Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Recent progress in extrusion 3D bioprinting of hydrogel biomaterials for tissue regeneration: a comprehensive review with focus on advanced fabrication techniques
Mohsen
Askari
 ab,
Moqaddaseh
Afzali Naniz
ab,
Moqaddaseh
Afzali Naniz
 ab,
Monireh
Kouhi
c,
Azadeh
Saberi
d,
Ali
Zolfagharian
e and
Mahdi
Bodaghi
ab,
Monireh
Kouhi
c,
Azadeh
Saberi
d,
Ali
Zolfagharian
e and
Mahdi
Bodaghi
 *a
*a
aDepartment of Engineering, School of Science and Technology, Nottingham Trent University, Nottingham NG11 8NS, UK. E-mail: mahdi.bodaghi@ntu.ac.uk
bDepartment of Textile Engineering, School of Material Engineering & Advanced Processes, Amirkabir University of Technology, Tehran, Iran
cBiomaterials Research Group, Department of Materials Engineering, Isfahan University of Technology, Isfahan, Iran
dNanotechnology and Advanced Materials Department, Materials and Energy Research Center, Tehran, Iran
eSchool of Engineering, Deakin University, Geelong, Victoria 3216, Australia
First published on 9th October 2020
Abstract
Over the last decade, 3D bioprinting has received immense attention from research communities for developing functional tissues. Thanks to the complexity of tissues, various bioprinting methods have been exploited to figure out the challenges of tissue fabrication, in which hydrogels are widely adopted as a bioink in cell printing technologies based on the extrusion principle. Thus far, there is a wealth of literature proposing the crucial parameters of extrusion-based bioprinting of hydrogel biomaterials (e.g., hydrogel properties, printing conditions, and tissue scaffold design) toward enhancing performance. Despite the growing research in this field, numerous challenges that hinder advanced applications still exist. Herein, the most recently reported hydrogel-based bioprinted scaffolds, i.e., skin, bone, cartilage, vascular, neural, and muscular (including skeletal, cardiac, and smooth) scaffolds, are systematically discussed with an emphasis on the advanced fabrication techniques from the tissue engineering perspective. The methods covered include multiple-dispenser, coaxial, and hybrid 3D bioprinting. The present work is a unique study to figure out the opportunities of the novel techniques to fabricate complicated constructs with structural and functional heterogeneity. Finally, the principal challenges of current studies and a vision of future research are presented.
Introduction
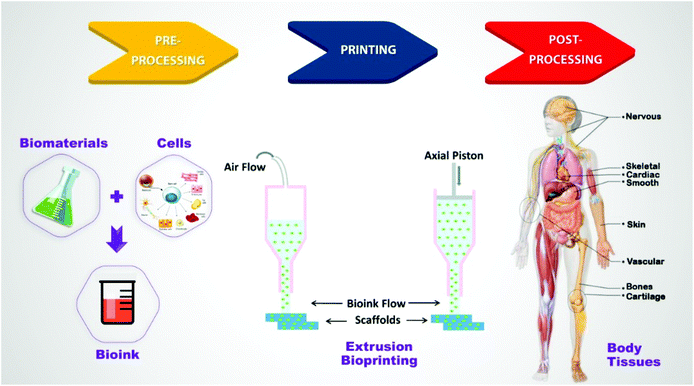
Tissue engineering (TE) is an interdisciplinary field that comprises applying principles of life sciences and materials engineering to restore, maintain, and enhance tissue function.1,2 By harvesting cells from a patient (or other resources) and seeding onto or incorporating into a tissue scaffold, the cell-scaffold construct tends to undergo maturation to being a functional construct. It could be implanted into the patient to help repair or heal the damaged tissues.3 The typical design of tissue scaffolds as functional constructs depends on the understanding of their composition and organization. Accordingly, appropriate architectures and biomaterials/cells to mimic the key properties of tissue should be carefully selected.4 In this regard, a wide variety of cells, biomaterials, growth factors, and other supporting components have been investigated to create functional constructs.5–8 However, scaffold-based strategies not only have often failed to imitate complex structures of native tissues but also remained ineffective for placing multiple types of cells in desired positions.9In recent years, three-dimensional (3D) bioprinting has occupied a prominent place among all other methods for producing tissue scaffolds to bridge the divergence between artificially engineered tissue constructs and native tissues.10–12 Due to increasing interest, its global market, which was estimated at nearly $ 487 million in 2014, is foreseen to reach $ 1.82 billion in 2022.13 Using 3D bioprinting techniques, bioinks (mainly comprising biomaterials, living cells, and/or bioactive molecules) are printed in a predesigned manner and incorporated with living cells as dynamic structures with functions (e.g., growth and proliferation) within scaffolds to regenerate target tissues.14–16 Besides, it is a rapid and inexpensive method to generate geometrically well-defined scaffolds,17 and offers precise control over the composition of cells and biomaterials, associated with spatial distribution, and architectural accuracy.12,18 Moreover, its ability for precise placement of high-density cells in the desired location and multiple types of cells in an orderly fashion mimics heterogeneous architectures of native tissues. It also allows the formation of vascular structures capable of recapitulating the structural features of human tissues.9
Current 3D bioprinting technologies for engineering functional human tissues and organs that recapitulate their native prototypes can be categorized based on four major governing approaches: (1) droplet-based, (2) extrusion-based, and (3) laser-induced forward transfer, and (4) stereolithography bioprinting, and each of them can be more sub-categorized based on the specific mechanisms with which materials and cells are positioned.19–21 Among these, one of the most interesting explored techniques is extrusion-based bioprinting (EBB), which extrudes or dispenses continuous strands or fibers of biomaterials to form 3D scaffold structures17,22 in a layer-by-layer manner.23 It should be mentioned that although novel bioprinting techniques are being developed (e.g., contactless24 and volumetric bioprinting25), EBB remains the most prevalently employed approach in research and commercial areas to fabricate 3D cell-laden scaffolds due to its cost-effectiveness, accessibility and capacity to replicate tissue complexity.20,21,26
The main advantages of EBB compared to other 3D printing methods have been concluded as follows: (1) producing tissue scaffolds using a wide variety of biomaterials and cell types, even hydrogel polymers with suspended cells;27 (2) successful layer-by-layer deposition of biomaterials with physiological cell density in a designed way;28 (3) relatively less process-induced cell damage compared to other techniques;22,29 and (4) great potential for regulating and conducting stem cell growth and differentiation for many applications.15 Despite some challenges such as limited strand resolution (typically greater than 100 μm),15 and restricted biomaterials choice,17 the stated advantages associated with economical aspects and commercial availability have made EBB the most popular technique amongst tissue engineers and researchers.30
Although various polymeric biomaterials have been employed as scaffold matrices, which had adequate qualities to provide necessary support and properties required for tissue growth, they had insufficient cell mimicking quality and inadequate interaction with stromal cells, which are essential in promoting tissue regeneration.31,32 An alternative approach to overcome the restrictions of these polymeric scaffolds was designing hydrogel-based bioprinted constructs.33 Hydrogels are well known as an appropriate environment for scaffold development because of their composition, their structure is somewhat similar to the extracellular matrix (ECM) of much human tissue and they are easily prepared using relatively mild conditions and aqueous chemistries. They have gained widespread popularity in recent years based on their ability to maintain a distinct and porous 3D structure, to provide mechanical support for cells in engineered tissues, to adapt to interchangeable sol–gel conditions, to simulate the native extracellular matrix, to retain high water content, and to achieve high cell seeding density and homogeneous cell distribution throughout the scaffold.34–36 Their high water content provides a hydrated tissue-like environment which is appropriate for cell incorporation, and enhances the cell viability in bioprinting in a hydrated and mechanically stable 3D environment.37 These structural properties enable hydrogels to be utilized as tissue scaffolds in the body by increasing the influx of cell metabolites and the disposal of cell waste through their pores.38,39 A large and growing body of the literature in recent years has investigated hydrogels concerning their origin, and structural, chemical, and biological characteristics.4,40–42 There are also systematic discussions in terms of suitable hydrogel-forming polymers for TE according to the origin and nature of the polymer, hydrogel-forming mechanisms, crosslinking mechanisms, modification approaches, their physical, chemical or biological properties, their functionality and printability and their mostly affected printing parameters.31,43,44
To answer the question as to what are the ideal properties of an extrusion-based hydrogel bioprinted scaffolds, there is a wealth of the literature concentrating on the crucial parameters of EBB such as hydrogel parameters, printing conditions, and tissue scaffold design.9,10,19,36,45–48 Also, some researchers have extended the discussion by investigating the optimized performance of bioprinting in native tissue development based on the simultaneous regulation of the main practical parameters of EBB.37 However, reviews on the limitations and potentials of tissue scaffolds in the EBB of polymeric hydrogels have not been well-documented. In this review, recently developed bioprinted scaffolds, i.e., skin, bone, cartilage, vascular, neural, and muscular (including skeletal, cardiac, and smooth) scaffolds, are discussed with a focus on novel approaches to building constructs (Fig. 1). Moreover, this review will provide recommendations for future challenges in 3D bioprinting and perspectives for advanced research on this framework. This review is not meant to be exhaustive but would offer the most prominent advances in their respective fields, and those with the most promise for prospective studies.
EBB strategies
In an EBB system, the positioning process allows the dispensing head to deposit the bioink onto the printing stage through three mechanisms: a pneumatic-, piston-, or screw-based system.49 Since the manufacturing process strongly affects the geometry of the scaffolds, there are numerous reports on the practical parameters in detail.9,10,19,42,46,50 Here, the focus is on the outcomes of various techniques on scaffold construction based on the TE perspective and fabrication methodology. From the TE perspective, direct and indirect, in situ and in vitro, and also scaffold-free versus scaffold-based bioprinting methods are considered for tissue fabrication. Besides, considering the limitations of conventional bioprinting technology to fulfil all the conditions, advanced EBB fabrication methods have been developed, which could be generally classified into multiple-dispenser, coaxial, and hybrid bioprinting.17One of the most prevalent methods for micro-extrusion of multiple materials is the application of multiple nozzles enabling simultaneous deposition of various bioinks with minimum cross-contamination.21,51 To be more specific, fabrication of practical constructs utilizing a multi-dispenser system provides the capability of simultaneous deposition of multiple biomaterials and cells in a uniformly blended form with minimum cross-contamination, which is promising for fabricating hydrogel-based composite scaffolds (e.g., combination of hydrogels with synthetic polymers or sacrificial materials).52
However, the complexity and high cost of assembling the required robotic system hinder the broad application of this method. Thus, advanced fabrication techniques are necessary to generate complex constructs with controlled architectures and adequate mechanical properties.53 Employing coaxial bioprinting (a configuration featuring two or more capillary nozzles connected in a coaxial fashion) would result in a more complex structure that would benefit TE applications such as vascularization.54 The core/shell geometry appears promising in creating vascular networks due to its specific characteristics: (1) capacity of fabricating hierarchical, multi-layer tissue constructs with desirable biological and mechanical properties using multi-material and cellular constructs, (2) increasing cell viability during cultivation, and (3) tuning the biophysical and biological properties of the vessel construct.55,56
One of the freshest trends in regenerative medicine is the improvement of 3D-printing hydrogel scaffolds with biomimetic structures. However, it has been almost difficult to achieve extremely biomimetic hydrogel constructs with proper mechanical properties resembling the natural tissue.57,58 Therefore, hybrid bioprinting techniques have been introduced to fabricate more complex constructs, e.g., a combination of a UV-light beam with EBB and integration of a multi-dispenser system with coaxial configurations or electrospinning technology.
Despite providing a controllable geometric configuration (macro-architecture), pore size, shape, interconnection, and spatial distribution (micro-architecture), 3D printing systems fail to create surface nanotopographies, which are beneficial in enhancing the performance of 3D printed constructs.59,60 On the other hand, for the electrospun nanofiber scaffolds, although the porosity is high, even up to 90%, the pore size is too small for cells to migrate and infiltrate. Besides, electrospun fibers typically form 2D membranes with low thicknesses rather than bulk 3D scaffolds, and fibrous scaffolds usually have poor mechanical properties due to their high surface-area-to-volume ratios and porosity.61–63 To overcome these issues, and also to mimic the ECM, the EBB technique has been consolidated with electrospinning to develop scaffolds possessing advantages of different kinds of materials only in one construction.64–67 In other words, combining 3D printing and electrospinning can make their particular advantages complementary and improve the capability of developing functional biomimetic scaffolds.68–70
Furthermore, the emerging microfluidic organ-on-a-chip platform with widespread applications has opened up a new window to create more complex constructs.71 The combination of bioprinting with organ-on-a-chip technology enables direct cell printing and/or patterning in microfluidic devices, and production of the biomimetic heterogeneous microenvironment, and complex 3D microstructures.72,73 It also enables the production of complex and biomimetic in vitro models for simulation, mechanistic biological studies and drug testing.74
An overview concerning the application of advanced fabrication strategies of EBB for TE is presented in Table 1.
| Strategies | Tissue | Biomaterialsa | Cellsb | Ref. | |
|---|---|---|---|---|---|
| a GelMA: Gelatin-methacryloyl; PVA: polyvinyl alcohol; PCL: poly(ε-caprolactone); PEG: polyethylene glycol; PEGDA: poly(ethylene glycol) diacrylate; HA: hyaluronic acid; dECM: decellularized extracellular matrix; HPMC: hydroxypropyl methyl cellulose; CNTs: carbon nanotubes; PEGTA: 4-arm poly(ethylene glycol)-tetra-acrylate; GPT: gelatin-PEG-tyramine; mdECM: skeletal muscle dECM; vdECM: vascular dECM; GelMA/C: blend of GelMA and nanofibrillar cellulose; PEO: poly(ethylene oxide); Collagen type I: collagen I; PLGA: polylactic-co-glycolic acid; PEGOA: PEG acrylate with a tripentaerythritol core; PF: polyethylene glycol monoacrylate-fibrinogen; and HAMa: hyaluronic acid–methacrylate. b hFBs: Human skin fibroblasts; hKCs: human keratinocytes; SaOS-2: sarcoma osteogenic; HUVECs: human umbilical vein endothelial cells; hMSCs: human mesenchymal stem cells; hNDFs: Human neonatal dermal fibroblasts; hBMSCs: human bone marrow mesenchymal stem cells; BMSCs: bone marrow mesenchymal stromal cells; ACPC: articular cartilage-resident chondroprogenitor cells; ADSCs: adipose-derived mesenchymal stem/stromal cells; hASCs: human adipose derived stem cells; NPCs: neuronal progenitor cells; OPCs: oligodendrocyte progenitor cells; hCPCs: human cardiac progenitor cells; hTMSCs: human turbinate tissue-derived MSCs; NRVCMs: neonatal rat ventricular cardiomyocytes; iPSCs-dCMs: induced pluripotent stem cell-derived cardiomyocytes; ECs: endothelial cells; RNCMs: rat neonatal cardiomyocytes, HUVECs: human umbilical vein endothelial cells; HDFs: human dermal fibroblasts; hNSCs: human neuronal stem cells; MNPCs: mouse neural progenitor cells; MSCs: mesenchymal stem cells; HCASMCs: human coronary artery smooth muscle cells; hSKMs: human skeletal muscle cells; HUVSMCs: human umbilical vein smooth muscle cells; CPCs: cartilage progenitor cells; hiPSC-CMs: human induced pluripotent stem cell cardiomyocytes; HASMCs: primary human airway smooth muscle cells; HISMCs: primary human intestinal smooth muscle cells; hAFSCs: human amniotic fluid-derived stem cells; hMPCs: human muscle progenitor cells; IPFP-ASCs: human infrapatellar fat pad derived adipose stem cells; and HCAECs: human coronary artery endothelial cells. | |||||
| Multi-dispenser bioprinting | Skin | Collagen type I (rat tail) | hFBs, hKCs | 103 | |
| Collagen type I (rat tail) | hFBs, hKCs | 104 | |||
| Bone | GelMA, PVA | SaOS-2 | 156 | ||
| GelMA, silicate nanoplatelets | HUVEC, hMSCs | 158 | |||
| Fibrinogen, gelatin, pluronic F127, silicon perfusion chips | HUVEC, hNDFs, hBMSCs | 157 | |||
| GELMA, pluronic F127 | Rat BMSCs | 342 | |||
| Alginate, PVA | Rat BMSCs | 343 | |||
| Alginate, PVA, HA | MC3T3-E1 | 142 | |||
| Gelatin, PVA | MG63 | 344 | |||
| Alginate, pluronic F127 | hBMSCs | 345 | |||
| RGD-γ alginate, PCL | Pig BMSCs | 133 | |||
| Alginate, gelatin, PCL, polydopamine modified calcium silicate | HUVEC Wharton's jelly MSCs | 134 | |||
| HA, gelatin, atelocollagen, PCL, PLGA | MC3T3-E1 | 137 | |||
| PCL, alginate | MC3T3-E1 | 136 | |||
| Cartilage | PCL, alginate | Chondrocytes | 193 | ||
| Gellan, alginate, BioCartilage (cartilage extracellular matrix particles) | Chondrocytes | 210 | |||
| PCL, PLGA, TGF 3, CTGF | MSCs | 346 | |||
| GelMA, Pluronic F-127 | BMSCs, chondrocytes, ACPCs | 206 | |||
| PCL, alginate, PEG | hASCs | 347 | |||
| Vascular | Gelatin, alginate, fibrinogen | ADSC, hepatocyte | 224 | ||
| Alginate, xanthan gum | — | 225 | |||
| Alginate | Human glioma U87-MG | 226 | |||
| Neural | Matrigel, gelatin, fibrin, GelMa, PEGDA, alginate, methylcellulose | NPCs, OPCs | 254 | ||
| Skeletal muscle | HA, gelatin, fibrinogen | C2C12, NIH/3T3 | 274 | ||
| Cardiac muscle | Heart dECM | hCPCs, hTMSCs | 314 | ||
| Fibrinogen, gelatin, aprotinin, glycerol, HA | NRVCMs | 307 | |||
| Alginate, calcium carbonate | iPSCs-dCMs, ECs, RNCMs, HUVECs, lumen-supporting fibroblasts | 318 | |||
| Coaxial bioprinting | Skin | Alginate, collagen | hFBs, hKCs | 106 | |
| Bone | Alginate, collagen | MG63, hASCs | 159 | ||
| Alginate, collagen, fibronectin | Rat BMSCs | 160 | |||
| HPMC, alginate | MC3T3-E1 | 161 | |||
| Collagen, GELMA, alginate | MC3T3-E1 | 162 | |||
| Cartilage | GelMa, HAMa | ADSCs | 215 | ||
| GelMa, HAMa | MSCs | 216 | |||
| Vascular | Alginate, CNTs | HCASMCs | 219 | ||
| Alginate | L929 | 229 | |||
| GPT | HUVECs, HDFs | 56 | |||
| GelMA, SA, PEGTA | HUVECs, hMSCs | 231 | |||
| Neural | Alginate, Matrigel | hNSCs | 251 | ||
| Alginate | MNPCs | 252 | |||
| Skeletal muscle | mdECM, vdECM | hSKMs, HUVECs | 289 | ||
| Smooth muscle | Alginate | HUVSMCs | 325 | ||
| GelMA/C | HCASMCs, hBMSCs, HUVECs | 328 | |||
| Hybrid bioprinting | Electrospinning + EBB | Skin | Nanofibers: PCL, silk sericin | hFBs | 118 |
| Struts: chitosan, alginate | |||||
| Bone | Nanofibers: PCL, gelatin | MC3T3-E1 | 165 | ||
| Struts: PCL | |||||
| Nanofibers: PCL | MG63 | 166 | |||
| Struts: alginate | |||||
| Skeletal muscle | Nanofibers: PCL, collagen I, | C2C12 | 296 | ||
| Struts: collagen I, PEO | |||||
| Nanofibers: PVA | C2C12 | 297 | |||
| Struts: PCL, collagen I | |||||
| Nanofibers: alginate, PEO, lecithin struts: alginate, PCL | MG63 | 298 | |||
| Nanofiber: alginate | C2C12, HUVECs | 299 | |||
| Struts: PCL, collagen | |||||
| Cartilage | Gelatin, PLGA | chondrocytes | 211 | ||
| Microfluidic + EBB | Skin | Alginate | hFBs | 116 | |
| Alginate, fibrin, collagen I, HA | hFBs, hKCs | 117 | |||
| Microfluidic + coaxial bioprinting | Bone | Collagen type I, GelMA, alginate | MC3T3E1, ATCC | 162 | |
| Vascular | Alginate, chitosan | CPCs | 227 | ||
| Alginate | HUVEC | 230 | |||
| PEGOA, GelMA, alginate | C2C12, skeletal myocytes, NIH/3T3, fibroblasts | 232 | |||
| Alginate | Fibroblasts, smooth muscle cells, ECs | 236 | |||
| Skeletal muscle | PEG, fibrinogen | C2C12 and BALB/3T3 | 293 | ||
| Alginate, PF | C2C12 | 294 | |||
| Cardiac muscle | GelMA, alginate | HUVECs, RNCMs, hiPSC-CMs | 315 | ||
| Alginate, PF | iPSCs-dCMs, HUVEC | 316 | |||
| Smooth muscle | Small intestine dECM | HASMCs, HISMCs | 324 | ||
| General cell culture | GelMA/alginate | HUVECs, MCF7 breast cancer cells, NIH/3T3 mouse fibroblasts | 72 | ||
| GelMA, alginate | HUVECs | 73 | |||
| Microfluidic + multi-dispenser bioprinting | Bone | PCL, Pluronic F-127, gelatin, fibrinogen, HA, glycerol | hAFSCs | 135 | |
| Cartilage | PCL, Pluronic F-127, gelatin, fibrinogen, HA, glycerol | Rabbit ear chondrocytes | 135 | ||
| Skeletal muscle | PCL, Pluronic F-127, gelatin, fibrinogen, HA, glycerol | Mouse C2C12 myoblasts | 135 | ||
| Gelatin, PCL | hMPCs | 292 | |||
| UV-light beam | Cartilage | GelMa, HAMa | IPFP-ASCs | 214 | |
| Cardiac muscle | Alginate, methacrylated collagen I, MeCol, CNTs | HCAECs | 317 | ||
Tissue bioprinting
Skin
As the largest and highly complex organ of the body, skin serves as a protective shield against pathogens, irritants, and antioxidants, physical and UV damage, and any external harmful agents.75,76 Being in a direct contact with the external environment makes it highly susceptible to different varieties of injuries.77,78 Regarding the wound size, extent, and depth, researchers have been developing numerous types of wound dressings or natural product-based skin substitutes.79,80 Despite all the advancements attained so far, several limitations with the use of autografts, allografts, and wound dressings81 have led to the development of tissue-engineered skin substitutes,82 so that they hold great promise for improving the treatment of skin defects.83,84 In response to the limitations of the mentioned techniques, combined with a foreseen higher demand for artificial skin,85,86 3D bioprinting was exploited to facilitate the simultaneous and highly specific deposition of multiple types of skin cells and biomaterials, i.e., a process that is lacking in conventional skin tissue-engineering approaches.87The skin that has almost a thin, layered, and structured nature, along with easy access to cell sources has promoted the immediate adoption of 3D bioprinting technology for the skin TE.88 Furthermore, 3D bioprinting serves as an innovative strategy to overcome the current impasses in the manufacturing of skin tissue, such as poor vascularization, and the absence of hair follicles, and sweat glands in the construct.42 Among various 3Dbioprinting techniques, to date, EBB has been accepted as the most promising approach for generating skin or soft tissue constructs.76,89
An ideal bioprinted skin should have specific characteristics such as biocompatibility, desired mechanical properties, proper surface chemistry, high porosity with a network of interconnected pores that will allow cells to attach and the capability of transferring nutrients and eliminating wound exudates.42 Accessible literature review reveals that a variety of biomaterials have been widely studied for the generation of skin grafts,90–92 in which the most common materials are hydrogels.93–98 However, the commonly available natural polymers besides synthetic polymers cannot provide the complex microenvironment analogous with the natural ECM.27 This complexity can be ascribed to the confined data on the dynamic assembly and interactions of such materials to create patterned and practical morphologies.99 To combat such issues, the use of a decellularized ECM (dECM) is currently receiving immense consideration as a promising alternative owing to its ability to preserve the complex functional and structural proteins of the ECM.100 Accordingly, a 3D cell-printed skin tissue utilizing skin-dECM (S-dECM) was presented by Cho's group. As porcine skin is highly similar to human skin, they successfully used decellularized porcine skin as a novel bioink, which contains intrinsic factors required for cell proliferation and showed that the new construct is highly stable for two weeks with a remarkable wound healing performance in vivo.100 However, the contradiction between the excellent biocompatibility and poor formability of dECMs limited their extensive applications. To overcome this challenge, a modified cryogenic free-form extrusion bioprinter was developed to directly print a simple decellularized small intestinal submucosal (dSIS) material extracted from porcine skin (Fig. 2(a)).101 Applying this approach, dSIS scaffolds with excellent physicochemical attributes and enhanced biocompatibility were fabricated. Owing to the similar chemical composition of dSIS to the components of dECM (mainly collagens and polysaccharides), this approach could open a new avenue for future studies.
