Open Access Article
Open Access ArticleDetection of falsified clopidogrel in the presence of excipients using voltammetry†
Ricoveer Singh
Shergill
 ,
Petra
Kristova
and
Bhavik Anil
Patel
,
Petra
Kristova
and
Bhavik Anil
Patel
 *
*
School of Applied Sciences, University of Brighton, Brighton, East Sussex, UK. E-mail: b.a.patel@brighton.ac.uk; Tel: +44(0)1273 642418
First published on 7th October 2021
Abstract
There has been a recent surge in the amount of substandard and falsified clopidogrel. Pharmacopeial based assays using high performance liquid chromatography and mass spectroscopy are widely used for the measurement of clopidogrel but are not accessible in low to middle income countries. Therefore, our study explored four different techniques (mid-infrared spectroscopy, thin layer chromatography, ultraviolet visible spectroscopy, and differential pulse voltammetry), which could be used in low to middle income countries. Differential pulse voltammetry showed the best performance for accurate and precise determination of clopidogrel in the presence of excipients. Clopidogrel tablets were fully crushed and sonicated in buffer for 30 seconds prior to differential pulse voltammetry measurements using a 3 mm glassy carbon electrode. Measurements were made without removing the excipients and the limit of detection was 0.08 mg ml−1 and the sensitivity was 15.7 μA mg ml−1. When conducting a blinded study, differential pulse voltammetry was able to identify varying types of substandard and falsified samples. Our findings highlight that voltammetry could be a vital analytical technique for the determination of substandard and falsified medicines in low- and middle-income countries.
Introduction
Substandard and falsified medicines are a major problem for society as they can cause considerable risk to public health, reduce confidence in healthcare systems, and cause economical loss.1–4 The major cause of substandard medicines is poor manufacturing and quality control practices,2,5,6 whilst for falsified medicines this is mainly for financial benefits for organised crime groups.7,8 The impact of such substandard and falsified medicines is mainly felt in low- and middle-income countries where accessibility to medicines is often poor.Clopidogrel, also commonly sold under the commercial name Plavix®, is a frontline anti-platelet medicine, which helps prevent platelets from sticking together and forming a dangerous blood clot. Therefore, this medicine is prescribed by practitioners to reduce the risk of myocardial infarction and stroke.9 The widespread use of clopidogrel makes it a target for counterfeit manufacture and there are a limited number of reliable methods offering a cheap convenient method for identifying and quantifying clopidogrel; identifying such a method would offer an invaluable tool for use in counterfeit detection in low- and middle-income countries. The production of falsified clopidogrel medication has recently occurred in Europe and the USA, where patients were given ineffective “Plavix” tablets leading to complaints from patients and subsequent testing showed that the product was falsified.10,11 Therefore, there is a clear need for a simple and effective analytical methodology for the determination of substandard and falsified clopidogrel.
The majority of substandard medicines have significant variations in the content of the active pharmaceutical ingredient,12 whilst in falsified medication, the majority of medicines contain little active pharmaceutical ingredient (API), no API or alternative APIs.13,14 Given that analytical techniques need to accurately monitor concentration and identity the presence of APIs, this makes it difficult for a uniform detection approach. The most widely used analytical techniques for the determination of substandard and falsified medicines are based on pharmacopoeial assays. Techniques such as high-performance liquid chromatography (HPLC) with ultraviolet spectroscopy (UV) and/or mass spectrometry detection or gas chromatography15–17 are also widely used. These methods can provide high sensitivity and selectivity but require a number of highly technical expensive instruments and expertise. Therefore, these robust approaches are not accessible for low- to middle-income countries where there is the greatest need for analytical detection.18 Rapid and simple approaches which can also be used in remote locations such as colorimetry and thin-layer chromatography (TLC)19–21 are usually less sensitive but require less expertise. Spectroscopic methods such as near-infrared, mid-infrared (MIR), Raman and benchtop nuclear magnetic resonance22–25 have shown great potential but provide more insight into the selectivity than the accurate analysis of concentration and are thus more likely suitable for falsified medicines than substandard medicines. Current non-analytical measurements are in use all around the world to identify counterfeit medication, and most such methods are based upon using track and trace or tamper resistant packing.26
Within our study we explored the suitability of voltammetry as a detection approach for monitoring the presence and concentration of clopidogrel within pharmaceutical tablets. Previous studies have shown that clopidogrel can be oxidised and therefore can be detected by this technique.27,28 We explored the suitability of voltammetry when compared to UV/visible spectroscopy, MIR, and TLC for accurate and precise determination of the concentration of clopidogrel. Additionally, we evaluated which method provided the simplest approach to sample preparation making the technique accessible to middle- to low- income countries. Finally, in a blinded study, we evaluated the ability of our established voltammetry method to determine a range of prepared substandard and falsified clopidogrel formulations.
