuri, Y. G. Spill, P. Weinkam, M. Hammel, J. A. Tainer, M. Nilges and A. Sali, ModBase, a database of annotated comparative protein structure models and associated resources, Nucleic Acids Res., 2014, 42, D336–D346 CrossRef CAS PubMed.
- M. J. Lucero, J. Vigo and V. M. León, Stability of hydrophilic gels of tretinoin, Int. J. Pharm., 1994, 110, 241–248 CrossRef CAS.
- S. Tsuiki and W. Pigman, The mucin of bovine sublingual glands, Arch. Oral Biol., 1960, 2, 1–14 CrossRef CAS PubMed.
- V. J. Schömig, B. T. Käsdorf, C. Scholz, K. Bidmon, O. Lieleg and S. Berensmeier, An optimized purification process for porcine gastric mucin with preservation of its native functional properties, RSC Adv., 2016, 6, P44932–P44943 RSC.
- W. E. G. Müller, E. Tolba, H. C. Schröder, B. Diehl-Seifert and X. H. Wang, Retinol encapsulated into amorphous Ca2+ polyphosphate nanospheres acts synergistically in MC3T3-E1 cells, Eur. J. Pharm. Biopharm., 2015, 93, 214–223 CrossRef PubMed.
- R. M. Eninger, A. Adhikari, T. Reponen, S. A. Grinshpun, C. J. Hogan and P. Biswas, Electrospray versus nebulization for aerosolization and filter testing with bacteriophage particles, Aerosol. Sci. Technol., 2009, 43, 298–304 CrossRef CAS.
- A. Laycock, M. D. Wright, I. Römer, A. Buckley and R. Smith, Characterisation of particles within and aerosols produced by nano-containing consumer spray products, Atmos. Environ.: X, 2020, 8, 100079 CAS.
- R. Goers, J. Thoma, N. Ritzmann, A. Di Silvestro, C. Alter, G. Gunkel-Grabole, D. Fotiadis, D. Müller and W. Meier, Optimized reconstitution of membrane proteins into synthetic membranes, Commun. Chem., 2018, 1, 35 CrossRef.
- D. A. Costello, J. K. Millet, C. Y. Hsia, G. R. Whittaker and S. Daniel, Single particle assay of coronavirus membrane fusion with proteinaceous receptor-embedded supported bilayers, Biomaterials, 2013, 34, 7895–7904 CrossRef CAS PubMed.
- O. H. Voss, H. N. Lee, L. Tian, K. Krzewski and J. E. Coligan, Liposome preparation for the analysis of lipid-receptor interaction and efferocytosis, Curr. Protoc. Immunol., 2018, 120, 14.44.1–14.44.21 CAS.
- C. M. Lee, B. Trevino and M. Chaiyawat, A simple and rapid solvent extraction method for determining total lipids in fish tissue, J. AOAC Int., 1996, 79, 487–492 CrossRef CAS PubMed.
- L. Patthy and E. L. Smith, Reversible modification of arginine residues. Application to sequence studies by restriction of tryptic hydrolysis to lysine residues, J. Biol. Chem., 1975, 250, 557–564 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2021 |