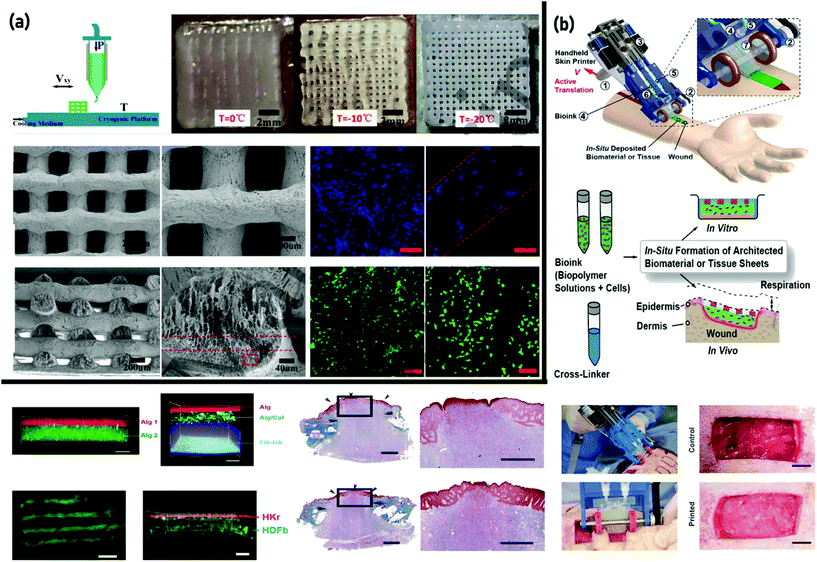 | ||
| Fig. 2 3D bioprinting of skin tissue: (a) cryogenic free-form extrusion bioprinting of decellularized small intestinal submucosal (dSIS) scaffolds with distinctive physicochemical characteristics and enhanced biocompatibility. Reproduced from ref. 101 with the permission of IOP Publishing, © 2018; and (b) employing a handheld bioprinter to generate skin cell-laden sheets with controllable thickness, width, and composition via incorporating dermal and epidermal cells into various cross-linkable hydrogels. Reproduced from ref.117 with the permission of the Royal Society of Chemistry, © 2018. | ||
Generally, there are two main approaches concerning the skin EBB for wound treatment:102 (1) in vitro bioprinting where the printed tissue is transplanted into the defect site and (2) in situ bioprinting where the bioinks are printed directly into the defect site. The feasibility of using bioprinting to fabricate skin constructs in vitro was first shown with multilayered engineered tissue composites of hFBs and hKCs deposited layer-by-layer within a collagen hydrogel, resulting in an inner layer of hFBs and an outer layer of hKCs.103 To be more specific, a four-nozzle bioprinter was developed utilizing pneumatic extrusion supported by microvalve control. Aiming to obtain multi-layered engineered composite tissues replicating natural skin layers, ten layers of the collagen hydrogel precursor were deposited, in which human skin fibroblasts (hFBs) were printed in the second layer, and human keratinocytes (hKCs) were printed in the eighth layer separately.103 By applying a similar bioprinting device (but for deploying eight nozzles), a variable number of layers of cross-linked collagen and collagen, including either hFBs or hKCs, were printed for expressing the epidermis, dermis, and dermal matrix of natural skin tissue. The printed tissue construct was comparable to human skin tissue biologically and morphologically and displayed better shape and form retention through in vitro cultures.104 Kim et al. engineered a collagen scaffold that had notably good cellular behavior but poor mechanical stability regarding the extremely porous structure (>95%) and poor mechanical characteristics of collagen.105 To overcome this insufficiency, they produced a core (alginate)/shell (collagen) scaffold which showed great structural stability, and optimum quantification of viable and proliferating hFB and hKC cells when cultured for a 7 day duration (in vitro and in vivo). The developed construct also demonstrated an approximate Young's modulus 6.7 times that of pure collagen, which mimics the skin modulus.106 In a study reported by Cubo et al., fibrin-based bilayer dermal constructs were fabricated utilizing human plasma and primary hFBs and hKCs taken from skin biopsies.107 The histological and immuno-histochemical in vitro and in vivo analyses indicated that the 3D-bioprinted skin constructs exhibited a high degree of similarity to the native human skin. Kim et al.108 used this method to fabricate collagen-based scaffolds with a poly(ε-caprolactone) PCL mesh, to form the dermal component of a skin substitute. It was exhibited that the incorporation of the PCL mesh could stabilize the dermal matrix, and prevent collagen shrinkage during the maturation process. In a recent study, a thermosensitive poly(N-isopropylacrylamide-co-acrylic acid) (p(NIPAAm-AA)) hydrogel was developed and implemented for various 3D printing methods (i.e, a single nozzle and a single syringe, coaxial needles and double syringes, and a single nozzle and double syringes). Relatively high cell viability of keratinocytes, fibroblasts and endothelial cells was achieved through 3D printing of the cell-laden hybrid bioink (p(NIPAAm-AA) and fibrin). Also, superficial cornification of the epidermis layer as well as sprouting and splitting of the subcutaneous endothelial cells were inspected.109
In comparison with the transplantation of in vitro fabricated constructs, in situ bioprinting avoids the risk of damaging the thin and fragile construct during transport and handling, and avoids potential issues related to the correct placement and orientation of a construct with a complex 3D topology. In one of the first descriptions of in situ bioprinting, human keratinocytes and fibroblasts were printed directly into a full-thickness mouse skin-wound model.110 The wounds were first scanned to obtain precise information on the wound topography, which then guided the print heads to deposit specified materials and cell types in appropriate locations. The first layer of a fibrinogen–collagen hydrogel precursor containing fibroblasts was bioprinted, followed by the simultaneous deposition of thrombin to form a fibrin–collagen hydrogel. An additional layer of keratinocytes was then bioprinted on top of the fibroblast layer via a similar deposition approach. In studies by Skardal et al., amniotic-fluid-derived stem cells were deposited on full-thickness skin wounds in mice, using either a fibrin–collagen bioink111 or a hyaluronic acid (HA)-based gel with tuneable properties tailored for extended cytokine release.112 The secretion of trophic factors accelerated wound-closure rates and promoted angiogenesis; however, the stem cells did not permanently integrate into the regenerated skin. The same approach was recently applied in a porcine model with large full-thickness wounds, where in situ bioprinting led to the complete re-epithelialization of the large wound after 8 weeks.113 The main advantage of this approach is the rapid coverage of large wounds with permanent skin tissue, and its accelerated healing.
From the fabrication point of view, advanced approaches have been considered to satisfy the complex necessities of the skin tissues. Accordingly, hybrid bioprinting by integrating the advantages of EBB and other techniques has emerged as a new method to create scaffolds that mimic targeted tissues.114 In 2012, Leng et al.115 developed a device consisting of a ten-layer microfluidic device with seven on-chip reservoirs that, in the following year, was applied to bioprinting of a fibroblast-laden hydrogel into wound dressings, which were subsequently implanted into murine wound models.116 Hence, accurate spatio-temporal control over the cell location and cell seeding was achieved, and the experimental results revealed enhanced wound healing, and keratinization was observed. In a remarkable report by Hakimi et al.117 (from the same research group), this device was developed into a portable skin printer (weight <0.8 kg) capable of being applied in swift repairing of deep wounds. The study demonstrated the in situ production of skin sheets in porcine and murine wound models as a direct therapy using skin-specific cells in the bioink. The skin cell-laden sheets with controllable thickness, width, and composition were produced by incorporating dermal and epidermal cells into different cross-linkable hydrogels containing alginate or fibrin mixed with collagen and HA (Fig. 2(b)).117 Such handheld 3D printers could be revolutionary in the prevailing healthcare market since patients do not have to wait for the laboratory-grown cellular skin grafts. Additionally, this technology could be utilized for emergency circumstances such as burn trauma cases and used for urgent treatment in real-time. As mentioned before, the preparation of electrospun fibers into 3D porous biomimetic scaffolds with accurately controllable shapes and large pores for tissue regeneration has attracted research attention.59,60 Accordingly, 3D skin asymmetric constructs (3D_SAC) were produced using electrospinning and 3D bioprinting techniques.118 A PCL and silk sericin blend was electrospun to produce a top layer aimed at mimicking the epidermal features. In turn, the dermis like layer was formed by printing a chitosan/sodium alginate (SA) hydrogel. The results obtained from the in vitro assays revealed that the 3D_SAC display a morphology, porosity, mechanical properties, wettability, antimicrobial activity, and a cytotoxic profile that enables their application as a skin substitute during the healing process.118
Over the past four decades, numerous researchers have undertaken many efforts in the design of human skin tissue though there are still shortcomings and challenges required to be overcome. Although the functionality of printed constructs can be improved through introducing more varieties of cells and cell numbers, there are still significant hurdles such as the formation of vascular networks and sensory receptors in addition to the proper development of hair follicles, pigmentation, and epidermis generation and maturation. Furthermore, the emerging organ-on-chip and microfluidic technologies can considerably assist in replicating as close as possible the heterogeneous cellular composition of native skin tissue.
Bone
Bone tissue as a dynamic structure is the main constituent of the musculoskeletal system, and its high mineralization of the ECM makes it different from other connective tissues in rigidity and hardness.119 The repair of bone tissue is a global clinical issue that causes high morbidity in trauma patients and imposes an enormous socioeconomic problem.120,121 The gold standard for bone restoration still generally is autogenous bone grafts that are harvested from intra- or extra-oral sites; however, this has the limitation of low graft quantity, donor site morbidity, and infection. Although many researchers have made attempts to develop therapeutic approaches for the fabrication of human bone120,122,123 as a highly ordered and vascularized tissue,124 few have succeeded and there is still no effective treatment for most cases.125–127 As a result, bone tissue engineering (BTE) is undergoing a booming advancement as an alternative to bone grafting, where graft substitutes are made using biomaterials to replace or repair damaged bone defects.124 Among different biomaterials, hydrogels are considered as promising materials for BTE due to their physical or structural similarity to natural tissues; however, hydrogels often suffer from poor mechanical properties especially in BTE applications.128 By reviewing the available literature, it can be observed that some researchers have concentrated on the requirements for bioinks in 3D-printed bone scaffolds.120,122,129 For instance, Turnbull and coworkers130 critically focused on materials and barriers to clinical translation. They reported the ideal properties of bioactive composite 3D scaffolds and examined the recent use of polymers, hydrogels, metals, ceramics, and bio-glasses in BTE. In addition to the general characteristics of the bioinks in EBB, they should satisfy the specifications for bone tissue regeneration.131The challenge of using hydrogels for the fabrication of the musculoskeletal system via 3D bioprinting should be seriously considered since a stiff and coherent hydrogel-based construct would be required for implantation in the human body.132 Accordingly, different strategies have been developed to enhance the strength of hydrogel-based bioprinted constructs, including utilizing toughened hydrogels and reinforcement of printed hydrogels with thermoplastic polymers133–140 or bioceramics,141–144 nanofibers, nanoparticles,145–148 microparticles, and microcarriers.149,150 Moreover, the crosslinking of bioprinted constructs by UV-rays and chemical agents not only improves their mechanical properties, but could also increase the stiffness, longevity, and thermal stability of 3D printed constructs.127,151,152 Despite various attempts having been made to increase the stiffness of the hydrogel, few have succeeded. For instance, preculturing of cells in the constructs has been rejected because of being not economically and practically possible. Similarly, increasing the hydrogel cross-link density was declined due to the delay in new tissue formation by restriction of the nutrients and waste product diffusion within the highly cross-linked hydrogel system.143
Scaffolds for BTE need to contain a mixture of macropores allowing cell and osteon ingrowth in vivo and micropores to encourage cell–scaffold ligand interactions.130 Increased scaffold macroporosity has been shown to improve angiogenesis in vivo, whilst a degree of microporosity (pores with diameters lower than 10 μm) can improve cell–scaffold interactions, resulting in osteogenic effects. Gupta et al.,146 using gelatin/carboxymethyl chitin/HA, produced a hierarchical 3D bioactive scaffold in a cryogenic environment followed by lyophilization. While the outer shape and macroporosity were controlled by the 3D printer, the desirable rough surface morphology and the microporous structure were obtained through lyophilization. Their result showed that the incorporation of bulk and surface porosity could lead to an increase in the water uptake ratio, cell retention capability, cell infiltration, attachment, proliferation, alkaline phosphatase (ALP) level, and mineralization.146 However, the microvasculature as a major challenge in engineering large bone graft substitutes153 is receiving considerable attention because bone is composed of an extensive vascular system in the medullary cavity that infiltrates into the bone containing osteocytes within a 100 μm distance. In traumatic injuries, necrosis of the blood vessels restricts the supply of nutrients and oxygen to the affected site, leading to tissue death.124 The current strategy is to implant synthetic bone grafts, which often fail in the case of critical-sized defects as the peripheral vasculature does not reach the core of the construct. Therefore, the formation of congruent bone largely depends upon the development of a functional vascular system, which remains a big hurdle in the fabrication of human-scale constructs.154,155 Several convergent bioprinting strategies used to handle this issue could be explained as follows: (1) multi-dispenser bioprinting with sacrificial materials or in combination with thermoplastic polymers and (2) coaxial bioprinting.
Applying sacrificial inks to create 3D vascular structures throughout thick bone constructs can increase nutrient diffusion into an engineered bone graft substitute. Materials with reversible crosslinking mechanisms (e.g., Pluronic F127, polyvinyl alcohol (PVA), agarose, and gelatin) are often employed as the sacrificial bioink.130 In such cases, the vascular network is fabricated through a fugitive bioink capable of being eliminated with suitable solvents or thermal modification resulting in a perfusable vasculature construct.124 The origin of these scaffolds can be traced back to the work by Sawyer et al.156 who scaled up a 3D thick perfused bone construct by printing cell-laden gelatin-methacryloyl (GelMA) with PVA as a sacrificial polymer. The construct was designed to have a central horizontal channel that supported a GelMA hydrogel laden with osteoblast-like cells. This study demonstrated the potential of using this technology to generate thick cell-laden constructs containing user-defined channels to aid the development of vascularized bone constructs.156 In another example of employing multi-dispenser printing,157 a 3D cell-laden vascularized tissue integrated parenchyma, stroma, and endothelium into a single thick tissue bioprinted in a perfusion chip. They printed cell-laden inks composed of human bone marrow-derived mesenchymal stem cells (hBMSCs) and human neonatal dermal fibroblasts (hNDFs) within a customized ECM alongside the embedded vasculature. It was subsequently seeded with human umbilical vein endothelial cells (HUVECs) in a crosslinking process to create a thick (1 cm) pervasive vascular network. Finally, it actively perfused with osteogenic media over more than six weeks. After 30 days, the printed hBMSCs expressed the highest osteocalcin expression in areas close to vessels perfused with osteogenic media. Collagen deposition was also found within printed filaments and around the circumference of the vasculature and alizarin staining also revealed a high degree of mineralization within the tissue (Fig. 3(a)).157 Byambaa and coworkers158 designed a complex bone-like 3D vasculature structure by printing a vascular endothelial growth factor (VEGF) functionalized GelMA bioink to fabricate bone and vascular tissues in one construct through a one-step bioprinting process.158 The central fiber of the construct formed a perfusable blood vessel of 500 μm after 12 days of in vitro incubation. The results demonstrated that synthetic silicate nanoplatelets can trigger osteogenesis and also induce the osteogenic differentiation of encapsulated human mesenchymal stem cells (hMSCs) within GelMA hydrogels. Furthermore, the approach of creating a central lumen using a composite GelMA-nanoplatelet hydrogel not only indicates the creation of a mechanically stable construct but also shows the perfusion with growth medium facilitated cell survival, proliferation, and osteogenic differentiation over 21 days.158 In brief, prominent advances in the production of multiscale channels with high accuracy and suitable biocompatibility have improved the sacrificial EBB of vascularized thick tissues. A broad range of channel sizes could be obtained based on the nozzle size and printability of bioinks. Among various bioinks, thermosensitive polymers are promising for printing cell-laden vascular constructs. However, the available literature lacks precise characterization of the effects of bioink combination and processing parameters such as pressure and light exposure on the biological characteristics of fabricated structures.
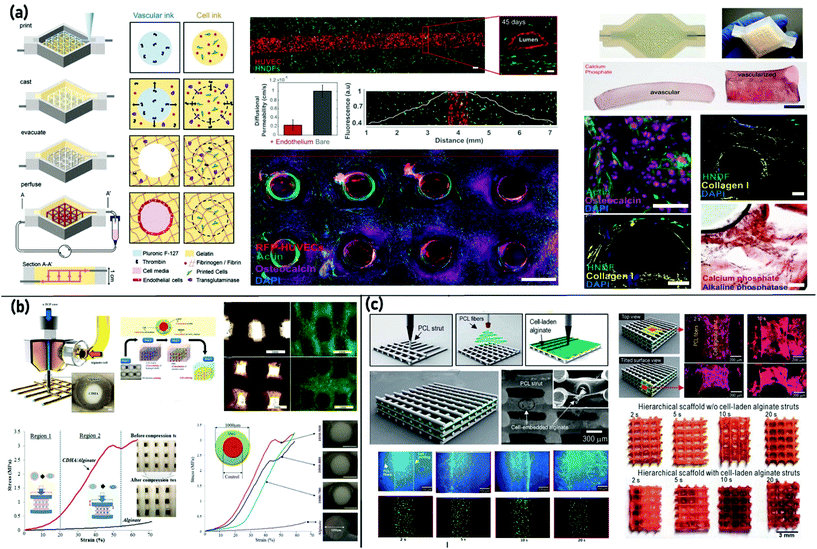 | ||
| Fig. 3 3D bioprinting of bone tissue (a) using a sacrificial ink to create 3D cell-laden vascularized tissue integrated parenchyma, stroma, and endothelium into a single thick tissue bioprinted in a perfusion chip. Reproduced from ref. 157 with the permission of the National Academy of Sciences, © 2016; (b) a cell printing process with a core (α-TCP)/shell (alginate + cell) geometry with a post-fabrication process, involving the crosslinking of the hydrogel shell and cementation of the ceramic core. Reproduced from ref. 161 with the permission of the Royal Society of Chemistry, © 2016; and (c) hierarchical scaffolds consisting of micro-sized struts with the appropriate inter-layered nanofibers between the struts supplemented with osteoblast-like cell-laden alginate struts. Reproduced from ref. 166 with the permission of the Royal Society of Chemistry, © 2014. | ||
As explained earlier, coaxial bioprinting is an exciting approach to fabricate hybrid and vasculature structures.159 The principal benefit of the core/shell construct is the potential of forming hierarchical, multi-layer tissue structures with desirable biological and mechanical attributes.160 Raja and Yun161 successfully provided bioprinted structures capable of homogeneous cell distribution along with performing a load-bearing function without breaking during tissue regeneration. It was the first simultaneous 3D printing of cells and bioceramics containing a core of α-TCP with a shell of alginate and pre-osteoblast bone cells. Accordingly, while the hydrogel shell prevented the immediate failure of the scaffold, even when the ceramic core was cracked, the construct showed greater mechanical stability than either brittle ceramics or weak hydrogels alone. Furthermore, data suggest that there is a direct connection between the shell thickness and mechanical properties in which the compressive modulus of each scaffold increased from 0.9 to 2.2 MPa with a decrease in shell thickness from 150 to 75 μm (Fig. 3(b)).161 As an innovative hybrid strategy, employment of the cell-laden core with a stable shell was introduced to produce vasculature bone constructs.162 Lee and Kim162 developed a low-temperature 3D bioprinting method improved with a microfluidic channel and a core/shell nozzle to fabricate cell-laden constructs for the cryopreservation of a cell suspension. The cryopreserved scaffold showed reasonable viability (∼85%), proliferation, and ALP activities similar to the non-cryopreserved scaffold.162 It should be noted that cryopreserved scaffolds have attracted considerable attention in TE since they can be considered ready-to-use “living” biomaterials, including a patient's cells.163
Following the hybrid bioprinting strategies, the combination of EBB and electrospinning has also been studied in TE of bone.164 For instance, a 3D composite scaffold was made through infusing PCL/gelatin dispersed nanofibers into the meshes of the PCL construct.165 According to the mechanical analysis outcomes, the compressive modulus of the scaffold (30.50 ± 0.82 MPa) was remarkably higher than that of the lyophilized electrospun scaffold (18.55 ± 0.56 MPa). Moreover, the microporous structure of the electrospun scaffold resulted in better cell proliferation and infiltration on the composite scaffold. In another study,166 a combination of a 3D printing system and an electrospinning device was utilized to fabricate a 3D cell embedded scaffold composed of perpendicular strands and a thin nanofiber sheet in the succeeding layer. The cell-laden alginate struts provided steady cell release to the layered nanofibers, resulting in a uniform cell distribution (Fig. 3(c)).166
Despite the progress in performing bone bioprinting, various challenges face the fabrication of clinically appropriate, functional bone grafts. The principal hurdles are (1) construct stability, (2) restricted construct size, (3) vascularization, (4) lack of mechanical characteristics, (5) integration to native tissue and (6) long-term function. Clinical translation will demand the application of integrated bioprinting platforms allowing the employment of multiple biomaterials to create biomimetic constructs at a clinically applicable scale. Besides, multidisciplinary strategies and continued funding are required to realize accomplishment in this developing research area.
Cartilage
Cartilaginous tissue is an avascular and aneural structure, including an almost low density of chondrocytes and an abundant water proportion (70%).167 It is a functional and very hydrated heterogeneous tissue for providing a low-friction, wear-resistant, and load-bearing surface in diarthrodial joints for an efficient joint move.36 According to the ECM composition, cartilage tissue can be classified into three categories, including elastic cartilage (if elastic fibres are present in the ECM), fibrous cartilage (if the matrix is rich in collagenous fibres), and hyaline cartilage (if the matrix is mainly composed of glycosaminoglycans (GAGs)).168 From the microscopic point of view, human cartilage is composed of a hydrated ECM, which is made of proteoglycans consisting of a core protein with covalently attached GAGs (accountable for the cartilages’ capacity to maintain high compressive loads), mainly chondroitin sulphates, and collagen type II fibrils (providing its high tensile strength and capability of tolerating shear stresses).169,170Trauma, accidents, or other infections could cause cartilage loss, due to its disability to self-repair because of avascularity, the low proliferation rate of chondrocytes, and its functional and structural complexity.171,172 Despite the existence of various treatments for chondral injuries, including autologous chondrocyte implantation, periosteal grafts, mosaicplasty, and microfracture, clinical investigations failed to exhibit reliable generation of normal hyaline cartilage and long-term solutions.173–175 Moreover, the generation of functional articular cartilage is challenging concerning the zonal structure of native tissue, including areas with different cell morphologies and arrangements, ECM arrangements, constituents, and distribution.176,177 The introduction of 3D bioprinting in TE has attained prominent progress in simulating the anatomy of articular cartilage tissue,178 and among various dispensing techniques, EBB is the most prevalent and affordable method.179,180 Applying this particular technique, researchers have reported the production of cartilage-like constructs through the combination of various hydrogels;46,181–187 However, the most efficient strategy has involved simultaneous deposition of thermoplastic polymers utilizing multi-dispenser systems, while structural materials are capable of maintaining mechanical forces, and hydrogels act as cell carriers.188–193 Besides, researchers have endeavored to modify bioinks’ attributes, such as their printability, mechanical properties, and degradation rates.176,177,194,195
For the generation of cartilage constructs, two main strategies of in vitro and in situ bioprinting have been considered in recent years. Employing the in vitro fabrication approach, chondrocytes, which can be harvested from various zones of the cartilage,196 have been deposited in hydrogels (e.g., gelatin and alginate, alginate, cartilage-dECM, and nanofibrillated cellulose)197–201 with high cell viability and zone-specific patterns.202,203 Printing of human chondrocytes in a shear-thinning nanofibrillated cellulose can also be combined with cross-linkable alginate to fabricate anatomically formed cartilage constructs, with high accuracy and permanence.186 Another approach includes the generation of constructs utilizing micromass chondrocyte pellets to make cartilage strands, with tubular penetrable alginate capsules serving as a repository for cell aggregation and tissue-strand maturation. This strategy resulted in ∼500 μm-diameter strands with notably enhanced cell density, and also increased post-transplantation maturation and function of the printed tissue.204 Combining various cell types may also improve the effectiveness of the engineered cartilage.205 In a research study reported by Levato et al.,206 three materials were loaded for printing via multi-dispenser heads: (1) a superficial zone-mimicking bioink, consisting of articular cartilage-resident chondroprogenitor cell (ACPC)-laden GelMA, (2) a middle/deep zone-mimicking bioink, composed of bone marrow mesenchymal stromal cell (MSC)-laden GelMA, and (3) Pluronic F-127 as a sacrificial ink to support (MSC)-laden GelMA during the process. The first seven layers and the last two were printed with the MSC-laden GelMA and ACPC-laden GelMA, respectively. The co-culture of cell types in multi-compartment hydrogels allowed generating constructs with a layered distribution of collagens and glycosaminoglycans, defining cartilage with shallow and deep areas, each with distinguished cellular and ECM combination.206 The combination of MSCs into a layered structure of natural and synthetic biomaterials can lead the cells to differentiate into zone-specific chondrocytes, producing native-like articular cartilage with mechanical and biochemical characteristics differing with depth.207,208 Similarly, hyaline-like cartilaginous tissue was created through the bioprinting of induced pluripotent stem cells (iPSCs) within a nanocellulose alginate bioink.209 In another example of employing multi-dispenser bioprinting, Kesti et al.210 fabricated cartilage grafts (i.e., 3D auricular, nasal, meniscal, and vertebral disk grafts) using a cartilage-specific bioink based on a blend of gellan, alginate, and a clinical product called BioCartilage (cartilage extracellular matrix particles). MRI and histological evaluation after 8 weeks in vitro revealed that this bioink supports the proliferation of chondrocytes and effective deposition of cartilage matrix proteins (in the presence of transforming growth factor beta-3). Besides, it was revealed that a cation-loaded transient support polymer improves physical gelation for structure stabilization.210 Utilizing a similar approach, Kundu et al.193 bioprinted cartilaginous tissue using PCL and chondrocyte cell-laden alginate. In vitro cell-based biochemical analysis was performed to determine glycosaminoglycans (GAGs), DNA, and total collagen contents from different PCL–alginate gel constructs. PCL–alginate gels, including transforming growth factor-b (TGFb), presented higher ECM formation. The histochemical and immunohistochemical analyses of the retrieved implants (after four weeks of implantation in the dorsal subcutaneous spaces of female nude mice) showed enhanced cartilage tissue and type II collagen fibril formation in the PCL–alginate gel (+TGFb) hybrid scaffold (Fig. 4(a)).193 In 2016, Kang et al.135 introduced an integrated tissue–organ printer (ITOP) for the reconstruction of ear cartilage tissue. The bioprinter was composed of multi-dispensing modules for delivering cells and various types of polymers. With the aim of facilitating the diffusion of nutrients into printed cells, the fabricated construct incorporated microchannels produced with the sacrificial molding of Pluronic1 F-127. To determine whether the printed ear constructs would mature in vivo, they were implanted in the dorsal subcutaneous space of athymic mice and were retrieved 1 and 2 months after implantation. It was confirmed that the shape was well sustained, with considerable cartilage generation upon gross examination. Also, the histological analysis showed the formation of cartilage tissue (Fig. 4(b)).135 In a recent study, a novel approach was presented by Chen and colleagues211 for the fabrication of electrospun fiber-based scaffolds with accurately controlled 3D shapes and large pores, as well as fibrous surface morphologies similar to that of the ECM, for cartilage regeneration. They processed gelatin/poly(lactic-co-glycolic acid) (PLGA) nanofibers into inks suitable for 3D printing, and then electrospun fiber-based inks were fabricated into printed constructs through combining 3D printing and freeze drying. The results exhibited good elasticity and water-induced shape memory, and scaffolds combined with chondrocytes attained satisfactory cartilage regeneration in vivo (Fig. 4(c)).211
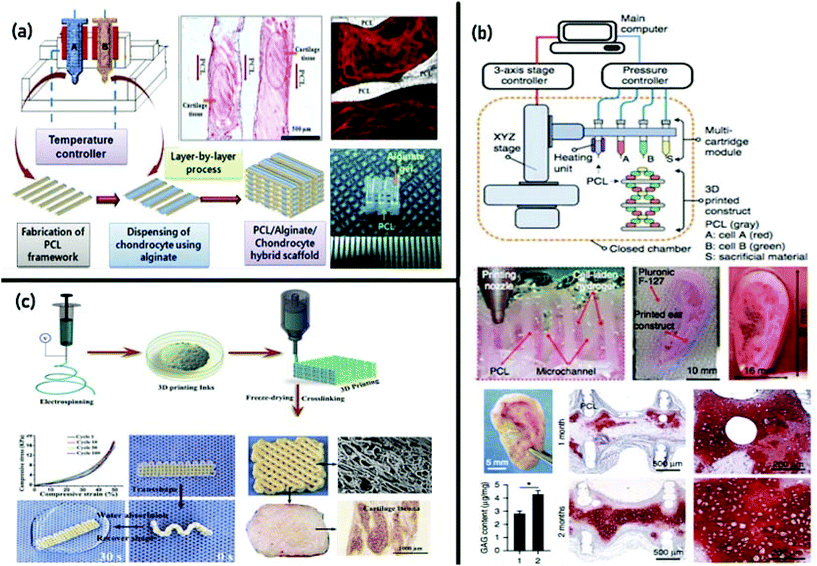 | ||
| Fig. 4 3D bioprinting of cartilage tissue: (a) fabrication of cartilaginous tissue using PCL and chondrocyte cell-laden alginate via multi-dispenser bioprinting. Enhanced cartilage tissue and collagen (type II) fibril formation was revealed via histochemical and immunohistochemical analyses of the retrieved implants after 4 weeks. Reproduced from ref. 193 with the permission of John Wiley & Sons, Ltd., © 2013; and (b) fabrication of cartilage tissues utilizing an integrated tissue–organ printer (ITOP). The results manifested the generation of ear-shaped cartilage with resilience characteristics similar to those of the rabbit ear. Reproduced from ref. 135 with the permission of Nature America, Inc., © 2016. (c) fabrication of electrospun fiber-based 3D scaffolds with controlled 3D shapes and large pores as well as an ECM biomimetic surface structure. The chondrocyte-laden scaffolds received satisfactory cartilage regeneration and form preservation in vivo. Reproduced from ref. 211 with the permission of Elsevier Ltd., © 2019. | ||
Regarding the shortcomings due to the implantation of the prefabricated construct, the concept of in situ bioprinting of cartilage tissue was introduced by Cohen et al.212 Applying geometric feedback-based approaches, they fabricated 3D implants using alginate and chondrocytes for in situ repair of cartilage injuries. In another study, Li et al.213 achieved the accurate size of defect regions of cartilage with the help of high-resolution 3D scanning and next applied in situ 3D bioprinting for injury rehabilitation ex vivo. Subsequently, a handheld pneumatic extrusion device “Biopen” was designed by O'Connell et al.214 concerning in vivo repair of osteochondral injuries. The novel nozzle design allowed the deposition of multiple inks in a collinear geometry. In vitro investigations revealed high viability (>97%) of human adipose stem cells in one-week post-printed hydrogels (GelMa + HAMa). Afterward, the same research group promoted Biopen via designing a co-axial nozzle that facilitated the simultaneous co-axial extrusion of the bioscaffold and cultured cells directly into the cartilage defect in a single session in vivo surgery.215 They tested Biopen to develop core/shell GelMa/HAMa bioscaffolds that have a mechanical strength of 200 kPa and high cell viability (>90%) for chondral repair. The results manifested that the core/shell geometry preserves the cells from the printing process and damaging consequences of the free radicals produced by the photo-activation process. This handheld Biopen was also employed to study the rehabilitation of full-thickness chondral defects in a sheep's stifle joints which exhibited safety and potential clinical effectiveness.216 The outcomes demonstrated that the in vivo 3D-printed bioscaffold bears better macroscopic and microscopic properties and shows an immediate configuration of hyaline-like cartilage. This study was significant as it involves primary in situ 3D bioprinting, which can be a key step toward the clinical employment of bioprinting technology.