Materials and methods
Chemicals and tablets
Clopidogrel tablets (75 mg dose) were purchased from the pharmaceutical manufacturer Torrent. Clopidogrel, magnesium stearate, microcrystalline cellulose (MCC), sunset-yellow dye, citric acid, sodium acetate, paracetamol, ethylenediaminetetraacetic acid (EDTA), sodium chloride and lactose were obtained from Sigma Aldrich. Solutions of clopidogrel were prepared in a citrate buffer (pH 3.0) made using 0.1 M citric acid, 0.1 M sodium acetate and 2.7 mM EDTA.Detection of clopidogrel using MIR
For MIR measurements, spectra were obtained from pure clopidogrel, a clopidogrel tablet (75 mg dose) and a clopidogrel tablet diluted further with lactose to the equivalent of a half dose. The samples were powdered using a glass pestle and mortar prior to analysis. Lactose was utilised to dilute the tablet as this was the same bulking agent utilised in the manufacturing of the tablet. Mid-infrared measurements were performed using a PerkinElmer, Spectrum 65 spectrometer, fitted with an attenuated total reflectance (ATR) accessory employing a ZnSe crystal. Measurements through the ATR accessory require a firm contact between the sample and the ZnSe crystal. This was achieved by light manually controlled compaction (using a pike). The samples were measured in the spectral range of 4000–550 cm−1 at a resolution of 4 cm−1. Each spectrum was collected from 16 scans. PerkinElmer Spectrum software was used for data analysis.TLC analysis of clopidogrel tablets
A powdered clopidogrel tablet was dissolved in 1 M methanol to make 2.5 mg ml−1 and 5 mg ml−1 solutions. Half of these solutions were filtered using Grade 601 filter paper to compare the filtered and unfiltered response. The samples of both concentrations, filtered and unfiltered, were spotted onto aluminium-backed silica gel 60F254 plates. The mobile phase was n-heptane-tetrahydrofuran, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v). The plate was visualised under UV light at 254 nm. A photograph was taken of the TLC plate and converted to the greyscale for densiometric analysis. Image J was utilised to measure the grey scale as a marker of the density of the spot.
1 (v/v). The plate was visualised under UV light at 254 nm. A photograph was taken of the TLC plate and converted to the greyscale for densiometric analysis. Image J was utilised to measure the grey scale as a marker of the density of the spot.
Determination of clopidogrel using UV/visible spectroscopy
A powdered clopidogrel tablet was dissolved in citrate buffer to make 0.5 mg ml−1 and 1 mg ml−1 concentration solutions. Half of these solutions were filtered using Grade 601 filter paper to compare the filtered and unfiltered responses. A UV/Visible 1601 double beam spectrophotometer with a fixed slit width (2 nm) and 1 cm matched quartz cuvettes were used for all the spectral measurements. The samples were scanned against a citrate buffer blank over the range of 900–200 nm wavelengths.Determination of clopidogrel using differential pulse voltammetry
Electrochemical measurements were carried out with a CHI630B potentiostat, controlled with CH Instruments software (CH Instruments, Austin, TX, USA). A three-electrode system was used, where a 3 mm glassy carbon electrode served as the working electrode, an Ag|AgCl (3 M NaCl) electrode as the reference electrode and a platinum wire as the counter electrode. Prior to the electrochemical measurements, the glassy carbon electrode was polished with alumina aqueous slurry. For all measurements, differential pulse voltammetry was used, where the pulse amplitude was 50 mV s−1, the pulse width was 0.06 s, and the potential window was between +0.7 and +1.4 V. To compare the difference in the current response from tablets which were filtered and unfiltered, a clopidogrel tablet was crushed to a fine powder using a pestle and mortar and then was dissolved in citrate buffer to make 1 and 0.5 mg ml−1 solutions and half of these solutions were filtered using Grade 601 filter paper. Calibration responses were determined using clopidogrel powder and conducting serial dilutions of the tablet. Calibrations were conducted in the concentration range of 0.1 to 1 mg ml−1. To explore the change in the current with sample preparation, we explored the duration that the crushed tablet samples needed to be sonicated from 5 to 45 s. For the analysis of the data, the anodic peak current was obtained using CH Instruments software.Preparation of falsified and substandard tablets