In a recent study, the application of a robotic-assisted in situ 3D bioprinting technology for cartilage regeneration was reported. A bio-ink including hyaluronic acid methacrylate and acrylate-terminated 4-armed polyethylene glycol was employed, and an in vitro experiment was conducted on a resin model. Also, to assess the cartilage treatment aptitude, the in vivo analysis was performed on rabbits. Based on the results, the osteochondral injury could be repaired in about 60 s, and the regenerated cartilage tissue exhibited the same biomechanical and biochemical performance in hydrogel implantation and in situ 3D bioprinting. It was observed that the presented method is very suitable for surgical procedure improvement, as well as enhancing cartilage rehabilitation.217
Further improvements in 3D bioprinting will permit the production of patterns of growth factors, mechanical gradients, and stem cells in each zonal region of cartilage, enhancing the function of bioengineered cartilage tissue. It has been shown that 3D-printed cartilage can possess the histological and mechanical properties of human auricles after implantation in vivo.135
Vascularization
Vascularization plays a critical role in governing the regeneration of thick tissues such as the heart, liver, pancreas, kidneys, and bone. It is required to provide oxygen and nutrients for cells and remove waste products out of tissue through a network.218,219 Despite the significant advancement in traditional biofabrication methods, the development of 3D vascular like networks remains a big challenge in the TE area. To address this issue, 3D bioprinting has been introduced as a promising approach to fabricate highly organized vascular structures within engineered tissue substitutes.220,221 The main features in engineering vascular tissue are the multi-scale and branched vasculature structure as well as proper mechanism of convective–diffusive transport.222 Bioprinting approaches for the fabrication of a vascularized tissue scaffold could be categorized into direct and indirect approaches. Applying the direct strategy, lumen-containing strands would be fabricated within the scaffolds, while using the indirect approach, vascular networks would be formed within the scaffolds through removing sacrificial strands.223Direct bioprinting of a vascular network allows biopolymers or hydrogels to dispense in the form of strands to form scaffolds. To the best of the authors’ knowledge, EBB of hydrogels for vascular network formation has been first reported by Li et al.224 They developed a double-nozzle assembling method to fabricate a vascular like network with embedded hybrid hydrogels according to predesigned digital models for the creation of liver-like constructs. Gelatin/alginate/fibrinogen encapsulated with adipose-derived stromal cells (ADSC) and hepatocytes were used as bioinks. A solution of thrombin/CaCl2/Na5P3O10 was used to allow the sol–gel transition of gelatin and crosslinking of fibrinogen and alginate. After two weeks of cell culture, the hepatocytes performed some liver like metabolic functions and ADSC showed some endothelium-like cell properties, while the construct maintained its integration. Application of multi-nozzle EBB in a vertical configuration for vascular reconstruction was later described by Tan et al.225 who designed a tubular alginate construct with 12 mm diameter and 15 mm length. In their work the crosslinking agent was provided through a concentric loop of 8 mm diameter. The quantifiable parameters such as the tubular length, wall thickness and roundness have been proposed to characterize the quality of the printed materials. Creating more complex structures including branched tubes with large diameters is one of the important advances in EBB which was reported by Ghanizadeh et al.226 Besides this development, they used a three-stage crosslinking process to provide better printability, more rigidity after printing, and long term stability of the alginate hydrogel in culture medium.
A coaxial nozzle assembling technique as a category of EBB has also been considered for 3D bioprinting of vascular networks. In a study by Zhang et al.,227 vessel-like cellular microfluidic channels were developed through coaxial 3D printing of the alginate hydrogels loaded with human umbilical vein smooth muscle cells (HUVSMCs) followed by a crosslinking process to form a hollow filament. The tubular filament was evaluated for its perfusion, permeability and cell viability. Regarding the application of an artificial vascular network, the engineered constituent should possess desirable mechanical elasticity and strength for pulsatile stress and suture retention.219,228 The mechanical properties of tubular constructs printed using a coaxial system have been proved to be improved by the incorporation of carbon nanotubes (CNT) in a study by Dolati et al.219 They reinforced the alginate based conduits with CNT to enhance their mechanical properties and bioprintability. The results showed that the tensile strength could be increased by ∼1.5–2.1 times with different concentrations of fillers. Gao et al.229 introduced a new configuration into coaxial bioprinted conduits, with a Z-shape platform for layer-by-layer deposition of alginate hollow filaments to form a 3D structure with built-in microchannels. Using this method, a high strength structure could be obtained by applying a higher concentration of alginate and a smaller distance between adjacent filaments. Moreover, the built-in microchannels resulted in higher cell viability. In a similar study by Attalla et al.,230 a multi-layered structure of alginate hollow filaments with a complex geometry was fabricated using an open-source 3D printer with a custom-built microfluidic nozzle. With this system, a precise control of the channel position, spacing, and diameter was possible. In another study, a coaxial EBB was used for the fabrication of cell laden vascular-like structures from a blended hydrogel system of GelMA/SA/4-arm poly(ethylene glycol)-tetra-acrylate (PEGTA).231 Two different crosslinking systems including ionic crosslinking (by CaCl2 solution) and photocrosslinking were applied to obtain stable constructs. This blended hydrogel system demonstrated the desired rheological properties and printability. Moreover, the 3D-printed constructs showed sufficient mechanical strength and biological properties. This work was further promoted by Pi et al.232 such that a more complicated hollow structure using GelMa-based bioinks was developed using a digitally tunable multi-layer coaxial nozzle printing. The GelMA/alginate hydrogel was printed in the form of a circumferentially multi-layered hollow tissue construct, and eight-arm poly(ethylene glycol) (PEG) acrylate with a tripentaerythritol core (PEGOA) was used to improve the mechanical strength and stability of the deposited hydrogels. Fig. 5(a) represents the schematic illustration of the components of the multichannel coaxial extrusion system and cross-sectional views of the hollow structures. The figure also shows the walls of a single-layered and a double-layered tube, colored fluorescently. The figure reveals that a wide range of cell types was tested for viability and proliferation which demonstrated favorable cell growth and maturation.232
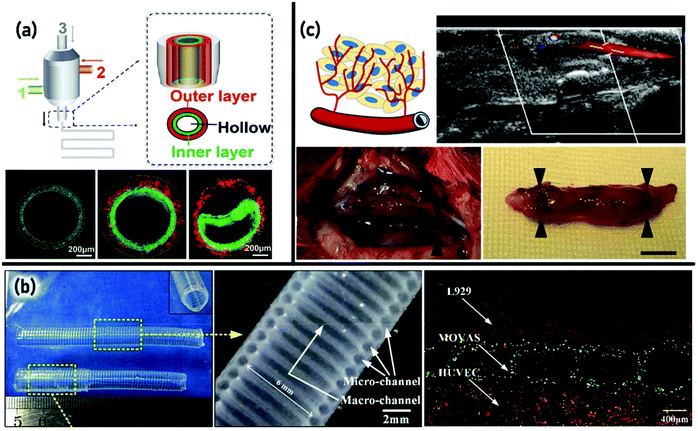 | ||
| Fig. 5 3D bioprinting of vascular tissue: (a) schematic showing the components of the multichannel coaxial extrusion system and cross-sectional views of the hollow structures of GelMa-based bioinks, showing walls of a single layered tube and a double-layered tube. Reproduced from ref. 232 with the permission of WILEY-VCH Verlag GmbH & Co., © 2018; (b) an overview of multi-level fluidic channels composed of macrochannels and microchannels, the longitudinal section of the single layer structure, and the printed vessel-like structure containing three kinds of vascular cells with three colors: red-L929, green-MOVAS, and orange-HUVEC. Reproduced from ref. 236 with the permission of American Chemical Society, © 2017; (c) schematic illustration of the inflammation-mediated process for vascular remodeling, optical images of the implanted grafts with the in vivo view (left) and in vitro view (right) after 1 month, and blood flow (39.4 cm s−1) assessed using ultrasonography 1 day after implantation. Reproduced from ref. 235 with the permission of American Chemical Society, © 2019. | ||
The ECM-related parameters such as the deposition and alignment of collagen and elastin are crucial in vascular tissue engineering. Regarding this, creation of a scaffold-based vascular substitute with a small diameter and mechanical properties close to native vascular tissue still faces general and specific challenges. Additionally, applying scaffolds causes extra problems, such that the mechanical strength of gels is naturally weak which may hinder the final strength of the tissue-engineered vascular like substitute. Also, the biodegradation by-products of the polymer can disrupt the normal organization of the vascular wall and even affect the smooth muscle cell phenotype. Such issues led to the introduction and investigation of scaffold-free bioprinting using cellular spheriods based on the self-assembly approach.233 In a study by Norotte et al., a fully biological engineered scaffold-free vascular substitute was developed using various vascular cell types. The cells were deposited simultaneously with agarose rode, used as the molding template. The distinct cellular units were further fused to create single- and double-layered vascular tubular grafts with small diameters (outer diameter: 0.9–2.5 mm). The method was shown to be accurate, reliable, and scalable.234
In a recent study, Zhou et al.235 introduced a convenient and efficient technique, designated as the interfacial diffusion for creating vascular tissue grafts. In this method, a hydrogel material was extruded into another medium and subjected to a diffusion gelation process. Upon changing the gelation time and nozzle size, the diameter of the printed tubes was changed. In order to increase the tube resistance again internal pressure, bacterial cellulose nanofibers were loaded into the hydrogel system. The developed vascular graft was evaluated for in vitro and in vivo assays which demonstrated the mechanical stability of the graft in rabbit carotid artery replacement. Fig. 5(c) shows a schematic illustration of the inflammation-mediated process for vascular remodeling and macroscopic observations of the vascular graft harvested after implantation for 1 month. Moreover, ultrasonography clearly shows that the blood flows normally at a speed of 39.4 cm s−1 in the grafted vascular 1 day after the implantation.235
Design and development of multi-level fluidic channels composed of macrochannels (for mechanical stimulation) and microchannels (for nutrient delivery) integrated into an organ-on-chip device have been reported by Gao et al.236 They 3D-printed alginate hollow filaments loading L929 mouse fibroblasts and smooth muscle cells (SMCS) as separate layers over a rod. Fig. 5(b) shows an overview of the printed device of a single-layer structure with a length of 70 mm, a double-layer structure with a length of 60 mm and a longitudinal section of the single-layer structure. The developed structures showed relatively strong mechanical properties (due to the progressive crosslinking reaction) and high cell viability (91.4% after 7 days of culture). A printed vessel-like structure containing three kinds of vascular cells is shown in Fig. 5(b).236 In conventional EBB, surface tension and gravity influence the filament formation, morphology and diameter which may cause defects during 3D printing. Jin et al.237 reported the application of a yield stress support bath for decreasing the effects of surface tension and gravity on filament formation. The alginate/gelatin blend as a hydrogel precursor was printed in a LAPONITE® nanoclay yield-stress bath. Their results demonstrated that the nanoclay concentration significantly influences the morphology of the printed filaments. They further used this deposition approach for producing branched vascular like structures. The cell viability was shown to be around 90% after 3 days of culture. Indirect EBB was introduced to avoid some limitations of direct EBB including flowing of low viscosity hydrogels (such as alginate, collagen and fibrin) in contact with the substrate or collapsing of printed layers. In this method, a slurry bath was applied in which the nozzle could move around to print the ink without any resistance. After printing, the slurry can be removed by thermal or chemical means, while the solidified hydrogel forms a vascular pattern.223 This method is also applicable in creating channels inside the bulk hydrogels. In this case, the printed tubes are removed from the hydrogels to form the channel embedded hydrogels.238,239 Using this approach, Bertassoni et al. reported a 3D micromolding method utilizing agarose fibers as a permissive template to create a perfusable microchannel network inside GelMA (gelatin methacryloyl) hydrogels.240 Their results indicated that the fabricated microchannel embedded hydrogels showed enhanced mass transport, cell viability (more than 90%) and differentiation.
Application of sacrificial moulding to produce rigid lattices of filaments using 3D bioprinting was reported by Eltaher et al.241 They described the development of high-resolution structures based on a flexible sugar–protein composite by casting during 3D printing to form sacrificial vessels. Thin endothelialized vessel walls were created by the incorporation of biocompatible crosslinkers. Moreover, it was demonstrated that the perfused vascular channels sustain the metabolic function of primary human cells. In a very recent work by Tsai et al.,242 a non-sacrificial gel system containing a sacrificial borate ester hydrogel was prepared to create tubular microchannels. In this hydrogel system, N-isopropylacrylamide, pentafluorophenyl acrylate, poly(vinyl alcohol), and cellulose nanofibrils were applied for thermoresponsiveness, post-modification, gel formation and 3D printing facilitation, respectively. To obtain 3D vascularized constructs, the non-sacrificial gel was cast on the sacrificial printed hydrogel followed by immersion into the culture medium, which resulted in creating interconnecting multiple channels in 5 min. The developed constructs exhibited vascular endothelial cell proliferation.
Biofabrication of living tissues and organs considerably relies on the vascularization. Despite the great advancement in common biofabrication approaches, creating a hierarchical perfusable vascular network with anatomical exactitude, and a heterocellular structure remains the main challenge. To date, significant progress has been made in generating perfusable branched vascular networks and vascularized tissue; however, much effort must be made in fabricating small-diameter vascular grafts with a complex microarchitecture and fully biological functions. Moreover, employing new bioinks based on functionalizing synthetic biomaterials, dECM, and autologous cells will result in clinically derived development in vascularized tissue substitutes. Furthermore, the engineered materials should possess superior mechanical properties such as elasticity, similar to native vascular tissue.
Neural
The regeneration of nerve defects/damage such as acute traumatic injuries (including brain injuries and spinal cord injuries) and neurological diseases (including stroke, Alzheimer's disease, Parkinson's disease, multiple sclerosis, and Huntington's disease) is one of the most challenging clinical issues worldwide.243,244 Development of nerve 3D models mimicking the native ECM has emerged as one of the promising strategies to reconstruct defective nervous tissues. Generally, the neural model should possess specific requirements including: neurocompatibility to allow attachment and proliferation of nerve cells, elastic properties/hierarchical microarchitecture to mimic the mechanical/physicochemical features of the native nervous tissue ECM, and ability to cause electroconductivity.245 Among all 3D bioprinting methods, EBB in particular showed advantages in developing neural tissue models due to its compatibility with processing the broad range of materials set, including cell suspensions, cell-laden hydrogels, solutions, thermoplastics, thermosets, and elastomers.246 A lack of appropriate neural bioinks which can properly mimic the mechanical/chemical characteristics of the native ECM is one reason for relatively fewer available reports on the application of EBB in neural regeneration. In a recent study by Haring et al.,247 a filler free bioink was developed. This bioink was made by crosslinking of thiolated Pluronic F-127 with dopamine-conjugated gelatin and dopamine-conjugated HA through a thiol/catechol reaction. Schwann cell-, rodent neuronal cell-, and human glioma cell encapsulated bioinks were used to form neural constructs. In another work on designing suitable bioinks that possess proper mechanical properties while promoting cell proliferation and tissue formation, Hsiao et al.248 synthesized a dual stimuli-responsive biodegradable polyurethane hydrogel. The advantage of the developed hydrogel bioink was its relatively low viscosity that could avoid excessive fluid shear stress and potential for jamming during extrusion. Moreover, the proper structure strength and shear yield stress of the hydrogels could bear the weight of ink without obviously changing the shape of stacking fibers. Their results also demonstrated that the printed constructs were conductive to proliferate and growth of NSCs as well as their differentiation into neural cells.The first attempt in biofabrication of a fully cellular nerve graft composed exclusively of cells and cell secreted material was reported by Owens et al.249 Mouse bone marrow stem cells and Schwann cells were printed in an agarose mould followed by the removal of the mould after 7 days. The developed graft was successfully implanted into rats suffering from sciatic nerve injury and tested for both motor and sensory functions. Lozano et al.250 utilized a handheld reactive bathless 3D printer to develop brain-like structures made of discrete layers of neural cells encapsulated in arginine–glycine–aspartate (RGD) peptide modified gellan gum hydrogels. Primary cortical neurons and glial cells were successfully encapsulated in the 3D-printed hydrogel, and higher survival and networking of cells were observed in RGD-coupled gellan gum than in pure gellan gum. Fig. 6(a) depicts the schematic representation of brain layer structures and cortical neurons encapsulated in RGD-gellan gum after 5 days of culture.250
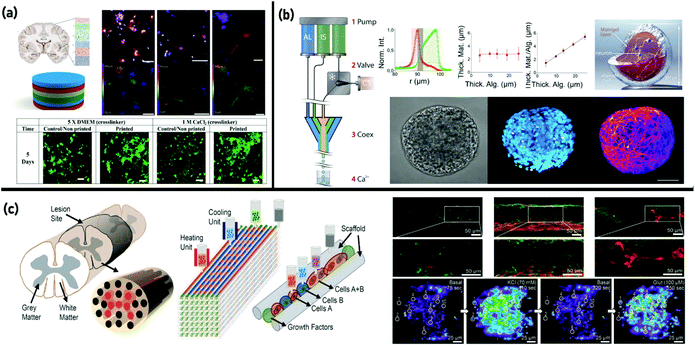 | ||
| Fig. 6 3D bioprinting of nerve tissue: (a) schematic representation of brain layer structures and cortical neurons encapsulated in RGD-gellan gum after 5 days of culture and confocal microscopy images of neuronal 3D culture models after 5 days of culture. Reproduced from ref. 250 with the permission of Elsevier Ltd., © 2015; (b) diagram of the co-extrusion set-up, schematic illustration of a neuronal capsule, and micrographs of a fixed neuronal capsule by bright field microscopy and fluorescence confocal microscopy. Reproduced from ref. 251 with the permission of the Royal Society of Chemistry, © 2016; and (c) schematic of the spinal cord designed for a 3D-bioprinted multichannel scaffold modeling, schematic overview of the 3D bioprinting process, and an image of 3D-printed different cell encapsulated channels showing mature neuronal marker expression. Reproduced from ref. 254 with the permission of WILEY-VCH Verlag GmbH & Co., © 2018. | ||
3D bioprinting was applied to develop a spatial cell culture system, in which the 3D-printed sub-millimetric hollow alginate spheres, encapsulated with neuronal stem cells (NSC) and coated internally with Matrigel (a layer of the ECM) a few microns thick, were generated.251 Utilizing a coaxial flow device, a multi-layered jet from the alginate hydrogel was formed. The inner wall of the capsules was shown to be decorated with a Matrigel layer anchored to the alginate hydrogel mimicking the basal membrane of the cellular niche. Fig. 6(b) exhibits a schematic illustration of the neuronal capsule. The developed 3D-printed microfluidic device was able to differentiate cells into neurons within the hydrogel, while maintaining the cell viability. The DAPI staining of the cell nuclei and tubulin subunit Beta3 staining of mature neuritis are illustrated in Fig. 6(b).251 Coaxial EBB was also used in another study to produce cell-encapsulated hydrogel structures and core–shell cell fibers as cell-laden frameworks in regeneration of neural tissue. In that study, SA was used as a bioink to encapsulate mouse neural progenitor cells. In cell-encapsulated structures, the cells were separated, while in cell fibers they were directly connected. The cells also showed a stronger tendency to undergo differentiation in cell fibers compared to another investigated structure.252
As discussed earlier, one of the main applications of 3D printing is creating cell-based tissue constructs. In this regard, the first work on direct-write printing of an hNSC encapsulated hydrogel to fabricate a 3D neural mini-tissue construct was reported by Gu et al.253 They applied a micro-EBB to print a cell encapsulated alginate/agarose/carboxymethyl cellulose-based construct. The cells showed desirable viability, differentiation into functional neurones, as well as formation of synaptic contacts and networks. Moreover, calcium imaging together with scanning electron microscopy (SEM) imaging of neurons and neuritis revealed that the cells can reasonably model the form and activity of human neural cells. Similarly, Joung et al.254 developed a 3D-printed neural tissue construct in the shape of spinal cord containing neuronal progenitor cells (NPCs) and oligodendrocyte progenitor cells (OPCs) using a one-pot printing process. Fig. 6(c) shows a schematic of the spinal cord designed for a 3D-bioprinted multichannel scaffold modeling along with a schematic overview of the 3D bioprinting process. The 3D scaffold was fabricated through the sequential deposition of a multiple cell laden bioink (cell containing a Matrigel matrix, gelatin/fibrin, GelMa) and a scaffold ink (poly(ethylene glycol) diacrylate, alginate, and methylcellulose) in a layer by layer manner to create multiple channels. This work was the first attempt in 3D printing of neuronal progenitor cells with differentiation into neurons with extended axons propagation. The printed construct showed cellular viability which maintained cell specific phenotype properties in response to the printed microenvironment, and the cell scaffold interactions are given in Fig. 6(c) as neural marker expression.254
To date, numerous research studies have been devoted to designing biomimetic constructs for nerve tissue engineering via integration of multiscale micro- and macroenvironments. Accordingly, a very recent study presented 3D bioprinted scaffolds based on GelMA/chitosan microspheres prepared through a microfluidic system. Cell–scaffold interactions were studied by co-culturing PC12 and Schwann cells.255 The results revealed that such a multiscale composite structure with hydrogel microspheres gave a decent 3D microenvironment for neurite growth enhancement, and the 3D printed hydrogel network provided a 3D macroenvironment resembling the epineurium layer for Schwann cell proliferation and nerve cell arrangement.255
In summary, although successful fabrication of the engineered nerve constructs was achieved through multiple-dispensers and coaxial extrusion bioprinting, future advances in materials will likely enable more flexibility to cell compatibility and adhesion while retaining printability. Furthermore, it seems that four-dimensional (4D) printing256,257 is also gaining attention as an emerging method for obtaining external stimuli-responsive constructs and overcoming some limitations of 3D bioprinting technologies in creating high-resolution constructs.
Muscular
Approximately half of the human body's weight is muscle. It is the only tissue in the body that can contract or shorten, so all body movements include muscle of some kind.258 In the muscular system, muscle tissue is classified into three primary types, i.e., skeletal, cardiac, and smooth, with a unique structure and a particular role.259 3D bioprinting has made exceptional progress in various fields, and it also provides an innovative approach in muscle TE. On reviewing the literature, it can be observed that the researchers worldwide investigated the regeneration of muscle tissues within the context of hydrogel-based EBB.Skeletal muscle
Serving about 45% of the human body weight including over 600 various types, skeletal muscles are involved in skeletal support, stability, movement, and even in the regulation of metabolism.260,261 Skeletal muscle TE (SMTE) intends to develop functional skeletal muscle constructs262,263 to replace or to restore damaged tissues, representing in vitro models for comprehending the growth mechanisms of the muscular system, and for examining different drugs for the remedy of ‘muscular injuries and illnesses’.264,265 Human skeletal muscle is composed of complex anatomical structures, including uniaxially ordered myotubes and widely distributed blood capillaries. Accordingly, vascularization is a crucial part of the successful development of engineered skeletal muscle tissue.106 Despite significant advances in SMTE using various conventional methods, the forces generated from engineered skeletal muscle tissues are yet low compared to their natural counterparts, and there is a lack of accurate 3D spatial cell organization.266–268 Mimicking the extremely packed and arranged cellular structure of the native muscle tissue, by employing natural or synthetic scaffolds and microscale technologies, is essential for successful SMTE.269,270 3D bioprinting has emerged as a powerful microscale technology for SMTE.271,272Hydrogels containing muscle precursor cells have been widely used as bioinks in combination with support structures in thermoplastic polymers273,274 and/or sacrificial materials135,275–277 to attain a proper arrangement of cell-laden fibers capable of mimicking the native muscle tissue.263 Different studies have investigated the propriety of the GelMA hydrogel and its composites with various nanomaterials for SMTE.72,264,278,279 Some solutions have been proposed to provide high cellular viability and function of skeletal muscle cells, such as applying optimized alginate concentration combined with a suitable crosslinking method.271,280 The administration of growth factors (locally or systemically) has also presented great promise to stimulate angiogenesis, stem cell recruitment and differentiation, cell survival and proliferation, a decrease of apoptosis, and adaptive remodeling.281,282 However, a significant restriction is that factor-eluting scaffolds ordinarily release a single factor and, based on their origin, these proteins are high-priced, which could result in disease transmission or inflammation. It has been demonstrated that platelet-rich plasma (PRP) could address these challenges by releasing biologically active proteins and growth factors over several days as a remedy for musculoskeletal diseases.283 For instance, a patient-specific bioink has been generated via loading an alginate bioink with PRP for angiogenesis enhancement, inflammation reduction, stem cell recruitment, and cardiovascular and skeletal muscle tissue regeneration.284
Skeletal muscle tissue has a complex multicellular anisotropic structure concerning the nervous and vascular networks. Such complexity can be achieved through the use of more complicated bioprinting processes combining various techniques, bioinks, and cell types.285,286 Moreover, the gelled bioink should have similar mechanical characteristics to skeletal muscle tissue. Although hydrogel-based 3D engineered muscles, dECM scaffolds, and acellular biological scaffolds have been widely investigated for volumetric muscle loss (VML) treatment,287,288 they have shown limited efficacy. Accordingly, Choi et al.289 proposed a novel VML treatment using a tissue-derived bioink for bioprinting of vascularized volumetric muscle constructs. Human skeletal muscle cells (hSKMs) and human umbilical vein endothelial cells (HUVECs) were blended in skeletal muscle dECM (mdECM) and vascular dECM (vdECM) bioinks, respectively, for coaxial bioprinting of them into thick constructs. The prevascularized muscle constructs exhibited enhanced cell viability without generating hypoxia, myotube formation, and de novo myofiber regeneration in a VML rat model. In vivo outcomes revealed that coaxial nozzle printing mimicked the hierarchical structure of vascularized muscles, and allogeneic human cells in the constructs increased vascularization, innervation, and also 85% of functional recovery witnessed in VML injury.289 But, due to the low mechanical properties of gels made with dECM-based bioinks, it may require stiffening utilizing crosslinking agents, or blending with different components.48,290 Despite promising outcomes, the disadvantages of dECM-based bioinks are the batch-to-batch variability and the possible immune responses they may induce in vivo upon implantation.272
Reviewing the available literature revealed that despite notable advances in SMTE through bioprinting, the level of organization of differentiated muscle precursor cells—i.e., the arrangement of sarcomeres, the production of long-range multinucleated myotubes and the degree of their alignment—was limited, most probably due to substrate mechanical characteristics and matrix density issues.272,291 A strategy that is gaining significance consists of employing the advantages of EBB combined with other scaffold fabrication technologies, to construct advanced structures that mimic skeletal muscle tissue.
In 2015, Lee and colleagues274 introduced a novel method for the 3D biofabrication of complex structures based on multi-dispenser bioprinting. Employing a 3D integrated organ printing (IOP) system, a C2C12 cell-laden hydrogel-based bioink was co-printed with polyurethane (PU) on one side, and an NIH/3T3 cell-laden hydrogel-based bioink was co-printed with PCL on the other side for elasticity and muscle development, and stiffness and tendon development, respectively. The results demonstrated the versatility of the IOP system to fabricate complex tissues such as the musculoskeletal system, which have regional diversity in cell types and mechanical characteristics.274 They promoted the system and presented the ITOP system capable of fabricating stable, human-scale tissue scaffolds of any shape, and providing microchannels with a porous lattice pattern that promoted nutrient and oxygen diffusion into the printed tissue scaffolds which resulted in enhanced tissue formation. Applying the ITOP, they fabricated organized skeletal muscle constructs (15 × 5 × 1 mm3) which were eventually implanted subcutaneously in athymic nude rats. The outcomes demonstrated evidence of vascularization without necrosis and newly formed oriented myofiber bundles.135 In the following, they extended their strategy to treat muscle defect injuries utilizing human cell-laden skeletal muscle constructs. Accordingly, 3D skeletal muscle constructs (up to 15 × 15 × 15 mm3) were fabricated that maintained long parallel multi-layered bundles of densely packed, extremely viable, and aligned myofibers.292 An in vivo study in a rodent model of tibialis anterior (TA) muscle defect after 8 weeks of post-implantation showed 82% of functional rehabilitation. Besides, histological and immunohistological analyses revealed the effective integration of bioprinted constructs with host vascular and neural networks. The results confirmed the potential application of the 3D-bioprinted skeletal muscle with a spatially organized structure in reconstructing extensive muscle injuries.292
Inspired by the native structural morphology of skeletal muscles, Costantini et al.293 introduced an innovative hybrid 3D bioprinting approach to fabricate skeletal muscle tissue with functional morphologies. The technique was based on a microfluidic printing head linked to a co-axial needle extruder for high-resolution 3D bioprinting of aligned hydrogel fibers encapsulating muscle precursor cells (C2C12). The muscle myofibers exhibited sarcomeric organization and improved muscle regeneration in immunocompromised mouse models. Applying such an approach could lead to an enhanced myogenic differentiation with the formation of parallelly aligned, long-range, and tightly packed, myotubes, hence mimicking the natural tissue morphology and organization more intimately. More recently, Testa et al.294 used the same approach and printed human muscle cells obtained from perivascular and pericyte stem cells to treat sphincter muscle injuries. The results of a pre-clinic study confirmed the feasibility of their innovative approach to treat the forms of fecal incontinence that are unresponsive to conservative therapies.
As a novel research line, Kim and colleagues have focused on the 3D fabrication of a group of muscle fibers forming a fascicle via EBB combined with electrospinning.295–299 They proposed a new cell-laden scaffold, including macro-sized struts for providing a 3D structural shape, aligned nanofibers, and cell-printed myoblasts. The results showed higher sarcomeric formation and differentiation on the seventh day of culture on collagen-coated aligned fibers and aligned fiber constructs in comparison with random fiber scaffolds. Besides, the incorporation of micro/nanofibers in the hierarchical scaffold significantly influenced myoblast proliferation and alignment, and even promoted the creation of myotubes.295,296 In another example of biomimetic muscle bundle fabrication, analysis of cells revealed a longitudinal cell alignment, high cell infiltration between the microfibers, and excellent cell proliferation on the surface, and a construct mimicking a muscle bundle section was obtained.297 Based on this initial success, they have recently studied the application of this platform in co-culturing HUVECs and C2C12 cells.299 To be more specific, the HUVEC-laden alginate bioink was uniaxially electrospun on the surface of PCL and collagen struts as mechanical supports by a topographical cue. The electrospun HUVECs exhibited high cell viability (90%), homogeneous cell distribution, and effective HUVEC growth. Moreover, the myoblasts, which were seeded on the vascularized structure (HUVEC-laden fibers), were co-cultured to help achieve myoblast regeneration. In comparison with the scaffold that comprised only myoblasts, the construct that included myoblasts and HUVECs expressed a high degree of the myosin heavy chain (MHC) with striated patterns and improved myogenic-specific gene expression (Fig. 7(c)).299 Their research has opened a new avenue for combining a novel scaffold design with an innovative cell-printing method to achieve myogenic tissue rehabilitation. In a recent study, Kim et al.300 investigated the probability of using the bioprinted human skeletal muscle scaffolds with neural cell integration to enhance the structural and functional regeneration of extensive muscle defect injuries. The neural input into the bioprinted skeletal muscle construct demonstrated the development of myofiber formation, long-term durability, and neuromuscular junction generation in vitro. Moreover, the bioprinted neural cell-laden human skeletal muscle scaffolds promoted rapid innervation and developed into organized muscle tissue that reconstructed normal muscle weight and function in a rat model of tibialis anterior (TA) muscle defect injury. The results showed that the 3D bioprinted human neural-skeletal muscle scaffolds could be quickly combined with the host neural network, resulting in accelerated muscle function rehabilitation.300
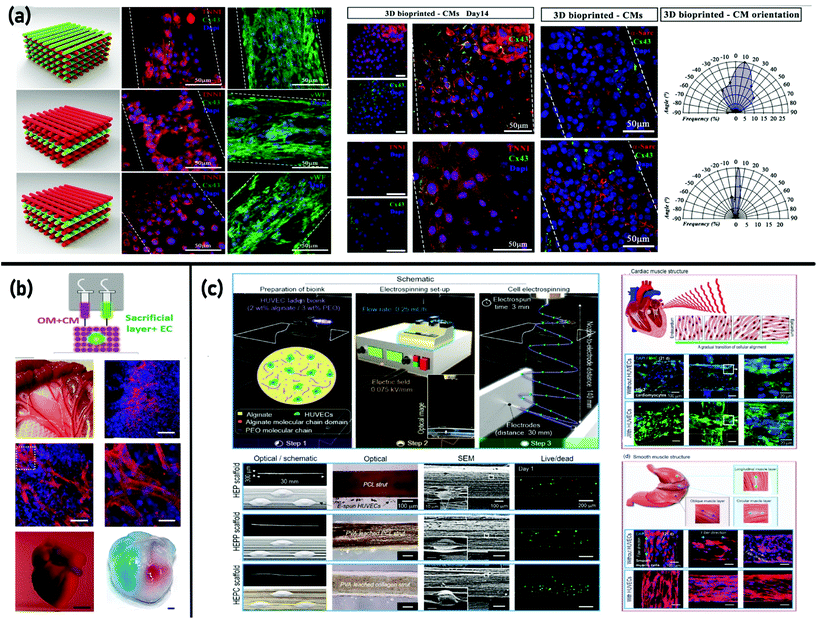 | ||
| Fig. 7 3D bioprinting of muscular tissue: (a) fabrication of heterogeneous, multi-cellular cardiac tissue composed of Human Umbilical Vein Endothelial Cells (HUVECs) and induced pluripotent cell-derived cardiomyocyte (iPSC-CM) cells via hybrid bioprinting (a microfluidic printing head (MPH) coupled to a co-axial nozzle extruder). Reproduced from ref. 316 with the permission of Springer Nature, © 2018; (b) development and application of thoroughly personalized contracting cardiac patches employing patient's cells. The structure and function of the patches were investigated in vitro, and the evaluation of cardiac cell morphology after transplantation exhibited elongated cardiomyocytes with massive actinin striation. Reproduced from ref. 318 with the permission of WILEY-VCH Verlag GmbH & Co., © 2019; and (c) development of scaffolds for co-culturing myoblasts and HUVECs via employing cell electrospinning and 3D printing. Striated patterns and enhanced myogenic gene markers showed a mature stage of myogenic differentiation with vascularization. Reproduced from ref. 299 with the permission of Elsevier Ltd., © 2020. | ||
Despite significant progress, the bioprinting of thick skeletal muscle tissue is still challenging concerning the need for an integrated vascular network. Besides, further improvements are necessary, such as the use of cells derived from patients, iPS cells, and stem cells, which will facilitate the development of patient-specific implants.301
Cardiac muscle
Most heart failures manifest cardiomyocyte loss, which is irreversible and leads to lethal heart diseases and high mortality rates.302 Currently, heart transplantation is the best choice at the end-stage of heart failure; though, substituting the damaged heart with a healthy one faces various limitations, such as insufficient organ availability, immune rejection, and surgical complexities.303 Accordingly, strategies to promote heart rehabilitation, notably through TE principles, have gained growing attention.304 Although bioengineering of a functional cardiac muscle composed of primary cardiomyocytes (CMs) is a promising approach for myocardial regeneration, its applications remain restricted because the cardiac tissue is an extremely organized structure with individual physiological, biomechanical, and electrical properties.305 Bioengineering cardiac tissue via bioprinting technology as a viable option for creating functional tissue constructs is gaining increasing importance owing to its complex build-up capability.306,3073D bioprinting has been adopted to produce cardiac patches that contain both cells and ECM proteins.280,291,308,309 Reviewing the literature, it is found that despite the origins of the base materials, hydrogels remain desirable materials for cardiac tissue regeneration.310 In some studies, a single ink and material formulations have been produced and applied using conventional bioprinting.311–313 For example, a porous patch was printed to support cell attachment and CM differentiation, and enhance left ventricular remodeling in mice by incorporating cardiac-derived progenitor cells into a gelatin/HA gel.313 Concerning the need for more complex tissues and the limitations of the available approaches, researchers have come up with the idea of hybrid structures generated using multiple-dispenser bioprinting and composite bioinks. Following this idea, Jang et al.314 reported the development of pre-vascularized and functional disk-shape constructs utilizing stem cell-laden dECM bioinks. In their research, multiple cell types were consolidated into dECM bioinks, plus soluble factors such as VEGF, to form composite 3D-printed patches. The printed structure composed of spatial patterning of dual stem cells (i.e., human cardiac progenitor cells (hCPCs) and human turbinate tissue-derived MSCs (hTMSCs)) improves cell-to-cell interactions and differentiation capability and functionality for tissue regeneration. The developed patterned patch promoted vascularization and tissue matrix formation in vivo and exhibited enhanced cardiac functions, reduced cardiac hypertrophy, and fibrosis, increased migration from the patch to the infarct zone, as well as advancements in cardiac functions. This method presented the spatial patterning of cells in a form that is in favor of rapid vascularization. Hence, the use of bioprinted stem cell patches has been shown to be a promising therapeutic approach for ischemic heart diseases.314 In another study, fabrication of a contractile cardiac tissue construct utilizing three dispensing modules was reported by Wang et al.307 They printed primary CMs incorporated into a fibrin-based bioink (including gelatin and HA) along with a sacrificial hydrogel and supporting polymeric frame (PCL). The fabricated constructs had a spontaneous synchronized contraction in culture, indicating in vitro cardiac tissue construction and maturation. Progressive cardiac tissue development was approved after one week of culture, and cardiac tissues were developed with uniformly aligned, dense, and electromechanically coupled cardiac cells after three weeks.307
Recapitulating the complexity of the myocardium within functional constructs with tailored biological and mechanical attributes is one of the current scientific preferences in the field of TE, which has stimulated researchers to design hybrid bioprinting methods. As a novel hybrid strategy, Zhang et al.315 fabricated endothelialized human myocardium employing coaxial bioprinting combined with a microfluidic perfusion bioreactor. The constructs were fabricated using a composite bioink, including GelMA, alginate, and induced iPSCs, and next seeded with CMs to induce myocardium development. Although the bioprinted microfibrous structures in this work were not perfusable, it was observed that the printed endothelialized microfibrous scaffold was capable of spontaneous and synchronous contraction.315 Following the hybrid bioprinting strategy, Maiullari et al.316 presented the fabrication of functional heart tissue with simultaneous bioprinting of iPSC-derived cardiomyocytes and HUVEC cells via applying a microfluidic printing head (MPH) coupled to a co-axial nozzle extruder. It was the first research that exposed vasculature development in transplanted tissue via printed endothelial cells. The resultant construct was better adapted for integration with the host's vasculature due to its combination of iPSC-CM with a high orientation index and HUVEC originated blood vessel-like shapes. Moreover, they showed the capability of multi-cellular bioprinted constructs to mature in vascularized functional tissues in vivo, which can be used in different translational applications316 (Fig. 7(a)).316 Izadifar and colleagues317 utilized a UV-integrated pneumatic 3D-Bioplotter system to construct human coronary artery endothelial cells (HCAECs) encapsulated in methacrylated collagen (MeCol). The CNT was incorporated into alginate and the MeCol bioink for building a cardiac patch with electrical and mechanical attributes. As a result, HCAECs in the MeCol gel presented significant cellular proliferation, migration, and differentiation over 10 days of incubation in in vitro cell culture.317
Despite meaningful advancements, the production of thick vascularized tissues that entirely match the case remains a challenge in cardiac TE. Lately, Dvir and colleagues318 fabricated 3D cellularized, vascularized, thick, and perfusable cardiac patches for the first time, which have been demonstrated to be a breakthrough in transplant science. They have exhibited bioprinting of fully personalized contracting cardiac patches utilizing patients’ cells, which decreases the risk of an immune response. Accordingly, they combined a personalized hydrogel, which was derived from the processing of the ECM obtained through biopsy of fatty tissue with the patient's cells (iPSC-derived CMs). The engineered cells in the fabricated cardiac patch were elongated and aligned, with massive striation, which showed their contractile capacity. Consequently, they demonstrated free-form printing of volumetric and anatomically heterogeneous-cellularized human hearts with major blood vessels (Fig. 7(b)).318 Although the printed patches could thoroughly match the anatomical, cellular, biochemical, and immunological characteristics of the patient, the printed blood vessel network is still limited and requires further investigation. To address this challenge, advanced technologies to accurately print small-diameter blood vessels within thick structures should be developed.
Smooth muscle
As a vital regulator of organ function, smooth muscle is an involuntary non-striated muscle in the walls of hollow organs like the bladder, uterus, stomach, intestines, and the walls of passageways, such as the arteries and veins of the circulatory system.319 Aberrant smooth muscle contraction plays a significant role in the pathology of a broad range of diseases. For instance, although asthma, COPD, and Crohn's illness are inflammatory in nature, each of them is characterized by changes to normal smooth muscle contraction.320,321 Despite significant efforts, research applying conventional 2D in vitro methods and animal models has failed to find a cure for the mentioned disorders of aberrant contraction,322,323 which resulted in the development of in vitro technologies (e.g., 3D bioprinting). With the aim of enhancement in the relevance of in vitro models for human illness, Dickman et al. investigated the efficacy of a unique microfluidic 3D bioprinting technology to generate viable and contractile smooth muscle tissue. The primary human airway and SMCs were printed into rings of muscle tissue in high density and viability. Based on the results, in response to physiologically relevant contractile agonists and clinically proven pharmacological triggers of relaxation, printed tissues regenerated the acute contractile function of smooth muscle. Utilizing an identified trigger of fibrosis (TGFβ) in airway muscle rings induced long-term alterations in tissue function similar to that seen in chronic lung infections. Furthermore, combining the dECM into intestinal smooth muscle constructs promotes contractile function relevant to a standard collagen-based hydrogel.324The ability to fabricate perfusable, small-diameter vasculature is a foundational step toward generating human tissues/organs for clinical applications. Cell-laden perfusable vascular conduits have been fabricated for employment in thick tissue regeneration. Employing a coaxial printing system, Zhang et al. developed branched vascular conduits using SA.325 It has been shown that HUVSMCs encapsulated in SA maintain their functions after printing. In another study, to replicate the cellular composition of natural blood vessels, HUVECs and MSCs were incorporated into a bioink comprising GelMA, SA and PEGTA which further differentiated into vascular SMCs in the presence of transforming growth factor-β1.231 Artificial valve conduits made from SMCs and aortic valve leaflet interstitial cells (VIC) have been fabricated and implemented to displace traditional prosthetic substitutes for the cure of heart valve illness.219,326 The alpha-smooth muscle actin and vimentin secreted by the printed cells showed the potential of EBB to produce valve-like tissue constructs.326 Similarly, constructs with high viability and the required function of hepatocytes have also been printed, confirming the capability of EBB techniques for rehabilitation of human liver function.327
Despite advances, it is very challenging to create vasculature integrated with smooth muscle and endothelium that mimic the complexity and functionality of natural vessels. Recently, an innovative method for coaxial extrusion printing of self-standing, small-diameter vasculature with smooth muscle and endothelium was performed by combining a tailored mussel-inspired bioink and a novel “fugitive-migration” approach, and its usefulness and satisfaction over other techniques were demonstrated. The outcomes exhibited that the bioprinted vascular construct possessed numerously desirable, biomimetic properties such as proper biomechanics, higher tissue affinity, vascularized tissue formation capacity, practical perfusability and permeability, and in vivo autonomous connection (∼2 weeks). Moreover, biofunctionalization and dynamic stimuli significantly enhanced vascular remodeling of both smooth muscle and endothelium (∼6 weeks). The desirable biocompatibility in vivo assured the safety of implantation, and investigations of vasculature tissue development in immunodeficient mice confirmed the design's effectiveness. The advancements in creating biomimetic, functional vasculature showed significant potential for producing a complex vascularized tissue/organ concerning clinical transplantation.328
Concluding remarks and future perspective
The 3D-bioprinting technology is accelerating innovation in a variety of disciplines and is making inroads into the fields of medicine and biology, particularly in the design and fabrication of 3D cell culture structures. It enables the rapid construction of scaffolds while sustaining a high level of control over the matrix architecture. Among various 3D-bioprinting approaches, EBB is the most convenient, affordable, and common one, which has been considered a revolutionary technique in tissue biofabrication. The rapidly expanding research area in this field is hydrogel-based EBB that stands out for its unique advantages, and hence has been extensively explored for the generation of different tissue constructs.Looking at the literature, the principal challenges of developing hydrogel-based EBB can be divided into four main categories: (1) bioink selection and process parameter optimization in the printing of various tissues, (2) enhancement in mechanical strength and bio-functionality of the printed constructs, (3) vascularization of the target tissue, and (4) commercialization and mass-market challenges. To address the first two issues, there is a wealth of current literature presenting laboratory studies to create functional 3D constructs. Accordingly, the capability of EBB to achieve personalization of target tissues through precise control over bioinks, printing processes, and architectural accuracy has been extensively reported. Although there is still an important requirement for the development of printable biomaterials and 3D printing mechanisms to replicate the functions of the tissues, it seems that focusing on the advantages and disadvantages of the reported fabrication methodology could open new avenues for future research. Thus, the present review manifests the recent progress in emerging technologies developed for the improvement of TE with a particular focus on most of the published 3D-printed tissues (i.e., skin, bone, cartilage, vascular, neural, and muscular tissue including skeletal, cardiac, and smooth tissue) exploring the TE perspective and fabrication methodology.
The potential of hydrogel-based EBB has been extended by researchers through developing coaxial nozzles and multi-dispenser apparatus. Coaxial nozzles have been extensively implemented in engineering microchannels and vascular networks. Multi-dispenser printing systems frequently facilitate the fabrication of tissues with high architectural and functional complexities (e.g., cartilages, bone, and muscle tissues). Furthermore, hybrid bioprinting approaches are advantageous for incorporating multiple biomaterials and fabricating complicated constructs with structural and functional heterogeneity. Through these improvements and novel techniques, it is possible to print artificial transplantable tissues in a short time with a fine micro- and macrostructure as well as practical functionalities.
The most commonly emerging methods reviewed in this article are as follows:
- Hybrid 3D bioprinting i.e., combination of the EBB with:
○ Electrospinning: Electrospinning and EBB have been known to have promising potential in the fabrication of complicated constructs such as bone and cartilage tissues. Combining these two techniques has successfully helped overcome some of the inherent limitations of each method (e.g., the tight intertwining of electrospun fibers that limits cell migration, and the insufficient resolution of EBB).
○ Microfluidic technologies: The microfluidic technologies and organ-on-chip platforms offer the capability of mimicking the physiological, mechanical, and chemical attributes of native tissues. Although the convergence of microfluidic technologies with EBB has led to a significant leap in the vascularization of engineered tissues, several issues have been reported to be solved. For instance, EBB achieves prevalence due to its low cost and mild printing conditions; however, it is not quite applicable for a microfluidic platform owing to its limited resolution and surface roughness. Although the microfluidic bioprinting approach is emerging to fabricate complex tissue constructs, further developments in the bioprinting processes and bioinks are required for its wide application in the generation of functional tissues.
- In situ bioprinting: The recent in situ bioprinting studies have pleasantly grasped the very conceptual idea of tissue biofabrication directly in the living body. Owing to its intrinsic advantages, increased efforts are being made to improve it through the development of more advanced bioinks, higher resolution of bioprinting methods, and automation of bioprinting processes. Besides, other capacities such as real-time monitoring, sensors for investigating critical parameters, miniaturization of the device, higher freedom, and printing on a dynamic surface, can be integrated with in situ bioprinting. Thus far, attempts have been made to fabricate tissues on the outer organs (i.e., skin, cartilage, and bone), which can be safely arrested and immobilized while printing.
Besides the abovementioned methods which have been extensively explored in vitro and in vivo, some innovative approaches have been reported recently, to overcome the reviewed challenges and accomplish more accurate and complicated architectures. For instance, the capability of printing multiple materials through a single nozzle helps overcome some restrictions of multi-nozzle 3D bioprinters (e.g., enhancement of the printing time while changing between materials, requiring specific calibration for all the printheads before every print).51 Accordingly, different techniques have been used for manufacturing continuous single-nozzle multi-material (SNMM) micro-extrusion bioprinters.329 Moreover, a novel multimaterial multinozzle 3D printing method (MM3D) has been introduced for generating voxelated soft materials, in which through a uniform printing process, complex architectures with controlled composition, function, and structure in a voxel-by-voxel manner could be fabricated. MM3D is capable of presenting an efficient approach to fabricate a broad range of high-performance structural, functional, and biological materials, which could exclude periodicity restrictions of the existing printhead design, progress feature resolution and minimise printing time.330
As EBB is hampered by the insufficiency of printing low-viscosity materials, the dual-step crosslinking method is implemented for cytocompatible bioprinting of a wide range of Gel-AGE bioink formulations, enabling the fabrication of soft and permissive constructs, e.g. vascular and neural tissue. This approach could open a promising gateway to produce complex constructs while maintaining a cell-permissive environment.331 Continuous chaotic printing is another approach that allows careful control of the spatial microstructures (i.e. the number of layers and the average spacing between them) within a single 3D printed fiber. The principal part of this technological platform is the use of an on-line static mixer in the printhead for presenting a partial mixing of various materials as they are coextruded through the nozzle.332
As the bioprinting matured, substantive roadblocks to obtaining the architecture and resolution of native tissues became obvious. Several groups have now demonstrated that innovations in the materials used for printing can promote transformative advances in both tissue architecture and resolution. Recently, the freeform reversible embedding of suspended hydrogel (FRESH) bioprinting technique223 was improved, and individual filaments of collagen as thin as 20 mm in diameter were printed.333 Although it is a notable improvement towards volumetric patterning of natural biomaterials at cellular resolution, and such astonishing improvement in resolution would lead the EBB field to a new era, the field still requires to address how to best seamlessly combine cells into FRESH-printed constructs.334 It should be considered that the FRESH method is the only technique to obtain volumetric patterning using EBB. The principal contrast between the FRESH method and other EBB approaches is that FRESH is achieved within a dissolvable support bath.334 The recent progress in the application of the jamming transition of granular hydrogels for supporting baths and bioinks expresses a potential paradigm shift in the EBB. They have appeared as a powerful platform for 3D bioprinting because of their dynamic structures, unique shear-thinning, and self-healing characteristics.335,336
In addition to the discussed current progress associated with EBB, 4D bioprinting, in which the concept of time is integrated with 3D bioprinting, has currently emerged as the next-generation solution of TE as it presents the possibility of constructing complex and functional structures.337 Integration of the fourth dimension “time” in 4D bioprinting allows for continued control over the evolution of 3D printed biomaterials and bioinks, and provides programming and regulation of the formation of biomimetic tissues from the printed constructs to achieve more native-like results.338 4D bioprinting can be used to fabricate dynamic 3D-patterned biological architectures that will change their shapes under various stimuli by employing stimuli-responsive materials. The functional transformation and maturation of printed cell-laden structures over time present an unprecedented potential for TE. The shape memory characteristics of the printed constructs could address the need for personalized tissue defect repairs.339 Applying this technique, researchers have fabricated bioconstructs capable of transforming into very complex structures which are difficult to directly achieve by 3D bioprinting or other systems. Despite the concise history of 4D bioprinting, the recent fast progress with a focus on developing novel 4D printable materials, exploring novel methods to precisely control the process, and seeking biomedical applications is testified in this field.340 In summary, 4D bioprinting has opened new windows for biofabrication, and it has shown magnificent potential to revolutionize tissue engineering, drug delivery, and other fields.337 However, it is in its infancy, and there is still a long way to achieve clinical applications. With the progress of materials science, printing technology, software, and numerical modelling, 4D bioprinting would take a huge step forward in achieving real applications.340,341
Finally, it should be highlighted that despite extensive efforts that have been made in recent years to develop hydrogel-based EBB and proposed notions by interdisciplinary researchers to overcome the challenges, it is still in the infancy stage. Besides, there is no clear picture of which method is best to overcome hurdles and accomplish more accurate and complicated architectures considering that all these methods in the field are still at early stages and many more studies should be performed in this area towards engineering functional human tissues and organs.