Table 1 shows the different substandard and falsified tablets that were prepared and subsequently tested using voltammetry. The commercial clopidogrel tablet approximately weighs 260 mg containing 75 mg of clopidogrel and further excipients, and it is a white tablet with a salmon pink colour coating. All falsified and substandard tablets were made to this weight/appearance, where alterative active compounds used had similar molecular weights. Acetyl salicylic acid (as aspirin tablets) and acetaminophen (as paracetamol tablets) were used due to their common availability and some structural similarities of the active ingredients, and aspirin also possesses a similar therapeutic effect. The falsified and substandard tablets were made as powders and thus did not require to be crushed prior to conducting differential pulse voltammetry measurements.| Falsified Tablet | Contents |
|---|---|
| 1 | 25 mg of clopidogrel (86.6 mg of crushed clopidogrel tablet) and 173.4 mg lactose (Granulac 200, Meggle) |
| 2 | 7.5 mg of clopidogrel (26 mg of crushed clopidogrel tablet) and 242 mg of crushed paracetamol tablet (the whole paracetamol tablet is 565 mg and contains 500 mg paracetamol) |
| 3 | 25 mg of clopidogrel (86.6 mg of crushed clopidogrel tablet) and 173.4 mg microcrystalline cellulose (FMC Biopolymer) |
| 4 | Aspirin tablet mixture and lactose (equivalent of 100 mg aspirin, total 115.3 mg of the aspirin tablet mixture, Aspar – 300 mg aspirin, tablet weight 346 mg) and lactose 144.7 mg (Granulac 200, Meggle) |
| 5 | Placebo tablet containing 1% of sunset yellow dye (130 mg excipients and 1.3 mg of sunset yellow) and lactose 130 mg (Granulac 200, Meggle) |
Data analysis
For all measurements, the data were plotted to show the mean ± standard deviation. Statistical analysis was carried out using GraphPad Prism, where data were compared using one-way or two-way ANOVA.Results and discussion
Comparing analytical techniques for the accurate and precise determination of the concentration of clopidogrel in the presence of excipients
Fig. 1 shows MIR, UV/Visible spectroscopy, TLC, and differential pulse voltammetry experiments. Within this experiment, we explored the suitability of these techniques for monitoring substandard and falsified medicines through understanding if these techniques will be able to accurately and precisely determine the concentration of clopidogrel in the presence of excipients.ESI Fig. 1† shows the entire IR spectra for raw powder of clopidogrel, a powdered commercial tablet, a powdered tablet diluted with lactose and lactose powder. There was a sharp, strong peak in the region around 1730–1750 cm−1, which shows the presence of a carbonyl group (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) ester stretching and thus provides a region for the identification of clopidogrel. No observable interference from the excipients present within the tablet was observed in this region. No other aspect of the IR spectra showed features which would provide a selective region for the determination of clopidogrel. Further analysis of the peak in the region around 1730–1750 cm−1 was conducted as shown in Fig. 1A, where the % transmission was measured to the baseline of the IR spectra. The results from multiple measurements are shown in Fig. 1B, where there was a significant difference between the original tablet and that diluted with lactose (n = 3, p < 0.05). The IR analysis was able to accurate measure half the concentration and thus suggests that in this specific region within the IR spectra it is feasible to monitor accurately the concentration of clopidogrel without the requirement of any sample preparation to remove the interference of the excipients. The average percentage coefficient of variation (CoV) was 28% for the measurements of the original and diluted tablets. This highlights that there is poor precision in repeated measurements which may be due to interference from other excipient compounds, which contain ester functional groups.
O) ester stretching and thus provides a region for the identification of clopidogrel. No observable interference from the excipients present within the tablet was observed in this region. No other aspect of the IR spectra showed features which would provide a selective region for the determination of clopidogrel. Further analysis of the peak in the region around 1730–1750 cm−1 was conducted as shown in Fig. 1A, where the % transmission was measured to the baseline of the IR spectra. The results from multiple measurements are shown in Fig. 1B, where there was a significant difference between the original tablet and that diluted with lactose (n = 3, p < 0.05). The IR analysis was able to accurate measure half the concentration and thus suggests that in this specific region within the IR spectra it is feasible to monitor accurately the concentration of clopidogrel without the requirement of any sample preparation to remove the interference of the excipients. The average percentage coefficient of variation (CoV) was 28% for the measurements of the original and diluted tablets. This highlights that there is poor precision in repeated measurements which may be due to interference from other excipient compounds, which contain ester functional groups.