The present review outlines that fast-developing fabrication technologies in the area of EBB could open up new avenues toward more innovative treatments in the future. Furthermore, challenges such as the economics of scale, the cost-effectiveness of the final product, regulatory standards, and ethical considerations are still the foremost issues for commercialization of bioprinted tissues for personalized medicine. It is expected that multidisciplinary approaches provide further convenient ways to overcome the mentioned hurdles.
Conflicts of interest
There are no conflicts to declare.References
- A. Eltom, G. Zhong and A. Muhammad, Scaffold Techniques and Designs in Tissue Engineering Functions and Purposes: A Review, Adv. Mater. Sci. Eng., 2019, 2019, 3429527, DOI:10.1155/2019/3429527.
- R. G. Pearson, R. Bhandari, R. A. Quirk and K. M. Shakesheff, Recent advances in tissue engineering, J. Long-Term Eff. Med. Implants, 2017, 27, 199–232, DOI:10.1615/JLongTermEffMedImplants.v27.i2–4.70.
- X. Zhang and Y. Zhang, Tissue Engineering Applications of Three-Dimensional Bioprinting, Cell Biochem. Biophys., 2015, 72, 777–782, DOI:10.1007/s12013-015-0531-x.
- D. X. B. Chen, Scaffold Design, in Extrus. Bioprinting Scaffolds Tissue Eng. Appl, Springer, Switzerland, 2019, pp. 15–30. DOI:10.1007/978-3-030-03460-3.
- K. Smetana, Cell biology of hydrogels, Biomaterials, 1993, 14, 1046–1050, DOI:10.1016/0142-9612(93)90203-E.
- P. A. Janmey and C. A. McCulloch, Cell Mechanics: Integrating Cell Responses to Mechanical Stimuli, Annu. Rev. Biomed. Eng., 2007, 9, 1–34, DOI:10.1146/annurev.bioeng.9.060906.151927.
- A. Skardal and A. Atala, Biomaterials for Integration with 3-D Bioprinting, Ann. Biomed. Eng., 2015, 43, 730–746, DOI:10.1007/s10439-014-1207-1.
- S. J. Hollister, Porous scaffold design for tissue engineering, Nat. Mater., 2005, 4, 518–524 CrossRef CAS.
- Z. Xia, S. Jin and K. Ye, Tissue and Organ 3D Bioprinting, SLAS Technol., 2018, 23, 301–314, DOI:10.1177/2472630318760515.
- J. M. Unagolla and A. C. Jayasuriya, Hydrogel-based 3D bioprinting: A comprehensive review on cell-laden hydrogels, bioink formulations, and future perspectives, Appl. Mater. Today, 2020, 18, 100479, DOI:10.1016/j.apmt.2019.100479.
- M. Moradi, M. K. Moghadam, M. Shamsborhan, M. Bodaghi and H. Falavandi, Post-processing of FDM 3d-printed polylactic acid parts by laser beam cutting, Polymers, 2020, 12, 550, DOI:10.3390/polym12030550.
- Y. S. Zhang, K. Yue, J. Aleman, K. Mollazadeh-Moghaddam, S. M. Bakht, J. Yang, W. Jia, V. Dell'Erba, P. Assawes, S. R. Shin, M. R. Dokmeci, R. Oklu and A. Khademhosseini, 3D Bioprinting for Tissue and Organ Fabrication, Ann. Biomed. Eng., 2017, 45, 148–163, DOI:10.1007/s10439-016-1612-8.
- M. Topuz, B. Dikici, M. Gavgali and H. Yilmazer, a Review on the Hydrogels Used in 3D Bio-Printing, Int. J. 3D Print. Technol. Digit. Ind, 2018, 2, 68–75 Search PubMed.
- P. S. Gungor-Ozkerim, I. Inci, Y. S. Zhang, A. Khademhosseini and M. R. Dokmeci, Bioinks for 3D bioprinting: An overview, Biomater. Sci., 2018, 6, 915–946, 10.1039/c7bm00765e.
- D. X. B. Chen, Extrusion Bioprinting of Scaffolds: An Introduction, in Extrus. Bioprinting Scaffolds Tissue Eng. Appl, Springer, Switzerland, 2019, pp. 1–13 Search PubMed.
- P. Rider, Ž P. Kačarević, S. Alkildani, S. Retnasingh and M. Barbeck, Bioprinting of tissue engineering scaffolds, J. Tissue Eng., 2018, 9, 2041731418802090, DOI:10.1177/2041731418802090.
- F. Pati, J. Jang, J. W. Lee and D. W. Cho, Extrusion bioprinting, in Essentials 3D Biofabrication Transl, 2015, pp. 123–152. DOI:10.1016/B978-0-12-800972-7.00007-4.
- T. Ahlfeld, V. Guduric, S. Duin, A. R. Akkineni, K. Schütz, D. Kilian, J. Emmermacher, N. Cubo-Mateo, S. Dani, M. V. Witzleben, J. Spangenberg, R. Abdelgaber, R. F. Richter, A. Lode and M. Gelinsky, Methylcellulose-a versatile printing material that enables biofabrication of tissue equivalents with high shape fidelity, Biomater. Sci., 2020, 8, 2102–2110, 10.1039/d0bm00027b.
- S. Kyle, Z. M. Jessop, A. Al-Sabah and I. S. Whitaker, Printability of Candidate Biomaterials for Extrusion Based 3D Printing: State-of-the-Art, Adv. Healthcare Mater., 2017, 6, 1–16, DOI:10.1002/adhm.201700264.
- P. Fisch, M. Holub and M. Zenobi-Wong, Improved accuracy and precision of bioprinting through progressive cavity pump-controlled extrusion, 2020, BioRxiv, 2020.01.23.915868. DOI: DOI:10.1101/2020.01.23.915868.
- I. Matai, G. Kaur, A. Seyedsalehi, A. McClinton and C. T. Laurencin, Progress in 3D bioprinting technology for tissue/organ regenerative engineering, Biomaterials, 2020, 226, 119536, DOI:10.1016/j.biomaterials.2019.119536.
- I. T. Ozbolat and M. Hospodiuk, Current advances and future perspectives in extrusion-based bioprinting, Biomaterials, 2016, 76, 321–343, DOI:10.1016/j.biomaterials.2015.10.076.
- V. Mironov, Printing technology to produce living tissue, Expert Opin. Biol. Ther., 2003, 3, 701–704 CrossRef.
- G. R. Souza, H. Tseng, J. A. Gage, A. Mani, P. Desai, F. Leonard, A. Liao, M. Longo, J. S. Refuerzo and B. Godin, Magnetically bioprinted human myometrial 3D cell rings as a model for uterine contractility, Int. J. Mol. Sci., 2017, 18, 683, DOI:10.3390/ijms18040683.
- P. N. Bernal, P. Delrot, D. Loterie, Y. Li, J. Malda, C. Moser and R. Levato, Volumetric Bioprinting of Complex Living–Tissue Constructs within Seconds, Adv. Mater., 2019, 31, 1904209, DOI:10.1002/adma.201904209.
- J. M. Lee, S. L. Sing, M. Zhou and W. Y. Yeong, 3D bioprinting processes: A perspective on classification and terminology, Int. J. Bioprint., 2018, 4, 151, DOI:10.18063/IJB.v4i2.151.
- F. Pati, J. Jang, D. H. Ha, S. W. Kim, J. W. Rhie, J. H. Shim, D. H. Kim and D. W. Cho, Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink, Nat. Commun., 2014, 5, 3935, DOI:10.1038/ncomms4935.
- V. Mironov, T. Trusk, V. Kasyanov, S. Little, R. Swaja and R. Markwald, Biofabrication: A 21st century manufacturing paradigm, Biofabrication, 2009, 1, 022001 CrossRef CAS.
- S. A. Skoog, P. L. Goering and R. J. Narayan, Stereolithography in tissue engineering, J. Mater. Sci. Mater. Med., 2014, 25, 845–856, DOI:10.1007/s10856-013-5107-y.
- A. Shapira, N. Noor, M. Asulin and T. Dvir, Stabilization strategies in extrusion-based 3D bioprinting for tissue engineering, Appl. Phys. Rev., 2018, 5, 041112, DOI:10.1063/1.5055659.
- S. Mantha, S. Pillai, P. Khayambashi, A. Upadhyay, Y. Zhang, O. Tao, H. M. Pham and S. D. Tran, Smart hydrogels in tissue engineering and regenerative medicine, Materials, 2019, 12, 3323, DOI:10.3390/ma12203323.
- S. Naghieh, M. D. Sarker, N. K. Sharma, Z. Barhoumi and X. Chen, Printability of 3D printed hydrogel scaffolds: Influence of hydrogel composition and printing parameters, Appl. Sci., 2020, 10, 292, DOI:10.3390/app10010292.
- E. Caló and V. V. Khutoryanskiy, Biomedical applications of hydrogels: A review of patents and commercial products, Eur. Polym. J., 2015, 65, 252–267, DOI:10.1016/j.eurpolymj.2014.11.024.
- D. X. B. Chen, Preparation of Scaffold Solutions and Characterization of Their Flow Behavior, in Extrus. Bioprinting Scaffolds Tissue Eng. Appl, 2019, pp. 91–114 Search PubMed.
- J. H. Y. Chung, S. Naficy, Z. Yue, R. Kapsa, A. Quigley, S. E. Moulton and G. G. Wallace, Bio-ink properties and printability for extrusion printing living cells, Biomater. Sci., 2013, 1, 763–773, 10.1039/c3bm00012e.
- F. You, B. F. Eames and X. Chen, Application of extrusion-based hydrogel bioprinting for cartilage tissue engineering, Int. J. Mol. Sci., 2017, 18, 8–14, DOI:10.3390/ijms18071597.
- D. X. B. Chen, Biomaterials for Bioprinting, in Extrus. Bioprinting Scaffolds Tissue Eng. Appl, Springer, Switzerland, 2019, pp. 33–48 Search PubMed.
- S. S. Athukoralalage, R. Balu, N. K. Dutta and N. R. Choudhury, 3D bioprinted nanocellulose-based hydrogels for tissue engineering applications: A brief review, Polymers, 2019, 11, 1–13, DOI:10.3390/polym11050898.
- M. Kouhi, J. Varshosaz, B. Hashemibeni and A. Sarmadi, Injectable gellan gum/lignocellulose nanofibrils hydrogels enriched with melatonin loaded forsterite nanoparticles for cartilage tissue engineering: Fabrication, characterization and cell culture studies, Mater. Sci. Eng., C, 2020, 115, 111114, DOI:10.1016/j.msec.2020.111114.
- H. A. Awad, M. Q. Wickham, H. A. Leddy, J. M. Gimble and F. Guilak, Chondrogenic differentiation of adipose-derived adult stem cells in agarose, alginate, and gelatin scaffolds, Biomaterials, 2004, 25, 3211–3222, DOI:10.1016/j.biomaterials.2003.10.045.
- D. S. W. Benoit, M. P. Schwartz, A. R. Durney and K. S. Anseth, Small functional groups for controlled differentiation of hydrogel-encapsulated human mesenchymal stem cells, Nat. Mater., 2008, 7, 816–823 CrossRef CAS.
- S. V. Murphy and A. Atala, 3D bioprinting of tissues and organs, Nat. Biotechnol., 2014, 32, 773–785, DOI:10.1038/nbt.2958.
- N. C. Negrini, N. Celikkin, P. Tarsini, S. Farè and W. Święszkowski, 3D printing of chemically crosslinked gelatin hydrogels for adipose tissue engineering, Biofabrication, 2019, 0–7 Search PubMed.
- J. Li, C. Wu, P. K. Chu and M. Gelinsky, 3D printing of hydrogels: Rational design strategies and emerging biomedical applications, Mater. Sci. Eng., R, 2020, 140, 100543, DOI:10.1016/j.mser.2020.100543.
- D. M. Kirchmajer, R. Gorkin and M. In Het Panhuis, An overview of the suitability of hydrogel-forming polymers for extrusion-based 3D-printing, J. Mater. Chem. B, 2015, 3, 4105–4117, 10.1039/c5tb00393h.
- U. Jammalamadaka and K. Tappa, Recent advances in biomaterials for 3D printing and tissue engineering, J. Funct. Biomater., 2018, 9, 22, DOI:10.3390/jfb9010022.
- J. K. Placone and A. J. Engler, Recent Advances in Extrusion-Based 3D Printing for Biomedical Applications, Adv. Healthcare Mater, 2018, 7, 1701161, DOI:10.1002/adhm.201701161.
- J. Jang, J. Y. Park, G. Gao and D. W. Cho, Biomaterials-based 3D cell printing for next-generation therapeutics and diagnostics, Biomaterials, 2018, 156, 88–106, DOI:10.1016/j.biomaterials.2017.11.030.
- L. Ning and X. Chen, A brief review of extrusion-based tissue scaffold bio-printing, Biotechnol. J., 2017, 12, 1–16, DOI:10.1002/biot.201600671.
- S. Naghieh, M. Sarker, M. Izadifar and X. Chen, Dispensing-based bioprinting of mechanically-functional hybrid scaffolds with vessel-like channels for tissue engineering applications – A brief review, J. Mech. Behav. Biomed. Mater, 2018, 78, 298–314, DOI:10.1016/j.jmbbm.2017.11.037.
- E. Sodupe-Ortega, A. Sanz-Garcia, A. Pernia-Espinoza and C. Escobedo-Lucea, Accurate calibration in multi-material 3D bioprinting for tissue engineering, Materials, 2018, 11, 1402, DOI:10.3390/ma11081402.
- B. Zhang, L. Gao, L. Ma, Y. Luo, H. Yang and Z. Cui, 3D Bioprinting: A Novel Avenue for Manufacturing Tissues and Organs, Engineering, 2019, 5, 777–794, DOI:10.1016/j.eng.2019.03.009.
- T. Xu, K. W. Binder, M. Z. Albanna, D. Dice, W. Zhao, J. J. Yoo and A. Atala, Hybrid Printing of Mechanically and Biologically Improved Constructs for Cartilage Tissue Engineering Applications, Biofabrication, 2013, 5, 015001, DOI:10.1088/1758-5082/5/1/015001.
- X. Dai, L. Liu, J. Ouyang, X. Li, X. Zhang, Q. Lan and T. Xu, Coaxial 3D bioprinting of self-assembled multicellular heterogeneous tumor fibers, Sci. Rep., 2017, 7, 1–12, DOI:10.1038/s41598-017-01581-y.
- A. K. Miri, A. Khalilpour, B. Cecen, S. Maharjan, S. R. Shin and A. Khademhosseini, Multiscale bioprinting of vascularized models, Biomaterials, 2019, 198, 204–216, DOI:10.1016/j.biomaterials.2018.08.006.
- S. Hong, J. S. Kim, B. Jung, C. Won and C. Hwang, Coaxial bioprinting of cell-laden vascular constructs using a gelatin-tyramine bioink, Biomater. Sci., 2019, 7, 4578–4587, 10.1039/c8bm00618k.
- L. Huang, X. Du, S. Fan, G. Yang, H. Shao, D. Li, C. Cao, Y. Zhu, M. Zhu and Y. Zhang, Bacterial cellulose nanofibers promote stress and fidelity of 3D-printed silk based hydrogel scaffold with hierarchical pores, Carbohydr. Polym., 2019, 221, 146–156, DOI:10.1016/j.carbpol.2019.05.080.
- D. Sooriyaarachchi, H. J. Minière, S. Maharubin and G. Z. Tan, Hybrid Additive Microfabrication Scaffold Incorporated with Highly Aligned Nanofibers for Musculoskeletal Tissues, Tissue Eng. Regener. Med., 2019, 16, 29–38, DOI:10.1007/s13770-018-0169-z.
- S. Bongiovanni Abel, F. Montini Ballarin and G. A. Abraham, Combination of electrospinning with other techniques for the fabrication of 3D polymeric and composite nanofibrous scaffolds with improved cellular interactions, Nanotechnology, 2020, 31, 172002, DOI:10.1088/1361-6528/ab6ab4.
- R. Li, A. McCarthy, Y. S. Zhang and J. Xie, Decorating 3D Printed Scaffolds with Electrospun Nanofiber Segments for Tissue Engineering, Adv. Biosyst., 2019, 3, 1900137, DOI:10.1002/adbi.201900137.
- Y. Si, J. Yu, X. Tang, J. Ge and B. Ding, Ultralight nanofibre-assembled cellular aerogels with superelasticity and multifunctionality, Nat. Commun., 2014, 5, 5802, DOI:10.1038/ncomms6802.
- C. Vaquette and J. J. Cooper-White, Increasing electrospun scaffold pore size with tailored collectors for improved cell penetration, Acta Biomater., 2011, 7, 2544–2557, DOI:10.1016/j.actbio.2011.02.036.
- M. Askari, B. Rezaei, A. M. Shoushtari, P. Noorpanah, M. Abdouss and M. Ghani, Fabrication of high performance chitosan/polyvinyl alcohol nanofibrous mat with controlled morphology and optimised diameter, Can. J. Chem. Eng., 2014, 92, 1008–1015, DOI:10.1002/cjce.21975.
- S. Naghieh, E. Foroozmehr, M. Badrossamay and M. Kharaziha, Combinational processing of 3D printing and electrospinning of hierarchical poly(lactic acid)/gelatin-forsterite scaffolds as a biocomposite: Mechanical and biological assessment, Mater. Des., 2017, 133, 128–135, DOI:10.1016/j.matdes.2017.07.051.
- N. Maurmann, D. P. Pereira, D. Burguez, F. D. A. de S. Pereira, P. I. Neto, R. A. Rezende, D. Gamba, J. V. L. da Silva, P. Pranke, F. C. Vazquez-Vazquez, O. A. Chanes-Cuevas, D. Masuoka, J. A. Alatorre, D. Chavarria-Bolaños, J. R. Vega-Baudrit, J. Serrano-Bello and M. A. Alvarez-Perez, Biocompatibility of Developing 3D-Printed Tubular Scaffold Coated with Nanofibers for Bone Applications, J. Nanomater., 2019, 2019, 1–13, DOI:10.1155/2019/6105818.
- A. M. Siegesmund, T. R. Torgerson, M. Oukka, D. M. Molina, L. Rajagopal, A. Discovery and E. Contribution, Mesenchymal stem cells cultivated on scaffolds formed by 3D printed PCL matrices, coated with PLGA electrospun nanofibers for use in tissue engineering, Biomed. Phys. Eng. Express, 2017, 1–28 Search PubMed.
- M. Rampichová, E. Košt’áková Kuželová, E. Filová, J. Chvojka, J. Šafka, M. Pelcl, J. Daňková, E. Prosecká, M. Buzgo, M. Plencner, D. Lukáš and E. Amler, Composite 3D printed scaffold with structured electrospun nanofibers promotes chondrocyte adhesion and infiltration, Cell Adhes. Migr., 2018, 12, 271–285, DOI:10.1080/19336918.2017.1385713.
- Y. Yoon, C. H. Kim, J. E. Lee, J. Yoon, N. K. Lee, T. H. Kim and S.-H. Park, 3D bioprinted complex constructs reinforced by hybrid multilayers of electrospun nanofiber sheets, Biofabrication, 2019, 11, 025015, DOI:10.1088/1758-5090/ab08c2.
- D. Sooriyaarachchi, J. Wu, A. Feng, M. Islam and G. Z. Tan, Hybrid Fabrication of Biomimetic Meniscus Scaffold by 3D Printing and Parallel Electrospinning, Procedia Manuf., 2019, 34, 528–534, DOI:10.1016/j.promfg.2019.06.216.
- N. H. A. Ngadiman, N. M. Yusof, A. Idris, E. Fallahiarezoudar and D. Kurniawan, Novel processing technique to produce three dimensional polyvinyl alcohol/maghemite nanofiber scaffold suitable for hard tissues, Polymers, 2018, 10, 353, DOI:10.3390/polym10040353.
- F. Yu and D. Choudhury, Microfluidic bioprinting for organ-on-a-chip models, Drug Discovery Today, 2019, 24, 1248–1257, DOI:10.1016/j.drudis.2019.03.025.
- W. Liu, Z. Zhong, N. Hu, Y. Zhou, L. Maggio, A. K. Miri, A. Fragasso, X. Jin, A. Khademhosseini and Y. S. Zhang, Coaxial extrusion bioprinting of 3D microfibrous constructs with cell-favorable gelatin methacryloyl microenvironments, Biofabrication, 2018, 10, 024102, DOI:10.1088/1758-5090/aa9d44.
- C. Colosi, S. R. Shin, V. Manoharan, S. Massa, M. Costantini, A. Barbetta, M. R. Dokmeci, M. Dentini and A. Khademhosseini, Microfluidic Bioprinting of Heterogeneous 3D Tissue Constructs Using Low-Viscosity Bioink, Adv. Mater., 2016, 28, 677–684, DOI:10.1002/adma.201503310.
- F. Yu, W. Hunziker and D. Choudhury, Engineering microfluidic organoid-on-a-chip platforms, Micromachines, 2019, 10, 165, DOI:10.3390/mi10030165.
- M. Yokouchi, T. Atsugi, M. Van Logtestijn, R. J. Tanaka, M. Kajimura, M. Suematsu, M. Furuse, M. Amagai and A. Kubo, Epidermal cell turnover across tight junctions based on Kelvin's tetrakaidecahedron cell shape, eLife, 2016, 5, e19593, DOI:10.7554/eLife.19593.
- S. Vijayavenkataraman, W. F. Lu and J. Y. H. Fuh, 3D bioprinting of skin: A state-of-the-art review on modelling, materials, and processes, Biofabrication, 2016, 8, 032001, DOI:10.1088/1758-5090/8/3/032001.
- P. A. J. Kolarsick, M. A. Kolarsick and C. Goodwin, Anatomy and Physiology of the Skin, J. Dermatol. Nurses’ Assoc., 2011, 3, 203–213, DOI:10.1097/JDN.0b013e31823cccbe.
- S. G. Priya, H. Jungvid and A. Kumar, Skin tissue engineering for tissue repair and regeneration, Tissue Eng., Part B, 2008, 14, 105–118, DOI:10.1089/teb.2007.0318.
- R. Sheridan, Closure of the Excised Burn Wound: Autografts, Semipermanent Skin Substitutes, and Permanent Skin Substitutes, Clin. Plast. Surg., 2009, 36, 643–651, DOI:10.1016/j.cps.2009.05.010.
- D. M. Supp and S. T. Boyce, Engineered skin substitutes: Practices and potentials, Clin. Dermatol., 2005, 23, 403–412, DOI:10.1016/j.clindermatol.2004.07.023.
- P. I. Morgado, A. Aguiar-Ricardo and I. J. Correia, Asymmetric membranes as ideal wound dressings: An overview on production methods, structure, properties and performance relationship, J. Membr. Sci., 2015, 490, 139–151, DOI:10.1016/j.memsci.2015.04.064.
- S. MacNeil, Progress and opportunities for tissue-engineered skin, Nature, 2007, 445, 874–880, DOI:10.1038/nature05664.
- R. V. Shevchenko, S. L. James and S. E. James, A review of tissue-engineered skin bioconstructs available for skin reconstruction, J. R. Soc., Interface, 2010, 7, 229–258, DOI:10.1098/rsif.2009.0403.
- N. Bhardwaj, D. Chouhan and B. B. Mandal, Tissue Engineered Skin and Wound Healing: Current Strategies and Future Directions, Curr. Pharm. Des., 2017, 23, 3455–3482, DOI:10.2174/1381612823666170526094606.
- 3D Bioprinting Market by Technology (Microextrusion, Inkjet, Laser, Magnetic), Material (Cells, Hydrogels, Extracellular Matrices, Biomaterials), Application (Clinical (Bone, Cartilage, Skin) & Research (Regenerative Medicine)) - Global Forecasts to 2021, 2017.
- F. Verseijden, S. J. Posthumus-van Sluijs, E. Farrell, J. W. Van Neck, S. E. R. Hovius, S. O. P. Hofer and G. J. V. M. Van Osch, Prevascular structures promote vascularization in engineered human adipose tissue constructs upon implantation, Cell Transplant., 2010, 19, 1007–1020, DOI:10.3727/096368910X492571.
- M. M. Stanton, J. Samitier and S. Sánchez, Bioprinting of 3D hydrogels, Lab Chip, 2015, 15, 3111–3115, 10.1039/c5lc90069g.
- R. Augustine, Skin bioprinting: a novel approach for creating artificial skin from synthetic and natural building blocks, Prog. Biomater, 2018, 7, 77–92, DOI:10.1007/s40204-018-0087-0.
- P. Liu, H. Shen, Y. Zhi, J. Si, J. Shi, L. Guo and S. G. Shen, 3D bioprinting and in vitro study of bilayered membranous construct with human cells-laden alginate/gelatin composite hydrogels, Colloids Surf., B, 2019, 181, 1026–1034, DOI:10.1016/j.colsurfb.2019.06.069.
- A. V. Do, B. Khorsand, S. M. Geary and A. K. Salem, 3D Printing of Scaffolds for Tissue Regeneration Applications, Adv. Healthcare Mater., 2015, 4, 1742–1762, DOI:10.1002/adhm.201500168.
- M. Guvendiren, J. Molde, R. M. D. Soares and J. Kohn, Designing Biomaterials for 3D Printing, ACS Biomater. Sci. Eng., 2016, 2, 1679–1693, DOI:10.1021/acsbiomaterials.6b00121.
- W. Aljohani, M. W. Ullah, X. Zhang and G. Yang, Bioprinting and its applications in tissue engineering and regenerative medicine, Int. J. Biol. Macromol., 2018, 107, 261–275, DOI:10.1016/j.ijbiomac.2017.08.171.
- S. V. Murphy, A. Skardal and A. Atala, Evaluation of hydrogels for bio-printing applications, J. Biomed. Mater. Res., Part A, 2013, 101A, 272–284, DOI:10.1002/jbm.a.34326.
- S. Wang, J. M. Lee and W. Y. Yeong, Smart hydrogels for 3D bioprinting, Int. J. Bioprint., 2015, 1, 3–14, DOI:10.18063/IJB.2015.01.005.
- S. Stratton, O. S. Manoukian, R. Patel, A. Wentworth, S. Rudraiah and S. G. Kumbar, Polymeric 3D printed structures for soft-tissue engineering, J. Appl. Polym. Sci., 2018, 135, 1–13, DOI:10.1002/app.45569.
- L. Shi, L. Xiong, Y. Hu, W. Li, Z. C. Chen, K. Liu and X. Zhang, Three-dimensional printing alginate/gelatin scaffolds as dermal substitutes for skin tissue engineering, Polym. Eng. Sci., 2018, 58, 1782–1790, DOI:10.1002/pen.24779.
- S. Datta, R. Sarkar, V. Vyas, S. Bhutoria, A. Barui, A. Roy Chowdhury and P. Datta, Alginate-honey bioinks with improved cell responses for applications as bioprinted tissue engineered constructs, J. Mater. Res., 2018, 33, 2029–2039, DOI:10.1557/jmr.2018.202.
- L. J. Pourchet, A. Thepot, M. Albouy, E. J. Courtial, A. Boher, L. J. Blum and C. A. Marquette, Human Skin 3D Bioprinting Using Scaffold-Free Approach, Adv. Healthcare Mater., 2017, 6, 1–8, DOI:10.1002/adhm.201601101.
- S. F. Badylak, D. O. Freytes and T. W. Gilbert, Extracellular matrix as a biological scaffold material: Structure and function, Acta Biomater., 2009, 5, 1–13, DOI:10.1016/j.actbio.2008.09.013.
- B. S. Kim, H. Kim, G. Gao, J. Jang and D. W. Cho, Decellularized extracellular matrix: A step towards the next generation source for bioink manufacturing, Biofabrication, 2017, 9, 034104, DOI:10.1088/1758-5090/aa7e98.
- L. Shi, Y. Hu, M. W. Ullah, I. Ullah, H. Ou, W. Zhang, L. Xiong and X. Zhang, Cryogenic free-form extrusion bioprinting of decellularized small intestinal submucosa for potential applications in skin tissue engineering, Biofabrication, 2019, 11, 035023, DOI:10.1088/1758-5090/ab15a9.
- W. L. Ng, S. Wang, W. Y. Yeong and M. W. Naing, Skin Bioprinting: Impending Reality or Fantasy?, Trends Biotechnol., 2016, 34, 689–699, DOI:10.1016/j.tibtech.2016.04.006.
- W. Lee, J. C. Debasitis, V. K. Lee, J. H. Lee, K. Fischer, K. Edminster, J. K. Park and S. S. Yoo, Multi-layered culture of human skin fibroblasts and keratinocytes through three-dimensional freeform fabrication, Biomaterials, 2009, 30, 1587–1595, DOI:10.1016/j.biomaterials.2008.12.009.
- V. Lee, G. Singh, J. P. Trasatti, C. Bjornsson, X. Xu, T. N. Tran, S. S. Yoo, G. Dai and P. Karande, Design and fabrication of human skin by three-dimensional bioprinting, Tissue Eng., Part C, 2014, 20, 473–484, DOI:10.1089/ten.tec.2013.0335.
- G. Kim, S. Ahn, H. Yoon, Y. Kim and W. Chun, A cryogenic direct-plotting system for fabrication of 3D collagen scaffolds for tissue engineering, J. Mater. Chem., 2009, 19, 8817–8823, 10.1039/b914187a.
- G. Kim, S. Ahn, Y. Kim, Y. Cho and W. Chun, Coaxial structured collagen-alginate scaffolds: Fabrication, physical properties, and biomedical application for skin tissue regeneration, J. Mater. Chem., 2011, 21, 6165–6172, 10.1039/c0jm03452e.
- N. Cubo, M. Garcia, J. F. Del Cañizo, D. Velasco and J. L. Jorcano, 3D bioprinting of functional human skin: Production and in vivo analysis, Biofabrication, 2017, 9, 1–12, DOI:10.1088/1758-5090/9/1/015006.
- B. S. Kim, J. S. Lee, G. Gao and D. W. Cho, Direct 3D cell-printing of human skin with functional transwell system, Biofabrication, 2017, 9, 025034, DOI:10.1088/1758-5090/aa71c8.
- J. Zhang, S. Yun, A. Karami, B. Jing, A. Zannettino, Y. Du and H. Zhang, 3D printing of a thermosensitive hydrogel for skin tissue engineering: A proof of concept study, Bioprinting, 2020, 19, e00089, DOI:10.1016/j.bprint.2020.e00089.
- K. W. Binder, W. Zhao, T. Aboushwareb, D. Dice, A. Atala and J. J. Yoo, In situ bioprinting of the skin for burns, J. Am. Coll. Surg., 2010, 211, S76, DOI:10.1016/j.jamcollsurg.2010.06.198.
- A. Skardal, D. Mack, E. Kapetanovic, A. Atala, J. D. Jackson, J. Yoo and S. Soker, Bioprinted Amniotic Fluid-Derived Stem Cells Accelerate Healing of Large Skin Wounds, Stem Cells Transl. Med., 2012, 1, 792–802, DOI:10.5966/sctm.2012-0088.
- A. Skardal, S. V. Murphy, K. Crowell, D. Mack, A. Atala and S. Soker, A tunable hydrogel system for long-term release of cell-secreted cytokines and bioprinted in situ wound cell delivery, J. Biomed. Mater. Res., Part B, 2017, 105, 1986–2000, DOI:10.1002/jbm.b.33736.
- M. Albanna, K. W. Binder, S. V. Murphy, J. Kim, S. A. Qasem, W. Zhao, J. Tan, I. B. El-Amin, D. D. Dice, J. Marco, J. Green, T. Xu, A. Skardal, J. H. Holmes, J. D. Jackson, A. Atala and J. J. Yoo, In Situ Bioprinting of Autologous Skin Cells Accelerates Wound Healing of Extensive Excisional Full-Thickness Wounds, Sci. Rep., 2019, 9, 1–15, DOI:10.1038/s41598-018-38366-w.
- D. X. B. Chen and D. X. B. Chen, Extrusion Bioprinting of Scaffolds, in Extrus. Bioprinting Scaffolds Tissue Eng. Appl, Springer International Publishing, 2019, pp. 117–145. DOI:10.1007/978-3-030-03460-3_6.
- L. Leng, A. McAllister, B. Zhang, M. Radisic and A. Günther, Mosaic hydrogels: One-step formation of multiscale soft materials, Adv. Mater., 2012, 24, 3650–3658, DOI:10.1002/adma.201201442.
- L. Leng, S. Amini-Nik, Q. Ba, M. Jeschke and A. Guenther, Skin printer: Microfluidic approach for skin regeneration and wound dressings, in 17th Int. Conf. Miniaturized Systems Chem. Life Sci, 2013, pp. 1833–1835 Search PubMed.
- N. Hakimi, R. Cheng, L. Leng, M. Sotoudehfar, P. Q. Ba, N. Bakhtyar, S. Amini-Nik, M. G. Jeschke and A. Günther, Handheld skin printer:: In situ formation of planar biomaterials and tissues, Lab Chip, 2018, 18, 1440–1451, 10.1039/c7lc01236e.
- S. P. Miguel, C. S. D. Cabral, A. F. Moreira and I. J. Correia, Production and characterization of a novel asymmetric 3D printed construct aimed for skin tissue regeneration, Colloids Surf., B, 2019, 181, 994–1003, DOI:10.1016/j.colsurfb.2019.06.063.
- Z. Hao, Z. Song, J. Huang, K. Huang, A. Panetta, Z. Gu and J. Wu, The scaffold microenvironment for stem cell based bone tissue engineering, Biomater. Sci., 2017, 5, 1382–1392, 10.1039/c7bm00146k.
- M. Larsen, R. Mishra, M. Miller and D. Dean, Bioprinting of Bone, in Essentials 3D Biofabrication Transl, Elsevier Inc., 2015, pp. 293–308. DOI:10.1016/B978-0-12-800972-7.00017-7.
- J. Wu, G. Miao, Z. Zheng, Z. Li, W. Ren, C. Wu, Y. Li, Z. Huang, L. Yang and L. Guo, 3D printing mesoporous bioactive glass/sodium alginate/gelatin sustained release scaffolds for bone repair, J. Biomater. Appl., 2019, 33, 755–765, DOI:10.1177/0885328218810269.
- C. Wang, W. Huang, Y. Zhou, L. He, Z. He, Z. Chen, X. He, S. Tian, J. Liao, B. Lu, Y. Wei and M. Wang, 3D printing of bone tissue engineering scaffolds, Bioact. Mater., 2020, 5, 82–91, DOI:10.1016/j.bioactmat.2020.01.004.
- M. Kouhi, V. Jayarama Reddy and S. Ramakrishna, GPTMS-Modified Bredigite/PHBV Nanofibrous Bone Scaffolds with Enhanced Mechanical and Biological Properties, Appl. Biochem. Biotechnol., 2019, 188, 357–368, DOI:10.1007/s12010-018-2922-0.
- F. Shahabipour, N. Ashammakhi, R. K. Oskuee, S. Bonakdar, T. Hoffman, M. A. Shokrgozar and A. Khademhosseini, Key components of engineering vascularized 3-dimensional bioprinted bone constructs, Transl. Res., 2020, 216, 57–76, DOI:10.1016/j.trsl.2019.08.010.
- A. De Mori, M. P. Fernández, G. Blunn, G. Tozzi and M. Roldo, 3D printing and electrospinning of composite hydrogels for cartilage and bone tissue engineering, Polymers, 2018, 10, 285, DOI:10.3390/polym10030285.
- M. Qasim, D. S. Chae and N. Lee, Advancements and frontiers in nano-based 3d and 4d scaffolds for bone and cartilage tissue engineering, Int. J. Nanomed., 2019, 14, 4333–4351, DOI:10.2147/IJN.S209431.
- N. Ashammakhi, A. Hasan, O. Kaarela, B. Byambaa, A. Sheikhi, A. K. Gaharwar and A. Khademhosseini, Advancing Frontiers in Bone Bioprinting, Adv. Healthcare Mater., 2019, 8, 1801048, DOI:10.1002/adhm.201801048.
- E. Y. Heo, N. R. Ko, M. S. Bae, S. J. Lee, B. J. Choi, J. H. Kim, H. K. Kim, S. A. Park and I. K. Kwon, Novel 3D printed alginate–BFP1 hybrid scaffolds for enhanced bone regeneration, J. Ind. Eng. Chem., 2017, 45, 61–67, DOI:10.1016/j.jiec.2016.09.003.
- J. Park, S. J. Lee, H. Lee, S. A. Park and J. Y. Lee, Three dimensional cell printing with sulfated alginate for improved bone morphogenetic protein-2 delivery and osteogenesis in bone tissue engineering, Carbohydr. Polym., 2018, 196, 217–224, DOI:10.1016/j.carbpol.2018.05.048.
- G. Turnbull, J. Clarke, F. Picard, P. Riches, L. Jia, F. Han, B. Li and W. Shu, 3D bioactive composite scaffolds for bone tissue engineering, Bioact. Mater., 2018, 3, 278–314, DOI:10.1016/j.bioactmat.2017.10.001.
- X. Zhai, C. Ruan, Y. Ma, D. Cheng, M. Wu, W. Liu, X. Zhao, H. Pan and W. W. Lu, 3D-Bioprinted Osteoblast-Laden Nanocomposite Hydrogel Constructs with Induced Microenvironments Promote Cell Viability, Differentiation, and Osteogenesis both In Vitro and In Vivo, Adv. Sci., 2018, 5, 1700550, DOI:10.1002/advs.201700550.
- Y. Luo, Y. Li, X. Qin and Q. Wa, 3D printing of concentrated alginate/gelatin scaffolds with homogeneous nano apatite coating for bone tissue engineering, Mater. Des., 2018, 146, 12–19, DOI:10.1016/j.matdes.2018.03.002.
- A. C. Daly, G. M. Cunniffe, B. N. Sathy, O. Jeon, E. Alsberg and D. J. Kelly, 3D Bioprinting of Developmentally Inspired Templates for Whole Bone Organ Engineering, Adv. Healthcare Mater., 2016, 5, 2353–2362, DOI:10.1002/adhm.201600182.
- Y. W. Chen, Y. F. Shen, C. C. Ho, J. Yu, Y. H. A. Wu, K. Wang, C. T. Shih and M. Y. Shie, Osteogenic and angiogenic potentials of the cell-laden hydrogel/mussel-inspired calcium silicate complex hierarchical porous scaffold fabricated by 3D bioprinting, Mater. Sci. Eng., C, 2018, 91, 679–687, DOI:10.1016/j.msec.2018.06.005.
- H. W. Kang, S. J. Lee, I. K. Ko, C. Kengla, J. J. Yoo and A. Atala, A 3D bioprinting system to produce human-scale tissue constructs with structural integrity, Nat. Biotechnol., 2016, 34, 312–319, DOI:10.1038/nbt.3413.
- H. Lee, S. Ahn, L. J. Bonassar and G. Kim, Cell(MC3T3-E1)-Printed Poly(ε-caprolactone)/Alginate Hybrid Scaffolds for Tissue Regeneration, Macromol. Rapid Commun., 2013, 34, 142–149, DOI:10.1002/marc.201200524.
- J. H. Shim, J. Y. Kim, M. Park, J. Park and D. W. Cho, Development of a hybrid scaffold with synthetic biomaterials and hydrogel using solid freeform fabrication technology, Biofabrication, 2011, 3, 034102, DOI:10.1088/1758-5082/3/3/034102.
- S. Hong, M. Kim and G. Kim, Collagen-β-TCP conjugated PCL biocomposites for bone tissue regeneration: Fabrication, physical properties, and cellular activities, J. Mater. Chem., 2012, 22, 22565–22574, 10.1039/c2jm34423h.
- M. A. Kuss, R. Harms, S. Wu, Y. Wang, J. B. Untrauer, M. A. Carlson and B. Duan, Short-term hypoxic preconditioning promotes prevascularization in 3D bioprinted bone constructs with stromal vascular fraction derived cells, RSC Adv., 2017, 7, 29312–29320, 10.1039/c7ra04372d.
- S. E. Bakarich, M. In Het Panhuis, S. Beirne, G. G. Wallace and G. M. Spinks, Extrusion printing of ionic-covalent entanglement hydrogels with high toughness, J. Mater. Chem. B, 2013, 1, 4939–4946, 10.1039/c3tb21159b.
- A. Wenz, K. Borchers, G. E. M. Tovar and P. J. Kluger, Bone matrix production in hydroxyapatite-modified hydrogels suitable for bone bioprinting, Biofabrication, 2017, 044103, DOI:10.1088/1758-5090/aa91ec.
- S. T. Bendtsen, S. P. Quinnell and M. Wei, Development of a novel alginate-polyvinyl alcohol-hydroxyapatite hydrogel for 3D bioprinting bone tissue engineered scaffolds, J. Biomed. Mater. Res., Part A, 2017, 105, 1457–1468, DOI:10.1002/jbm.a.36036.
- M. Neufurth, X. Wang, H. C. Schröder, Q. Feng, B. Diehl-Seifert, T. Ziebart, R. Steffen, S. Wang and W. E. G. Müller, Engineering a morphogenetically active hydrogel for bioprinting of bioartificial tissue derived from human osteoblast-like SaOS-2 cells, Biomaterials, 2014, 35, 8810–8819, DOI:10.1016/j.biomaterials.2014.07.002.
- S. Wüst, M. E. Godla, R. Müller and S. Hofmann, Tunable hydrogel composite with two-step processing in combination with innovative hardware upgrade for cell-based three-dimensional bioprinting, Acta Biomater., 2014, 10, 630–640, DOI:10.1016/j.actbio.2013.10.016.
- X. F. Wang, P. J. Lu, Y. Song, Y. C. Sun, Y. G. Wang and Y. Wang, Nano hydroxyapatite particles promote osteogenesis in a three-dimensional bio-printing construct consisting of alginate/gelatin/hASCs, RSC Adv., 2016, 6, 6832–6842, 10.1039/c5ra21527g.
- D. Gupta, A. K. Singh, A. Dravid and J. Bellare, Multiscale Porosity in Compressible Cryogenically 3D Printed Gels for Bone Tissue Engineering, ACS Appl. Mater. Interfaces, 2019, 11, 20437–20452, DOI:10.1021/acsami.9b05460.
- J. Shen, W. Wang, X. Zhai, B. Chen, W. Qiao, W. Li, P. Li, Y. Zhao, Y. Meng, S. Qian, X. Liu, P. K. Chu and K. W. K. Yeung, 3D-printed nanocomposite scaffolds with tunable magnesium ionic microenvironment induce in situ bone tissue regeneration, Appl. Mater. Today, 2019, 16, 493–507, DOI:10.1016/j.apmt.2019.07.012.
- H. Cui, Y. Yu, X. Li, Z. Sun, J. Ruan, Z. Wu, J. Qian and J. Yin, Direct 3D printing of a tough hydrogel incorporated with carbon nanotubes for bone regeneration, J. Mater. Chem. B, 2019, 7, 7207–7217, 10.1039/c9tb01494b.
- M. J. Sawkins, P. Mistry, B. N. Brown, K. M. Shakesheff, L. J. Bonassar and J. Yang, Cell and protein compatible 3D bioprinting of mechanically strong constructs for bone repair, Biofabrication, 2015, 7, 35004, DOI:10.1088/1758-5090/7/3/035004.
- H. P. Dang, C. Vaquette, T. Shabab, R. A. Pérez, Y. Yang, T. R. Dargaville, A. Shafiee and P. A. Tran, Porous 3D Printed Scaffolds For Guided Bone Regeneration In a Rat Calvarial Defect Model, Appl. Mater. Today, 2020, 20, 100706, DOI:10.1016/j.apmt.2020.100706.
- X. Zhai, Y. Ma, C. Hou, F. Gao, Y. Zhang, C. Ruan, H. Pan, W. W. Lu and W. Liu, 3D-Printed High Strength Bioactive Supramolecular Polymer/Clay Nanocomposite Hydrogel Scaffold for Bone Regeneration, ACS Biomater. Sci. Eng., 2017, 3, 1109–1118, DOI:10.1021/acsbiomaterials.7b00224.
- Y. B. Kim, H. Lee and G. H. Kim, Strategy to Achieve Highly Porous/Biocompatible Macroscale Cell Blocks, Using a Collagen/Genipin-bioink and an Optimal 3D Printing Process, ACS Appl. Mater. Interfaces, 2016, 8, 32230–32240, DOI:10.1021/acsami.6b11669.
- G. Cidonio, C. R. Alcala-Orozco, K. S. Lim, M. Glinka, I. Mutreja, Y.-H. Kim, J. I. Dawson, T. B. F. Woodfield and R. O. C. Oreffo, Osteogenic and angiogenic tissue formation in high fidelity nanocomposite Laponite-gelatin bioinks, Biofabrication, 2018, 035027, DOI:10.1088/1758-5090/ab19fd.
- S. Midha, M. Dalela, D. Sybil, P. Patra and S. Mohanty, Advances in three-dimensional bioprinting of bone: Progress and challenges, J. Tissue Eng. Regener. Med., 2019, 13, 925–945, DOI:10.1002/term.2847.
- S. Patra and V. Young, A Review of 3D Printing Techniques and the Future in Biofabrication of Bioprinted Tissue, Cell Biochem. Biophys., 2016, 74, 93–98, DOI:10.1007/s12013-016-0730-0.
- S. W. Sawyer, S. V. Shridhar, K. Zhang, L. D. Albrecht, A. B. Filip, J. A. Horton and P. Soman, Perfusion directed 3D mineral formation within cell-laden hydrogels, Biofabrication, 2018, 10, 035013, DOI:10.1088/1758-5090/aacb42.
- D. B. Kolesky, K. A. Homan, M. A. Skylar-Scott and J. A. Lewis, Three-dimensional bioprinting of thick vascularized tissues, Proc. Natl. Acad. Sci. U. S. A., 2016, 113, 3179–3184, DOI:10.1073/pnas.1521342113.
- B. Byambaa, N. Annabi, K. Yue, G. Trujillo-de Santiago, M. M. Alvarez, W. Jia, M. Kazemzadeh-Narbat, S. R. Shin, A. Tamayol and A. Khademhosseini, Bioprinted Osteogenic and Vasculogenic Patterns for Engineering 3D Bone Tissue, Adv. Healthcare Mater., 2017, 6, 1700015, DOI:10.1002/adhm.201700015.
- M. G. Yeo, J. S. Lee, W. Chun and G. H. Kim, An Innovative Collagen-Based Cell-Printing Method for Obtaining Human Adipose Stem Cell-Laden Structures Consisting of Core-Sheath Structures for Tissue Engineering, Biomacromolecules, 2016, 17, 1365–1375, DOI:10.1021/acs.biomac.5b01764.
- R. A. Perez, M. Kim, T. H. Kim, J. H. Kim, J. H. Lee, J. H. Park, J. C. Knowles and H. W. Kim, Utilizing core-shell fibrous collagen-alginate hydrogel cell delivery system for bone tissue engineering, Tissue Eng., Part A, 2014, 20, 103–114, DOI:10.1089/ten.tea.2013.0198.
- N. Raja and H. S. Yun, A simultaneous 3D printing process for the fabrication of bioceramic and cell-laden hydrogel core/shell scaffolds with potential application in bone tissue regeneration, J. Mater. Chem. B, 2016, 4, 4707–4716, 10.1039/c6tb00849f.
- J. Lee and G. Kim, A cryopreservable cell-laden GelMa-based scaffold fabricated using a 3D printing process supplemented with an in situ photo-crosslinking, J. Ind. Eng. Chem., 2020, 85, 249–257, DOI:10.1016/j.jiec.2020.02.007.
- K. Arai, D. Murata, S. Takao, A. R. Verissiomo and K. Nakayama, Cryopreservation method for spheroids and fabrication of scaffold-free tubular constructs, PLoS One, 2020, 15, e0230428, DOI:10.1371/journal.pone.0230428.
- G. H. Kim, J. G. Son, S. Park and W. D. Kim, Hybrid process for fabricating 3D hierarchical scaffolds combining rapid prototyping and electrospinning, Macromol. Rapid Commun., 2008, 29, 1577–1581, DOI:10.1002/marc.200800277.
- Y. Yu, S. Hua, M. Yang, Z. Fu, S. Teng, K. Niu, Q. Zhao and C. Yi, Fabrication and characterization of electrospinning/3D printing bone tissue engineering scaffold, RSC Adv., 2016, 6, 110557–110565, 10.1039/C6RA17718B.
- M. Yeo and G. Kim, Cell-printed hierarchical scaffolds consisting of micro-sized polycaprolactone (PCL) and electrospun PCL nanofibers/cell-laden alginate struts for tissue regeneration, J. Mater. Chem. B, 2014, 2, 314–324, 10.1039/c3tb21163k.
- A. J. Sophia Fox, A. Bedi and S. A. Rodeo, The basic science of articular cartilage: Structure, composition, and function, Sports Health, 2009, 1, 461–468, DOI:10.1177/1941738109350438.
- A. De Mori, M. P. Fernández, G. Blunn, G. Tozzi and M. Roldo, 3D printing and electrospinning of composite hydrogels for cartilage and bone tissue engineering, Polymers, 2018, 10, 1–26, DOI:10.3390/polym10030285.
- N. Asadi, E. Alizadeh, R. Salehi, B. Khalandi, S. Davaran and A. Akbarzadeh, Nanocomposite hydrogels for cartilage tissue engineering: a review, Artif. Cells, Nanomed., Biotechnol., 2018, 46, 465–471, DOI:10.1080/21691401.2017.1345924.
- A. Kosik-Kozioł, M. Costantini, T. Bolek, K. Szöke, A. Barbetta, J. Brinchmann and W. Święszkowski, PLA short sub-micron fiber reinforcement of 3D bioprinted alginate constructs for cartilage regeneration, Biofabrication, 2017, 9, 044105 CrossRef.
- A. R. Poole, T. Kojima, T. Yasuda, F. Mwale, M. Kobayashi and S. Laverty, Composition and structure of articular cartilage: A template for tissue repair, in Clin. Orthop. Relat. Res, Lippincott Williams and Wilkins, 2001. DOI:10.1097/00003086-200110001-00004.
- K. Ye, C. Di Bella, D. E. Myers and P. F. M. Choong, The osteochondral dilemma: review of current management and future trends, Aust. N. Z. J. Surg., 2014, 84, 211–217, DOI:10.1111/ans.12108.
- G. D. Smith, G. Knutsen and J. B. Richardson, A clinical review of cartilage repair techniques, J. Bone Jt. Surg., Ser. B, 2005, 87, 445–449, DOI:10.1302/0301-620X.87B4.15971.
- B. Sharma, S. Fermanian, M. Gibson, S. Unterman, D. A. Herzka, B. Cascio, J. Coburn, A. Y. Hui, N. Marcus, G. E. Gold and J. H. Elisseeff, Human cartilage repair with a photoreactive adhesive-hydrogel composite, Sci. Transl. Med., 2013, 5, 167ra6, DOI:10.1126/scitranslmed.3004838.
- J. Brian, C. H. Huang and A. A. Kyriacos, Cell-based tissue engineering startegies used in the clinical articular cartilage, Biomaterials, 2016, 98, 1–22 CrossRef.
- K. Ye, R. Felimban, K. Traianedes, S. E. Moulton, G. G. Wallace, J. Chung, A. Quigley, P. F. M. Choong and D. E. Myers, Chondrogenesis of infrapatellar fat pad derived adipose stem cells in 3D printed chitosan scaffold, PLoS One, 2014, 9, e102638, DOI:10.1371/journal.pone.0099410.
- T. Billiet, E. Gevaert, T. De Schryver, M. Cornelissen and P. Dubruel, The 3D printing of gelatin methacrylamide cell-laden tissue-engineered constructs with high cell viability, Biomaterials, 2014, 35, 49–62, DOI:10.1016/j.biomaterials.2013.09.078.
- A. Munaz, R. K. Vadivelu, J. St. John, M. Barton, H. Kamble and N. T. Nguyen, Three-dimensional printing of biological matters, J. Sci.: Adv. Mater. Devices, 2016, 1, 1–17, DOI:10.1016/j.jsamd.2016.04.001.
- Y. Wu, P. Kennedy, N. Bonazza, Y. Yu, A. Dhawan and I. Ozbolat, Three-Dimensional Bioprinting of Articular Cartilage: A Systematic Review, Cartilage, 2018, 1947603518809410, DOI:10.1177/1947603518809410.
- C. Antich, J. De Vicente, G. Jim, C. Chocarro, E. Carrillo, E. Monta, G. Patricia, J. A. Marchal and C. Antich, Bio-inspired hydrogel composed of hyaluronic acid and alginate as a potential bioink for 3D bioprinting of articular cartilage engineering constructs, Acta Biomater., 2020, 106, 114–123 CrossRef CAS.
- Z. Izadifar, T. Chang, W. Kulyk, X. Chen and B. F. Eames, Analyzing biological performance of 3D-printed, cell-impregnated hybrid constructs for cartilage tissue engineering, Tissue Eng., Part C, 2016, 22, 173–188, DOI:10.1089/ten.tec.2015.0307.
- X. Ren, F. Wang, C. Chen, X. Gong, L. Yin and L. Yang, Engineering zonal cartilage through bioprinting collagen type II hydrogel constructs with biomimetic chondrocyte density gradient, BMC Musculoskeletal Disord., 2016, 17, 301, DOI:10.1186/s12891-016-1130-8.
- V. H. M. Mouser, R. Levato, A. Mensinga, W. J. A. Dhert, D. Gawlitta and J. Malda, Bio-ink development for three-dimensional bioprinting of hetero-cellular cartilage constructs, Connect. Tissue Res., 2020, 61, 137–151, DOI:10.1080/03008207.2018.1553960.
- A. C. Daly, S. E. Critchley, E. M. Rencsok and D. J. Kelly, A comparison of different bioinks for 3D bioprinting of fibrocartilage and hyaline cartilage, Biofabrication, 2016, 8, 045002, DOI:10.1088/1758-5090/8/4/045002.
- A. Abbadessa, V. H. M. Mouser, M. M. Blokzijl, D. Gawlitta, W. J. A. Dhert, W. E. Hennink, J. Malda and T. Vermonden, A Synthetic Thermosensitive Hydrogel for Cartilage Bioprinting and Its Biofunctionalization with Polysaccharides, Biomacromolecules, 2016, 17, 2137–2147, DOI:10.1021/acs.biomac.6b00366.
- K. Markstedt, A. Mantas, I. Tournier, H. Martínez Ávila, D. Hägg and P. Gatenholm, 3D bioprinting human chondrocytes with nanocellulose-alginate bioink for cartilage tissue engineering applications, Biomacromolecules, 2015, 16, 1489–1496, DOI:10.1021/acs.biomac.5b00188.
- S. Abdulghani and P. G. Morouço, Biofabrication for osteochondral tissue regeneration: bioink printability requirements, J. Mater. Sci. Mater. Med., 2019, 30, 20, DOI:10.1007/s10856-019-6218-x.
- B. Holmes, W. Zhu, J. Li, J. D. Lee and L. G. Zhang, Development of novel three-dimensional printed scaffolds for osteochondral regeneration, Tissue Eng., Part A, 2015, 21, 403–415, DOI:10.1089/ten.tea.2014.0138.
- J. Baena, Volume-by-volume bioprinting of chondrocytes-alginate bioinks in high temperature thermoplastic scaffolds for cartilage regeneration, Cytotherapy, 2019, 21, S23, DOI:10.1016/j.jcyt.2019.03.326.
- X. Cui, K. Breitenkamp, M. Lotz and D. D'Lima, Synergistic action of fibroblast growth factor-2 and transforming growth factor-beta1 enhances bioprinted human neocartilage formation, Biotechnol. Bioeng., 2012, 109, 2357–2368, DOI:10.1002/bit.24488.
- R. Levato, J. Visser, J. A. Planell, E. Engel, J. Malda and M. A. Mateos-Timoneda, Biofabrication of tissue constructs by 3D bioprinting of cell-laden microcarriers, Biofabrication, 2014, 6, 035020, DOI:10.1088/1758-5082/6/3/035020.
- L. Pescosolido, W. Schuurman, J. Malda, P. Matricardi, F. Alhaique, T. Coviello, P. R. Van Weeren, W. J. A. Dhert, W. E. Hennink and T. Vermonden, Hyaluronic acid and dextran-based semi-IPN hydrogels as biomaterials for bioprinting, Biomacromolecules, 2011, 12, 1831–1838, DOI:10.1021/bm200178w.
- J. Kundu, J. H. Shim, J. Jang, S. W. Kim and D. W. Cho, An additive manufacturing-based PCL-alginate-chondrocyte bioprinted scaffold for cartilage tissue engineering, J. Tissue Eng. Regener. Med., 2015, 9, 1286–1297, DOI:10.1002/term.1682.
- J. Jia, D. J. Richards, S. Pollard, Y. Tan, J. Rodriguez, R. P. Visconti, T. C. Trusk, M. J. Yost, H. Yao, R. R. Markwald and Y. Mei, Engineering alginate as bioink for bioprinting, Acta Biomater., 2014, 10, 4323–4331, DOI:10.1016/j.actbio.2014.06.034.
- Z. Li, X. Zhang, T. Yuan, Y. Zhang, C. Luo, J. Zhang, Y. Liu and W. Fan, Addition of Platelet-Rich Plasma to Silk Fibroin Hydrogel Bioprinting for Cartilage Regeneration, Tissue Eng., Part A, 2020, 1–30, DOI:10.1089/ten.tea.2019.0304.
- B. Sharma, C. G. Williams, T. K. Kim, D. Sun, A. Malik, M. Khan, K. Leong and J. H. Elisseeff, Designing zonal organization into tissue-engineered cartilage, Tissue Eng., 2007, 13, 405–414, DOI:10.1089/ten.2006.0068.
- Y. Meng, J. Cao, Y. Chen, Y. Yu and L. Ye, 3D printing of a poly(vinyl alcohol)-based nano-composite hydrogel as an artificial cartilage replacement and the improvement mechanism of printing accuracy, J. Mater. Chem. B, 2020, 8, 677–690, 10.1039/c9tb02278c.
- S. Schwarz, S. Kuth, T. Distler, C. Gögele, K. Stölzel, R. Detsch, A. R. Boccaccini and G. Schulze-Tanzil, 3D printing and characterization of human nasoseptal chondrocytes laden dual crosslinked oxidized alginate-gelatin hydrogels for cartilage repair approaches, Mater. Sci. Eng., C, 2020, 116, 111189, DOI:10.1016/j.msec.2020.111189.
- W. Schuurman, P. A. Levett, M. W. Pot, P. R. van Weeren, W. J. A. Dhert, D. W. Hutmacher, F. P. W. Melchels, T. J. Klein and J. Malda, Gelatin-methacrylamide hydrogels as potential biomaterials for fabrication of tissue-engineered cartilage constructs, Macromol. Biosci., 2013, 13, 551–561, DOI:10.1002/mabi.201200471.
- H. Martínez Ávila, S. Schwarz, N. Rotter and P. Gatenholm, 3D bioprinting of human chondrocyte-laden nanocellulose hydrogels for patient-specific auricular cartilage regeneration, Bioprinting, 2016, 1–2, 22–35, DOI:10.1016/j.bprint.2016.08.003.
- M. Kesti, C. Eberhardt, G. Pagliccia, D. Kenkel, D. Grande, A. Boss and M. Zenobi-Wong, Bioprinting Complex Cartilaginous Structures with Clinically Compliant Biomaterials, Adv. Funct. Mater., 2015, 25, 7406–7417, DOI:10.1002/adfm.201503423.
- E. Coates and J. P. Fisher, Gene expression of alginate-embedded chondrocyte subpopulations and their response to exogenous IGF-1 delivery, J. Tissue Eng. Regener. Med., 2012, 6, 179–192, DOI:10.1002/term.411.
- N. S. Hwang, S. Varghese, H. J. Lee, P. Theprungsirikul, A. Canver, B. Sharma and J. Elisseeff, Response of zonal chondrocytes to extracellular matrix-hydrogels, FEBS Lett., 2007, 581, 4172–4178, DOI:10.1016/j.febslet.2007.07.049.
- Y. Yu, K. K. Moncal, J. Li, W. Peng, I. Rivero, J. A. Martin and I. T. Ozbolat, Three-dimensional bioprinting using self-Assembling scalable scaffold-free “tissue strands” as a new bioink, Sci. Rep., 2016, 6, 28714, DOI:10.1038/srep28714.
- C. T. Hsieh, C. Y. Liao, N. T. Dai, C. S. Tseng, B. L. Yen and S. h. Hsu, 3D printing of tubular scaffolds with elasticity and complex structure from multiple waterborne polyurethanes for tracheal tissue engineering, Appl. Mater. Today, 2018, 12, 330–341, DOI:10.1016/j.apmt.2018.06.004.
- R. Levato, W. R. Webb, I. A. Otto, A. Mensinga, Y. Zhang, M. van Rijen, R. van Weeren, I. M. Khan and J. Malda, The bio in the ink: cartilage regeneration with bioprintable hydrogels and articular cartilage-derived progenitor cells, Acta Biomater., 2017, 61, 41–53, DOI:10.1016/j.actbio.2017.08.005.
- L. H. Nguyen, A. K. Kudva, N. S. Saxena and K. Roy, Engineering articular cartilage with spatially-varying matrix composition and mechanical properties from a single stem cell population using a multi-layered hydrogel, Biomaterials, 2011, 32, 6946–6952, DOI:10.1016/j.biomaterials.2011.06.014.
- L. H. Nguyen, A. K. Kudva, N. L. Guckert, K. D. Linse and K. Roy, Unique biomaterial compositions direct bone marrow stem cells into specific chondrocytic phenotypes corresponding to the various zones of articular cartilage, Biomaterials, 2011, 32, 1327–1338, DOI:10.1016/j.biomaterials.2010.10.009.
- D. Nguyen, D. A. Hgg, A. Forsman, J. Ekholm, P. Nimkingratana, C. Brantsing, T. Kalogeropoulos, S. Zaunz, S. Concaro, M. Brittberg, A. Lindahl, P. Gatenholm, A. Enejder and S. Simonsson, Cartilage Tissue Engineering by the 3D Bioprinting of iPS Cells in a Nanocellulose/Alginate Bioink, Sci. Rep., 2017, 7, 1–10, DOI:10.1038/s41598-017-00690-y.
- M. Kesti, C. Eberhardt, G. Pagliccia, D. Kenkel, D. Grande, A. Boss and M. Zenobi-Wong, Bioprinting Complex Cartilaginous Structures with Clinically Compliant Biomaterials, Adv. Funct. Mater., 2015, 25, 7406–7417, DOI:10.1002/adfm.201503423.
- W. Chen, Y. Xu, Y. Liu, Z. Wang, Y. Li, G. Jiang, X. Mo and G. Zhou, Three-dimensional printed electrospun fiber-based scaffold for cartilage regeneration, Mater. Des., 2019, 179, 107886, DOI:10.1016/j.matdes.2019.107886.
- D. L. Cohen, J. I. Lipton, L. J. Bonassar and H. Lipson, Additive manufacturing for in situ repair of osteochondral defects, Biofabrication, 2010, 2, 035004, DOI:10.1088/1758-5082/2/3/035004.
- L. Li, F. Yu, J. Shi, S. Shen, H. Teng, J. Yang, X. Wang and Q. Jiang, In situ repair of bone and cartilage defects using 3D scanning and 3D printing, Sci. Rep., 2017, 7, 9416, DOI:10.1038/s41598-017-10060-3.
- C. D. O'Connell, C. Di Bella, F. Thompson, C. Augustine, S. Beirne, R. Cornock, C. J. Richards, J. Chung, S. Gambhir, Z. Yue, J. Bourke, B. Zhang, A. Taylor, A. Quigley, R. Kapsa, P. Choong and G. G. Wallace, Development of the Biopen: a handheld device for surgical printing of adipose stem cells at a chondral wound site., Biofabrication, 2016, 8, 015019, DOI:10.1088/1758-5090/8/1/015019.
- S. Duchi, C. Onofrillo, C. D. O'Connell, R. Blanchard, C. Augustine, A. F. Quigley, R. M. I. Kapsa, P. Pivonka, G. Wallace, C. Di Bella and P. F. M. Choong, Handheld Co-Axial Bioprinting: Application to in situ surgical cartilage repair, Sci. Rep., 2017, 7, 5837, DOI:10.1038/s41598-017-05699-x.
- C. Di Bella, S. Duchi, C. D. O'Connell, R. Blanchard, C. Augustine, Z. Yue, F. Thompson, C. Richards, S. Beirne, C. Onofrillo, S. H. Bauquier, S. D. Ryan, P. Pivonka, G. G. Wallace and P. F. Choong, In situ handheld three-dimensional bioprinting for cartilage regeneration, J. Tissue Eng. Regener. Med., 2018, 12, 611–621, DOI:10.1002/term.2476.
- K. Ma, T. Zhao, L. Yang, P. Wang, J. Jin, H. Teng, D. Xia, L. Zhu, L. Li, Q. Jiang and X. Wang, Application of robotic-assisted in situ 3D printing in cartilage regeneration with HAMA hydrogel: An in vivo study, J. Adv. Res., 2020, 23, 123–132, DOI:10.1016/j.jare.2020.01.010.
- V. K. Lee, A. M. Lanzi, H. Ngo, S.-S. Yoo, P. A. Vincent and G. Dai, Generation of Multi-scale Vascular Network System Within 3D Hydrogel Using 3D Bio-printing Technology, Cell. Mol. Bioeng., 2014, 7, 460–472, DOI:10.1007/s12195-014-0340-0.
- F. Dolati, Y. Yu, Y. Zhang, A. M. De Jesus, E. A. Sander and I. T. Ozbolat, In vitro evaluation of carbon-nanotube-reinforced bioprintable vascular conduits., Nanotechnology, 2014, 25, 145101, DOI:10.1088/0957-4484/25/14/145101.
- D. B. Kolesky, R. L. Truby, A. S. Gladman, T. A. Busbee, K. A. Homan and J. A. Lewis, 3D Bioprinting of Vascularized, Heterogeneous Cell-Laden Tissue Constructs, Adv. Mater., 2014, 26, 3124–3130, DOI:10.1002/adma.201305506.
- V. K. Lee, D. Y. Kim, H. Ngo, Y. Lee, L. Seo, S.-S. Yoo, P. A. Vincent and G. Dai, Creating perfused functional vascular channels using 3D bio-printing technology, Biomaterials, 2014, 35, 8092–8102, DOI:10.1016/j.biomaterials.2014.05.083.
- A. Athirasala, F. Lins, A. Tahayeri, M. Hinds, A. J. Smith, C. Sedgley, J. Ferracane and L. E. Bertassoni, A Novel Strategy to Engineer Pre-Vascularized Full-Length Dental Pulp-like Tissue Constructs, Sci. Rep., 2017, 7, 3323, DOI:10.1038/s41598-017-02532-3.
- T. J. Hinton, Q. Jallerat, R. N. Palchesko, J. H. Park, M. S. Grodzicki, H.-J. Shue, M. H. Ramadan, A. R. Hudson and A. W. Feinberg, Three-dimensional printing of complex biological structures by freeform reversible embedding of suspended hydrogels, Sci. Adv., 2015, 1, e1500758 CrossRef.
- S. Li, Z. Xiong, X. Wang, Y. Yan, H. Liu and R. Zhang, Direct Fabrication of a Hybrid Cell/Hydrogel Construct by a Double-nozzle Assembling Technology, J. Bioact. Compat. Polym., 2009, 24, 249–265, DOI:10.1177/0883911509104094.
- E. Y. S. Tan and W. Y. Yeong, Concentric bioprinting of alginate-based tubular constructs using multi-nozzle extrusion-based technique, Int. J. Bioprint., 2015, 1(1), 49–56, DOI:10.18063/IJB.2015.01.003.
- A. G. Tabriz, M. A. Hermida, N. R. Leslie and W. Shu, Three-dimensional bioprinting of complex cell laden alginate hydrogel structures, Biofabrication, 2015, 7, 45012 CrossRef.
- Y. Zhang, Y. Yu and I. T. Ozbolat, Direct Bioprinting of Vessel-Like Tubular Microfluidic Channels, J. Nanotechnol. Eng. Med., 2013, 4, 0210011–0210017, DOI:10.1115/1.4024398.
- K. Yow, J. Ingram, S. A. Korossis, E. Ingham and S. Homer-Vanniasinkam, Tissue engineering of vascular conduits, Br. J. Surg., 2006, 93, 652–661 CrossRef.
- Q. Gao, Y. He, J. Fu, A. Liu and L. Ma, Coaxial nozzle-assisted 3D bioprinting with built-in microchannels for nutrients delivery, Biomaterials, 2015, 61, 203–215, DOI:10.1016/j.biomaterials.2015.05.031.
- R. Attalla, C. Ling and P. Selvaganapathy, Fabrication and characterization of gels with integrated channels using 3D printing with microfluidic nozzle for tissue engineering applications, Biomed. Microdevices, 2016, 18, 17, DOI:10.1007/s10544-016-0042-6.
- W. Jia, P. S. Gungor-Ozkerim, Y. S. Zhang, K. Yue, K. Zhu, W. Liu, Q. Pi, B. Byambaa, M. R. Dokmeci, S. R. Shin and A. Khademhosseini, Direct 3D bioprinting of perfusable vascular constructs using a blend bioink, Biomaterials, 2016, 106, 58–68, DOI:10.1016/j.biomaterials.2016.07.038.
- Q. Pi, S. Maharjan, X. Yan, X. Liu, B. Singh, A. M. van Genderen, F. Robledo-Padilla, R. Parra-Saldivar, N. Hu, W. Jia, C. Xu, J. Kang, S. Hassan, H. Cheng, X. Hou, A. Khademhosseini and Y. S. Zhang, Digitally Tunable Microfluidic Bioprinting of Multilayered Cannular Tissues, Adv. Mater., 2018, 30, 1706913, DOI:10.1002/adma.201706913.
- A. Patel, B. Fine, M. Sandig and K. Mequanint, Elastin biosynthesis: The missing link in tissue-engineered blood vessels, Cardiovasc. Res., 2006, 71, 40–49, DOI:10.1016/j.cardiores.2006.02.021.
- C. Norotte, F. S. Marga, L. E. Niklason and G. Forgacs, Scaffold-free vascular tissue engineering using bioprinting, Biomaterials, 2009, 30, 5910–5917, DOI:10.1016/j.biomaterials.2009.06.034.
- Y. Zhou, Q. Gui, W. Yu, S. Liao, Y. He, X. Tao, Y. Yu and Y. Wang, Interfacial Diffusion Printing: An Efficient Manufacturing Technique for Artificial Tubular Grafts, ACS Biomater. Sci. Eng., 2019, 5, 6311–6318, DOI:10.1021/acsbiomaterials.9b01293.
- Q. Gao, Z. Liu, Z. Lin, J. Qiu, Y. Liu, A. Liu, Y. Wang, M. Xiang, B. Chen, J. Fu and Y. He, 3D Bioprinting of Vessel-like Structures with Multilevel Fluidic Channels, ACS Biomater. Sci. Eng., 2017, 3, 399–408, DOI:10.1021/acsbiomaterials.6b00643.
- Y. Jin, W. Chai and Y. Huang, Printability study of hydrogel solution extrusion in nanoclay yield-stress bath during printing-then-gelation biofabrication, Mater. Sci. Eng., C, 2017, 80, 313–325, DOI:10.1016/j.msec.2017.05.144.
- S. Wüst, R. Müller and S. Hofmann, 3D Bioprinting of complex channels—Effects of material, orientation, geometry, and cell embedding, J. Biomed. Mater. Res., Part A, 2015, 103, 2558–2570 CrossRef.
- Y. S. Zhang, F. Davoudi, P. Walch, A. Manbachi, X. Luo, V. Dell'Erba, A. K. Miri, H. Albadawi, A. Arneri, X. Li, X. Wang, M. R. Dokmeci, A. Khademhosseini and R. Oklu, Bioprinted thrombosis-on-a-chip, Lab Chip, 2016, 16, 4097–4105, 10.1039/C6LC00380J.
- L. E. Bertassoni, M. Cecconi, V. Manoharan, M. Nikkhah, J. Hjortnaes, A. L. Cristino, G. Barabaschi, D. Demarchi, M. R. Dokmeci, Y. Yang and A. Khademhosseini, Hydrogel bioprinted microchannel networks for vascularization of tissue engineering constructs, Lab Chip, 2014, 14, 2202–2211, 10.1039/C4LC00030G.
- H. M. Eltaher, F. E. Abukunna, L. Ruiz-Cantu, Z. Stone, J. Yang and J. E. Dixon, Human-scale tissues with patterned vascular networks by additive manufacturing of sacrificial sugar-protein composites, Acta Biomater., 2020, 113, 339–349, DOI:10.1016/j.actbio.2020.06.012.
- Y. L. Tsai, P. Theato, C. F. Huang and S. h. Hsu, A 3D-printable, glucose-sensitive and thermoresponsive hydrogel as sacrificial materials for constructs with vascular-like channels, Appl. Mater. Today, 2020, 20, 100778, DOI:10.1016/j.apmt.2020.100778.
- S.-J. Lee, T. Esworthy, S. Stake, S. Miao, Y. Y. Zuo, B. T. Harris and L. G. Zhang, Advances in 3D Bioprinting for Neural Tissue Engineering, Adv. Biosyst., 2018, 2, 1700213, DOI:10.1002/adbi.201700213.
- F.-Y. Hsieh and S. Hsu, 3D bioprinting: A new insight into the therapeutic strategy of neural tissue regeneration, Organogenesis, 2015, 11, 153–158, DOI:10.1080/15476278.2015.1123360.
- L. Binan, A. Ajji, G. De Crescenzo and M. Jolicoeur, Approaches for Neural Tissue Regeneration, Stem Cell Rev. Rep., 2014, 10, 44–59, DOI:10.1007/s12015-013-9474-z.
- A. P. Haring, H. Sontheimer and B. N. Johnson, Microphysiological Human Brain and Neural Systems-on-a-Chip: Potential Alternatives to Small Animal Models and Emerging Platforms for Drug Discovery and Personalized Medicine, Stem Cell Rev. Rep., 2017, 13, 381–406, DOI:10.1007/s12015-017-9738-0.
- A. P. Haring, E. G. Thompson, Y. Tong, S. Laheri, E. Cesewski, H. Sontheimer and B. N. Johnson, Process- and bio-inspired hydrogels for 3D bioprinting of soft free-standing neural and glial tissues, Biofabrication, 2019, 11, 25009, DOI:10.1088/1758-5090/ab02c9.
- S.-H. H. Hsiao and S. H. Hsu, Synthesis and characterization of dual stimuli-sensitive biodegradable polyurethane soft hydrogels for 3D cell-laden bioprinting, ACS Appl. Mater. Interfaces, 2018, 10, 29273–29287, DOI:10.1021/acsami.8b08362.
- C. M. Owens, F. Marga, G. Forgacs and C. M. Heesch, Biofabrication and testing of a fully cellular nerve graft, Biofabrication, 2013, 5, 45007, DOI:10.1088/1758-5082/5/4/045007.
- R. Lozano, L. Stevens, B. C. Thompson, K. J. Gilmore, R. Gorkin, E. M. Stewart, M. in het Panhuis, M. Romero-Ortega and G. G. Wallace, 3D printing of layered brain-like structures using peptide modified gellan gum substrates, Biomaterials, 2015, 67, 264–273, DOI:10.1016/j.biomaterials.2015.07.022.
- K. Alessandri, M. Feyeux, B. Gurchenkov, C. Delgado, A. Trushko, K.-H. Krause, D. Vignjević, P. Nassoy and A. Roux, A 3D printed microfluidic device for production of functionalized hydrogel microcapsules for culture and differentiation of human Neuronal Stem Cells (hNSC), Lab Chip, 2016, 16, 1593–1604, 10.1039/C6LC00133E.
- X. Li, X. Wang, H. Chen, Z. Jin, X. Dai, X. Zhang, L. Zhang and T. Xu, A comparative study of the behavior of neural progenitor cells in extrusion-based in vitro hydrogel models, Biomed. Mater., 2019, 14, 65001 CrossRef CAS.
- Q. Gu, E. Tomaskovic-Crook, R. Lozano, Y. Chen, R. M. Kapsa, Q. Zhou, G. G. Wallace and J. M. Crook, Functional 3D Neural Mini-Tissues from Printed Gel-Based Bioink and Human Neural Stem Cells, Adv. Healthcare Mater., 2016, 5, 1429–1438, DOI:10.1002/adhm.201600095.
- D. Joung, V. Truong, C. C. Neitzke, S.-Z. Guo, P. J. Walsh, J. R. Monat, F. Meng, S. H. Park, J. R. Dutton, A. M. Parr and M. C. McAlpine, 3D Printed Stem-Cell Derived Neural Progenitors Generate Spinal Cord Scaffolds, Adv. Funct. Mater., 2018, 28, 1801850, DOI:10.1002/adfm.201801850.
- J. Chen, D. Huang, L. Wang, J. Hou, H. Zhang, Y. Li, S. Zhong, Y. Wang, Y. Wu and W. Huang, 3D bioprinted multiscale composite scaffolds based on gelatin methacryloyl (GelMA)/chitosan microspheres as a modular bioink for enhancing 3D neurite outgrowth and elongation, J. Colloid Interface Sci., 2020, 574, 162–173, DOI:10.1016/j.jcis.2020.04.040.
- M. Bodaghi, A. R. Damanpack and W. H. Liao, Self-expanding/shrinking structures by 4D printing, Smart Mater. Struct., 2016, 25, 1–15, DOI:10.1088/0964-1726/25/10/105034.
- A. Zolfagharian, A. Kaynak, M. Bodaghi, A. Z. Kouzani, S. Gharaie and S. Nahavandi, Control-based 4D printing: Adaptive 4D-printed systems, Appl. Sci., 2020, 10, 3020, DOI:10.3390/app10093020.
- G. J. Stewart, The Skeletal And Muscuar Systems, in Skelet. Muscuar Syst, Chelsea House, New York, 2009, pp. 10–14 Search PubMed.
- G. J. Stewart, Muscles, Muscle Cells, and Muscle Tissues, in Skelet. Muscuar Syst, 2009, pp. 82–97 Search PubMed.
- S. Ostrovidov, V. Hosseini, S. Ahadian, T. Fujie, S. P. Parthiban, M. Ramalingam, H. Bae, H. Kaji and A. Khademhosseini, Skeletal muscle tissue engineering: methods to form skeletal myotubes and their applications., Tissue Eng., Part B., 2014, 20, 403–436, DOI:10.1089/ten.TEB.2013.0534.
- M. Beldjilali-Labro, A. G. Garcia, F. Farhat, F. Bedoui, J. F. Grosset, M. Dufresne and C. Legallais, Biomaterials in tendon and skeletal muscle tissue engineering: Current trends and challenges, Materials, 2018, 11, 1116, DOI:10.3390/ma11071116.
- S. Ostrovidov, S. Ahadian, J. Ramon-Azcon, V. Hosseini, T. Fujie, S. P. Parthiban, H. Shiku, T. Matsue, H. Kaji, M. Ramalingam, H. Bae and A. Khademhosseini, Three-dimensional co-culture of C2C12/PC12 cells improves skeletal muscle tissue formation and function, J. Tissue Eng. Regener. Med., 2017, 11, 582–595, DOI:10.1002/term.1956.
- V. Hosseini, S. Ahadian, S. Ostrovidov, G. Camci-Unal, S. Chen, H. Kaji, M. Ramalingam and A. Khademhosseini, Engineered contractile skeletal muscle tissue on a microgrooved methacrylated gelatin substrate, Tissue Eng., Part A, 2012, 18, 2453–2465, DOI:10.1089/ten.TEA.2012.0181.
- S. Ahadian, J. Ramón-Azcón, M. Estili, R. Obregón, H. Shiku and T. Matsue, Facile and rapid generation of 3D chemical gradients within hydrogels for high-throughput drug screening applications, Biosens. Bioelectron., 2014, 59, 166–173, DOI:10.1016/j.bios.2014.03.031.
- S. Ahadian, J. Ramón-Azcón, H. Chang, X. Liang, H. Kaji, H. Shiku, K. Nakajima, M. Ramalingam, H. Wu, T. Matsue and A. Khademhosseini, Electrically regulated differentiation of skeletal muscle cells on ultrathin graphene-based films, RSC Adv., 2014, 4, 9534–9541, 10.1039/c3ra46218h.
- S. G. M. Uzel, A. Pavesi and R. D. Kamm, Microfabrication and microfluidics for muscle tissue models, Prog. Biophys. Mol. Biol., 2014, 115, 279–293, DOI:10.1016/j.pbiomolbio.2014.08.013.
- R. G. Dennis, P. E. Kosnik, M. E. Gilbert and J. A. Faulkner, Excitability and contractility of skeletal muscle engineered from primary cultures and cell lines, Am. J. Physiol.: Cell Physiol., 2001, 280, C288–C295, DOI:10.1152/ajpcell.2001.280.2.c288.
- T. H. Qazi, D. J. Mooney, M. Pumberger, S. Geißler and G. N. Duda, Biomaterials based strategies for skeletal muscle tissue engineering: Existing technologies and future trends, Biomaterials, 2015, 53, 502–521, DOI:10.1016/j.biomaterials.2015.02.110.
- M. Del Monico, M. Tahriri, M. D. Fahmy, H. Ghassemi, D. Vashaee and L. Tayebi, Cartilage and facial muscle tissue engineering and regeneration: a mini review, Bio-Des. Manuf., 2018, 1, 115–122, DOI:10.1007/s42242-018-0011-4.
- J. M. Fishman, A. Tyraskis, P. Maghsoudlou, L. Urbani, G. Totonelli, M. A. Birchall and P. De Coppi, Skeletal Muscle Tissue Engineering: Which Cell to Use?, Tissue Eng., Part B, 2013, 19, 503–515, DOI:10.1089/ten.teb.2013.0120.
- R. Seyedmahmoud, B. Celebi-Saltik, N. Barros, R. Nasiri, E. Banton, A. Shamloo, N. Ashammakhi, M. R. Dokmeci and S. Ahadian, Three-Dimensional Bioprinting of Functional Skeletal Muscle Tissue Using Gelatin Methacryloyl-Alginate Bioinks, Micromachines, 2019, 10, 679, DOI:10.3390/mi10100679.
- S. Ostrovidov, S. Salehi, M. Costantini, K. Suthiwanich, M. Ebrahimi, R. B. Sadeghian, T. Fujie, X. Shi, S. Cannata, C. Gargioli, A. Tamayol, M. R. Dokmeci, G. Orive, W. Swieszkowski and A. Khademhosseini, 3D Bioprinting in Skeletal Muscle Tissue Engineering, Small, 2019, 15, 1–14, DOI:10.1002/smll.201805530.
- Y. J. Choi, T. G. Kim, J. Jeong, H. G. Yi, J. W. Park, W. Hwang and D. W. Cho, 3D Cell Printing of Functional Skeletal Muscle Constructs Using Skeletal Muscle-Derived Bioink, Adv. Healthcare Mater., 2016, 5, 2636–2645, DOI:10.1002/adhm.201600483.
- T. K. Merceron, M. Burt, Y. J. Seol, H. W. Kang, S. J. Lee, J. J. Yoo and A. Atala, A 3D bioprinted complex structure for engineering the muscle-tendon unit, Biofabrication, 2015, 7, 35003, DOI:10.1088/1758-5090/7/3/035003.
- S. Giannitelli, P. Mozetic, M. Trombetta and A. Rainer, Additive Manufacturing of Pluronic/Alginate Composite Thermogels for Drug and Cell Delivery, in Addit. Manuf, CRC Press, 2015, pp. 403–412. DOI:10.1201/b19360-17.
- A. Tamayol, A. H. Najafabadi, B. Aliakbarian, E. Arab-Tehrany, M. Akbari, N. Annabi, D. Juncker and A. Khademhosseini, Hydrogel Templates for Rapid Manufacturing of Bioactive Fibers and 3D Constructs, Adv. Healthcare Mater., 2015, 4, 2146–2153, DOI:10.1002/adhm.201500492.
- P. Mozetic, S. M. Giannitelli, M. Gori, M. Trombetta and A. Rainer, Engineering muscle cell alignment through 3D bioprinting, J. Biomed. Mater. Res., Part A, 2017, 105, 2582–2588, DOI:10.1002/jbm.a.36117.
- S. Ahadian, J. Ramón-Azcón, M. Estili, X. Liang, S. Ostrovidov, H. Shiku, M. Ramalingam, K. Nakajima, Y. Sakka, H. Bae, T. Matsue and A. Khademhosseini, Hybrid hydrogels containing vertically aligned carbon nanotubes with anisotropic electrical conductivity for muscle myofiber fabrication, Sci. Rep., 2014, 4, 4271, DOI:10.1038/srep04271.
- L. E. Bertassoni, J. C. Cardoso, V. Manoharan, A. L. Cristino, N. S. Bhise, W. A. Araujo, P. Zorlutuna, N. E. Vrana, A. M. Ghaemmaghami, M. R. Dokmeci and A. Khademhosseini, Direct-write bioprinting of cell-laden methacrylated gelatin hydrogels, Biofabrication, 2014, 6, 024105, DOI:10.1088/1758-5082/6/2/024105.
- K. Zhu, S. R. Shin, T. van Kempen, Y.-C. C. Li, V. Ponraj, A. Nasajpour, S. Mandla, N. Hu, X. Liu, J. Leijten, Y.-D. D. Lin, M. A. Hussain, Y. S. Zhang, A. Tamayol and A. Khademhosseini, Gold Nanocomposite Bioink for Printing 3D Cardiac Constructs, Adv. Funct. Mater., 2017, 27, 1605352, DOI:10.1002/adfm.201605352.
- K. Lee, E. A. Silva and D. J. Mooney, Growth factor delivery-based tissue engineering: General approaches and a review of recent developments, J. R. Soc., Interface, 2011, 8, 153–170, DOI:10.1098/rsif.2010.0223.
- C. H. Lee, S. A. Rodeo, L. A. Fortier, C. Lu, C. Erisken and J. J. Mao, Protein-releasing polymeric scaffolds induce fibrochondrocytic differentiation of endogenous cells for knee meniscus regeneration in sheep, Sci. Transl. Med., 2014, 6, 266ra171, DOI:10.1126/scitranslmed.3009696.
- V. Cervelli, P. Gentile, M. G. Scioli, M. Grimaldi, C. U. Casciani, L. G. Spagnoli and A. Orlandi, Application of platelet-rich plasma in plastic surgery: Clinical and in vitro evaluation, Tissue Eng., Part C, 2009, 15, 625–634, DOI:10.1089/ten.tec.2008.0518.
- N. Faramarzi, I. K. Yazdi, M. Nabavinia, A. Gemma, A. Fanelli, A. Caizzone, L. M. Ptaszek, I. Sinha, A. Khademhosseini, J. N. Ruskin and A. Tamayol, Patient-Specific Bioinks for 3D Bioprinting of Tissue Engineering Scaffolds, Adv. Healthcare Mater., 2018, 7, e1701347, DOI:10.1002/adhm.201701347.
- V. K. Lee and G. Dai, Printing of Three-Dimensional Tissue Analogs for Regenerative Medicine, Ann. Biomed. Eng., 2017, 45, 115–131, DOI:10.1007/s10439-016-1613-7.
- W. J. Kim and G. H. Kim, 3D bioprinting of functional cell-laden bioinks and its application for cell-alignment and maturation, Appl. Mater. Today, 2020, 19, 100588, DOI:10.1016/j.apmt.2020.100588.
- B. M. Sicari, J. Peter Rubin, C. L. Dearth, M. T. Wolf, F. Ambrosio, M. Boninger, N. J. Turner, D. J. Weber, T. W. Simpson, A. Wyse, E. H. P. Brown, J. L. Dziki, L. E. Fisher, S. Brown and S. F. Badylak, An acellular biologic scaffold promotes skeletal muscle formation in mice and humans with volumetric muscle loss, Sci. Transl. Med., 2014, 6, 234ra58, DOI:10.1126/scitranslmed.3008085.
- J. Zhang, Z. Q. Hu, N. J. Turner, S. F. Teng, W. Y. Cheng, H. Y. Zhou, L. Zhang, H. W. Hu, Q. Wang and S. F. Badylak, Perfusion-decellularized skeletal muscle as a three-dimensional scaffold with a vascular network template, Biomaterials, 2016, 89, 114–126, DOI:10.1016/j.biomaterials.2016.02.040.
- Y. J. Choi, Y. J. Jun, D. Y. Kim, H. G. Yi, S. H. Chae, J. Kang, J. Lee, G. Gao, J. S. Kong, J. Jang, W. K. Chung, J. W. Rhie and D. W. Cho, A 3D cell printed muscle construct with tissue-derived bioink for the treatment of volumetric muscle loss, Biomaterials, 2019, 206, 160–169, DOI:10.1016/j.biomaterials.2019.03.036.
- J. Jang, T. G. Kim, B. S. Kim, S. W. Kim, S. M. Kwon and D. W. Cho, Tailoring mechanical properties of decellularized extracellular matrix bioink by vitamin B2-induced photo-crosslinking, Acta Biomater., 2016, 33, 88–95, DOI:10.1016/j.actbio.2016.01.013.
- B. Yilmaz, A. Tahmasebifar and E. T. Baran, Bioprinting Technologies in Tissue Engineering, in Curr. Appl. Pharm. Biotechnol, Springer, Cham, 2019, pp. 279–319 Search PubMed.
- J. H. Kim, Y. J. Seol, I. K. Ko, H. W. Kang, Y. K. Lee, J. J. Yoo, A. Atala and S. J. Lee, 3D Bioprinted Human Skeletal Muscle Constructs for Muscle Function Restoration, Sci. Rep., 2018, 8, 1–15, DOI:10.1038/s41598-018-29968-5.
- M. Costantini, S. Testa, P. Mozetic, A. Barbetta, C. Fuoco, E. Fornetti, F. Tamiro, S. Bernardini, J. Jaroszewicz, W. Święszkowski, M. Trombetta, L. Castagnoli, D. Seliktar, P. Garstecki, G. Cesareni, S. Cannata, A. Rainer and C. Gargioli, Microfluidic-enhanced 3D bioprinting of aligned myoblast-laden hydrogels leads to functionally organized myofibers in vitro and in vivo, Biomaterials, 2017, 131, 98–110, DOI:10.1016/j.biomaterials.2017.03.026.
- S. Testa, C. Fuoco, M. Costantini, R. Belli, F. Fascetti Leon, L. Vitiello, A. Rainer, S. Cannata and C. Gargioli, Designing a 3D printed human derived artificial myo-structure for anal sphincter defects in anorectal malformations and adult secondary damage, Mater. Today Commun., 2018, 15, 120–123, DOI:10.1016/j.mtcomm.2018.02.011.
- M. Yeo, H. Lee and G. H. Kim, Combining a micro/nano-hierarchical scaffold with cell-printing of myoblasts induces cell alignment and differentiation favorable to skeletal muscle tissue regeneration, Biofabrication, 2016, 8, 035021, DOI:10.1088/1758-5090/8/3/035021.
- M. Yeo and G. Kim, Three-Dimensional Microfibrous Bundle Structure Fabricated Using an Electric Field-Assisted/Cell Printing Process for Muscle Tissue Regeneration, ACS Biomater. Sci. Eng., 2018, 4, 728–738, DOI:10.1021/acsbiomaterials.7b00983.
- W. Kim, M. Kim and G. H. Kim, 3D-Printed Biomimetic Scaffold Simulating Microfibril Muscle Structure, Adv. Funct. Mater., 2018, 28, 1800405, DOI:10.1002/adfm.201800405.
- M. G. Yeo and G. H. Kim, Fabrication of cell-laden electrospun hybrid scaffolds of alginate-based bioink and PCL microstructures for tissue regeneration, Chem. Eng. J., 2015, 275, 27–35, DOI:10.1016/j.cej.2015.04.038.
- M. Yeo and G. H. Kim, Micro/nano-hierarchical scaffold fabricated using a cell electrospinning/3D printing process for co-culturing myoblasts and HUVECs to induce myoblast alignment and differentiation, Acta Biomater., 2020, 107, 102–114, DOI:10.1016/j.actbio.2020.02.042.
- J. H. Kim, I. Kim, Y. J. Seol, I. K. Ko, J. J. Yoo, A. Atala and S. J. Lee, Neural cell integration into 3D bioprinted skeletal muscle constructs accelerates restoration of muscle function, Nat. Commun., 2020, 11, 1025, DOI:10.1038/s41467-020-14930-9.
- J. Jang, H. G. Yi and D. W. Cho, 3D Printed Tissue Models: Present and Future, ACS Biomater. Sci. Eng., 2016, 2, 1722–1731, DOI:10.1021/acsbiomaterials.6b00129.
- M. G. St. John Sutton and N. Sharpe, Left ventricular remodeling after myocardial infarction: Pathophysiology and therapy, Circulation, 2000, 101, 2981–2988, DOI:10.1161/01.cir.101.25.2981.
- G. Vunjak-Novakovic, N. Tandon, A. Godier, R. Maidhof, A. Marsano, T. P. Martens and M. Radisic, Challenges in cardiac tissue engineering, Tissue Eng., Part B, 2010, 16, 169–187, DOI:10.1089/ten.teb.2009.0352.
- W. H. Zimmermann, I. Melnychenko, G. Wasmeier, M. Didié, H. Naito, U. Nixdorff, A. Hess, L. Budinsky, K. Brune, B. Michaelis, S. Dhein, A. Schwoerer, H. Ehmke and T. Eschenhagen, Engineered heart tissue grafts improve systolic and diastolic function in infarcted rat hearts, Nat. Med., 2006, 12, 452–458, DOI:10.1038/nm1394.
- S. D. Adams, A. Ashok, R. K. Kanwar, J. R. Kanwar and A. Z. Kouzani, Integrated 3D printed scaffolds and electrical stimulation for enhancing primary human cardiomyocyte cultures, Bioprinting, 2017, 6, 18–24, DOI:10.1016/j.bprint.2017.04.003.
- J. J. Liu, J. He, J. J. Liu, X. Ma, Q. Chen, N. Lawrence, W. Zhu, Y. Xu and S. Chen, Rapid 3D bioprinting of in vitro cardiac tissue models using human embryonic stem cell-derived cardiomyocytes, Bioprinting, 2019, 13, 1–15, DOI:10.1016/j.bprint.2019.e00040.Rapid.
- Z. Wang, S. J. Lee, H. J. Cheng, J. J. Yoo and A. Atala, 3D bioprinted functional and contractile cardiac tissue constructs, Acta Biomater., 2018, 70, 48–56, DOI:10.1016/j.actbio.2018.02.007.
- G. Gao, J. H. Lee, J. Jang, D. H. Lee, J.-S. Kong, B. S. Kim, Y.-J. Choi, W. B. Jang, Y. J. Hong, S.-M. Kwon and D.-W. Cho, Tissue Engineered Bio-Blood-Vessels Constructed Using a Tissue-Specific Bioink and 3D Coaxial Cell Printing Technique: A Novel Therapy for Ischemic Disease, Adv. Funct. Mater., 2017, 27, 1700798, DOI:10.1002/adfm.201700798.
- L. H. Kang, P. A. Armstrong, L. J. Lee, B. Duan, K. H. Kang and J. T. Butcher, Optimizing Photo-Encapsulation Viability of Heart Valve Cell Types in 3D Printable Composite Hydrogels, Ann. Biomed. Eng., 2017, 45, 360–377, DOI:10.1007/s10439-016-1619-1.
- R. A. Hortensius, W. H. Lin and B. M. Ogle, Cardiac tissue engineering: A pathway for repair, in Eng. Med. Adv. Challenges, Elsevier Inc., 2018, pp. 3–33. DOI:10.1016/B978-0-12-813068-1.00001-4.
- M. Izadifar, P. Babyn, M. E. Kelly, D. Chapman and X. Chen, Bioprinting Pattern-Dependent Electrical/Mechanical Behavior of Cardiac Alginate Implants: Characterization and Ex Vivo Phase-Contrast Microtomography Assessment, Tissue Eng., Part C, 2017, 23, 548–564, DOI:10.1089/ten.tec.2017.0222.
- B. Duan, E. Kapetanovic, L. A. Hockaday and J. T. Butcher, Three-dimensional printed trileaflet valve conduits using biological hydrogels and human valve interstitial cells, Acta Biomater., 2014, 10, 1836–1846, DOI:10.1016/j.actbio.2013.12.005.
- R. Gaetani, D. A. M. Feyen, V. Verhage, R. Slaats, E. Messina, K. L. Christman, A. Giacomello, P. A. F. M. Doevendans and J. P. G. Sluijter, Epicardial application of cardiac progenitor cells in a 3D-printed gelatin/hyaluronic acid patch preserves cardiac function after myocardial infarction, Biomaterials, 2015, 61, 339–348, DOI:10.1016/j.biomaterials.2015.05.005.
- J. Jang, H. J. Park, S. W. Kim, H. Kim, J. Y. Park, S. J. Na, H. J. Kim, M. N. Park, S. H. Choi, S. H. Park, S. W. Kim, S. M. Kwon, P. J. Kim and D. W. Cho, 3D printed complex tissue construct using stem cell-laden decellularized extracellular matrix bioinks for cardiac repair, Biomaterials, 2017, 112, 264–274, DOI:10.1016/j.biomaterials.2016.10.026.
- Y. S. Zhang, A. Arneri, S. Bersini, S.-R. Shin, K. Zhu, Z. Goli-Malekabadi, J. Aleman, C. Colosi, F. Busignani, V. Dell'Erba, C. Bishop, T. Shupe, D. Demarchi, M. Moretti, M. Rasponi, M. R. Dokmeci, A. Atala and A. Khademhosseini, Bioprinting 3D microfibrous scaffolds for engineering endothelialized myocardium and heart-on-a-chip., Biomaterials, 2016, 110, 45–59, DOI:10.1016/j.biomaterials.2016.09.003.
- F. Maiullari, M. Costantini, M. Milan, V. Pace, M. Chirivì, S. Maiullari, A. Rainer, D. Baci, H. E. S. Marei, D. Seliktar, C. Gargioli, C. Bearzi and R. Rizzi, A multi-cellular 3D bioprinting approach for vascularized heart tissue engineering based on HUVECs and iPSC-derived cardiomyocytes, Sci. Rep., 2018, 8, 1–15, DOI:10.1038/s41598-018-31848-x.
- M. Izadifar, D. Chapman, P. Babyn, X. Chen and M. E. Kelly, UV-Assisted, 3D Bioprinting of Nanoreinforced Hybrid Cardiac Patch for Myocardial Tissue Engineering, Tissue Eng., Part C, 2018, 24, 74–88, DOI:10.1089/ten.tec.2017.0346.
- N. Noor, A. Shapira, R. Edri, I. Gal, L. Wertheim and T. Dvir, 3D Printing of Personalized Thick and Perfusable Cardiac Patches and Hearts, Adv. Sci., 2019, 6, 1900344, DOI:10.1002/advs.201900344.
- W. Mitzner, Airway smooth muscle: The appendix of the lung, Am. J. Respir. Crit. Care Med., 2004, 169, 787–790, DOI:10.1164/rccm.200312-1636PP.
- C. Severi, R. Sferra, A. Scirocco, A. Vetuschi, N. Pallotta, A. Pronio, R. Caronna, G. Di Rocco, E. Gaudio, E. Corazziari and P. Onori, Contribution of intestinal smooth muscle to Crohn's disease fibrogenesis, Eur. J. Histochem., 2014, 58, 307–314, DOI:10.4081/ejh.2014.2457.
- S. S. An, T. R. Bai, J. H. T. Bates, J. L. Black, R. H. Brown, V. Brusasco, P. Chitano, L. Deng, M. Dowell, D. H. Eidelman, B. Fabry, N. J. Fairbank, L. E. Ford, J. J. Fredberg, W. T. Gerthoffer, S. H. Gilbert, R. Gosens, S. J. Gunst, A. J. Halayko, R. H. Ingram, C. G. Irvin, A. L. James, L. J. Janssen, G. G. King, D. A. Knight, A. M. Lauzon, O. J. Lakser, M. S. Ludwig, K. R. Lutchen, G. N. Maksym, J. G. Martin, T. Mauad, B. E. McParland, S. M. Mijallovich, H. W. Mitchell, R. W. Mitchell, W. Mitzner, T. M. Murphy, P. D. Paré, R. Pellegrino, M. J. Sanderson, R. R. Schellenberg, C. Y. Seow, P. S. P. Silveira, P. G. Smith, J. Solway, N. L. Stephens, P. J. Sterk, A. G. Stewart, D. D. Tang, R. S. Tepper, T. Tran and L. Wang, Airway smooth muscle dynamics: A common pathway of airway obstruction in asthma, Eur. Respir. J., 2007, 29, 834–860, DOI:10.1183/09031936.00112606.
- P. McGonigle and B. Ruggeri, Animal models of human disease: Challenges in enabling translation, Biochem. Pharmacol., 2014, 87, 162–171, DOI:10.1016/j.bcp.2013.08.006.
- P. Perel, I. Roberts, E. Sena, P. Wheble, C. Briscoe, P. Sandercock, M. Macleod, L. E. Mignini, P. Jayaram and K. S. Khan, Comparison of treatment effects between animal experiments and clinical trials: Systematic review, Br. Med. J., 2007, 334, 197–200, DOI:10.1136/bmj.39048.407928.BE.
- C. T. D. Dickman, V. Russo, K. Thain, S. Pan, S. T. Beyer, K. Walus, S. Getsios, T. Mohamed and S. J. Wadsworth, Functional characterization of 3D contractile smooth muscle tissues generated using a unique microfluidic 3D bioprinting technology, FASEB J., 2020, 34, 1652–1664, DOI:10.1096/fj.201901063RR.
- Y. Zhang, Y. Yu, A. Akkouch, A. Dababneh, F. Dolati and I. T. Ozbolat, In vitro study of directly bioprinted perfusable vasculature conduits, Biomater. Sci., 2015, 3, 134–143, 10.1039/c4bm00234b.
- B. Duan, L. A. Hockaday, K. H. Kang and J. T. Butcher, 3D Bioprinting of heterogeneous aortic valve conduits with alginate/gelatin hydrogels, J. Biomed. Mater. Res., Part A, 2013, 101, 1255–1264, DOI:10.1002/jbm.a.34420.
- X. Wang, Y. Yan and R. Zhang, Rapid prototyping as a tool for manufacturing bioartificial livers, Trends Biotechnol., 2007, 25, 505–513, DOI:10.1016/j.tibtech.2007.08.010.
- H. Cui, W. Zhu, Y. Huang, C. Liu, Z. X. Yu, M. Nowicki, S. Miao, Y. Cheng, X. Zhou, S. J. Lee, Y. Zhou, S. Wang, M. Mohiuddin, K. Horvath and L. G. Zhang, In Vitro and In Vivo Evaluation of 3D Bioprinted Small-diameter Vasculature with Smooth Muscle and Endothelium, Biofabrication, 2019, 12, 015004, DOI:10.1088/1758-5090/ab402c.In.
- T. Cameron, E. Naseri, B. MacCallum and A. Ahmadi, Development of a disposable single-nozzle printhead for 3D bioprinting of continuous multi-material constructs, Micromachines, 2020, 11, 459, DOI:10.3390/MI11050459.
- M. A. Skylar-Scott, J. Mueller, C. W. Visser and J. A. Lewis, Voxelated soft matter via multimaterial multinozzle 3D printing, Nature, 2019, 575, 330–335, DOI:10.1038/s41586-019-1736-8.
- B. G. Soliman, G. C. J. Lindberg, T. Jungst, G. J. Hooper, J. Groll, T. B. F. Woodfield and K. S. Lim, Stepwise Control of Crosslinking in a One–Pot System for Bioprinting of Low–Density Bioinks, Adv. Healthcare Mater., 2020, 9, 1901544, DOI:10.1002/adhm.201901544.
- C. Chávez-Madero, M. D. De León-Derby, M. Samandari, C. F. Ceballos-González, E. J. Bolívar-Monsalve, C. Mendoza-Buenrostro, S. Holmberg, N. A. Garza-Flores, M. A. Almajhadi, I. González-Gamboa, J. F. Yee-De León, S. O. Martínez-Chapa, C. A. Rodríguez, H. K. Wickramasinghe, M. Madou, D. Dean, A. Khademhosseini, Y. S. Zhang, M. M. Alvarez and G. Trujillo-De Santiago, Using chaotic advection for facile high-throughput fabrication of ordered multilayer micro-and nanostructures: Continuous chaotic printing, Biofabrication, 2020, 12, 035023, DOI:10.1088/1758-5090/ab84cc.
- A. Lee, A. R. Hudson, D. J. Shiwarski, J. W. Tashman, T. J. Hinton, S. Yerneni, J. M. Bliley, P. G. Campbell and A. W. Feinberg, 3D bioprinting of collagen to rebuild components of the human heart, Science, 2019, 365, 482–487, DOI:10.1126/science.aav9051.
- D. C. Corbett, E. Olszewski and K. Stevens, A FRESH Take on Resolution in 3D Bioprinting, Trends Biotechnol., 2019, 37, 1153–1155, DOI:10.1016/j.tibtech.2019.09.003.
- A. McCormack, C. B. Highley, N. R. Leslie and F. P. W. Melchels, 3D Printing in Suspension Baths: Keeping the Promises of Bioprinting Afloat, Trends Biotechnol., 2020, 38, 584–593, DOI:10.1016/j.tibtech.2019.12.020.
- W. Cheng, J. Zhang, J. Liu and Z. Yu, Granular hydrogels for 3D bioprinting applications, View, 2020, 20200060, DOI:10.1002/VIW.20200060.
- Z. Wan, P. Zhang, Y. Liu, L. Lv and Y. Zhou, Four-dimensional bioprinting: Current developments and applications in bone tissue engineering, Acta Biomater., 2020, 101, 26–42, DOI:10.1016/j.actbio.2019.10.038.
- C. S. Ong, P. Yesantharao and N. Hibino, 3D and 4D Printing in Biomedical Applications, in 3D 4D Print. Biomed. Appl. Process Eng. Addit. Manuf, ed. M. Maniruzzaman, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2019, pp. 317–343. DOI:10.1002/9783527813704.
- B. Gao, Q. Yang, X. Zhao, G. Jin, Y. Ma and F. Xu, 4D Bioprinting for Biomedical Applications, Trends Biotechnol., 2016, 34, 746–756, DOI:10.1016/j.tibtech.2016.03.004.
- Q. Yang, B. Gao and F. Xu, Recent Advances in 4D Bioprinting, Biotechnol. J., 2020, 15, 1900086, DOI:10.1002/biot.201900086.
- N. Ashammakhi, S. Ahadian, F. Zengjie, K. Suthiwanich, F. Lorestani, G. Orive, S. Ostrovidov and A. Khademhosseini, Advances and Future Perspectives in 4D Bioprinting, Biotechnol. J., 2018, 13, e1800148, DOI:10.1002/biot.201800148.
- A. C. Daly, P. Pitacco, J. Nulty, G. M. Cunniffe and D. J. Kelly, 3D printed microchannel networks to direct vascularisation during endochondral bone repair, Biomaterials, 2018, 162, 34–46, DOI:10.1016/j.biomaterials.2018.01.057.
- Y. Luo, G. Luo, M. Gelinsky, P. Huang and C. Ruan, 3D bioprinting scaffold using alginate/polyvinyl alcohol bioinks, Mater. Lett., 2017, 189, 295–298, DOI:10.1016/j.matlet.2016.12.009.
- H. Kim, G. H. Yang, C. H. Choi, Y. S. Cho and G. H. Kim, Gelatin/PVA scaffolds fabricated using a 3D-printing process employed with a low-temperature plate for hard tissue regeneration: Fabrication and characterizations, Int. J. Biol. Macromol., 2018, 120, 119–127, DOI:10.1016/j.ijbiomac.2018.07.159.
- J. P. K. Armstrong, M. Burke, B. M. Carter, S. A. Davis and A. W. Perriman, 3D Bioprinting Using a Templated Porous Bioink, Adv. Healthcare Mater., 2016, 5, 1724–1730, DOI:10.1002/adhm.201600022.
- Y. Sun, Y. You, W. Jiang, Q. Wu, B. Wang and K. Dai, Generating ready-to-implant anisotropic menisci by 3D-bioprinting protein-releasing cell-laden hydrogel-polymer composite scaffold, Appl. Mater. Today, 2020, 18, 100469, DOI:10.1016/j.apmt.2019.100469.
- J.-S. Lee, J. M. Hong, J. W. Jung, J.-H. Shim, J.-H. Oh and D.-W. Cho, 3D printing of composite tissue with complex shape applied to ear regeneration, Biofabrication, 2014, 6, 024103, DOI:10.1088/1758-5082/6/2/024103.
| This journal is © The Royal Society of Chemistry 2021 |