Fig. 1C shows the UV visible spectra of unfiltered and filtered clopidogrel tablet solutions made at two concentrations. The maximal peak response was observed for 270 nm and used to measure the absorbance. Fig. 1D shows a significant decrease in the absorbance observed in the unfiltered samples when compared to the filtered samples at both concentrations (p < 0.01, n = 3). The CoV for filtered samples was 4% and for unfiltered samples it was 16%. The reduction in the absorbance and the reduced precision may be due to the scattering of the light from excipient particles, thus increasing the observed absorbance. However, there was good accuracy in the ability to monitor the concentration in both unfiltered and filtered samples.
Fig. 1E shows the response of the TLC plate with the densitometric analysis superimposed on the plate. Fig. 1F shows the analysis of the TLC plate for the samples, which were filtered and unfiltered at the two concentrations. There was no significant different in the greyscale index observed for the filtered and unfiltered samples. However, there was no clear relationship between the greyscale index and concentration, with the concentration utilised at the top of the potential linear range. However, when 0.5 and 1 mg ml−1 samples were run they also provided no concentration relationship indicating a very smaller linear range for densitometry measurements. The CoV was 7% for the filtered samples and 9% for the unfiltered samples and thus indicated that the precision was identical and thus TLC can be used for the measurement of drug compounds in the presence of excipients.
Differential pulse voltammograms for the filtered and unfiltered clopidogrel tablets at the two different concentrations are shown in Fig. 1G. The oxidation peak potential for clopidogrel was 1.06 V. Fig. 1H shows that there was no difference observed in the current response between the filtered and unfiltered samples at both concentrations. The current observed for 0.5 mg ml−1 clopidogrel was directly half that observed for the measurement of 1 mg ml−1 solutions of clopidogrel tablets. The CoV was 0.9% for the filtered samples and 1.6% for the unfiltered samples. These findings clearly showed that voltammetry is suitable for accurate and precise measurement of clopidogrel concentration without requiring the removal of the excipients. These findings support other voltammetry studies where accurate measurement of the concentration was achieved in the presence of excipients.29,30
When comparing the four techniques, which would all be suitable for remote measurement of substandard and falsified clopidogrel in low to middle income countries, we considered three important factors. They were the ability to accurately monitor the concentration of clopidogrel present, the precision of the technique when conducted without extensive sample preparation, and the application of the technique in the presence of common tableting excipients. Table 2 shows the performance differences between the four techniques, which highlights that only voltammetry showed excellent results in all criteria, whilst the other techniques showed some potential, however performed well in fewer categories. These findings highlight the ability of differential pulse voltammetry to be the most suitable approach for the identification of many substandard and falsified medicines, in which we observe that little or no active drug is present.13,14 However, where falsified medicines contain alternative drugs, MIR may be more suitable to distinguish counterfeits structurally similar to original compounds.
| Criteria | FTIR | UV/visible spectroscopy | TLC | Differential pulse voltammetry |
|---|---|---|---|---|
| Accurate determination of concentration | Good | Excellent | Poor | Excellent |
| Precision for routine monitoring | Poor | Good | Good | Excellent |
| Minimal sample preparation | Excellent | Poor | Good | Good |
Calibration of clopidogrel using voltammetry
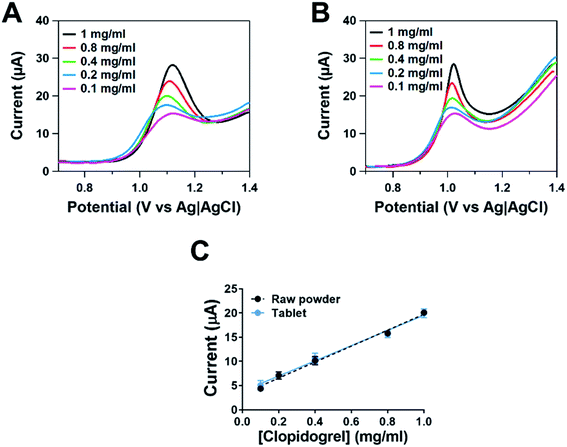
Following our comparison between the four techniques, differential pulse voltammetry was chosen to be the most suitable, and therefore we conducted studies to establish a robust method of measurement. Calibration studies were conducted to understand the limit of detection and sensitivity for the detection of clopidogrel. Studies were conducted using the clopidogrel standard and creation of a calibration using serial dilution of the pharmaceutical tablet. This approach was taken as previous studies have shown that excipients can reduce the current response of the active drug.30Fig. 2A shows differential pulse voltammograms for the clopidogrel standard. There is a clear relationship between the current and concentration of clopidogrel. There is a gradual increase in the oxidation peak potential with increasing concentration, most likely due to mass transfer effects. Fig. 2B shows differential pulse voltammograms for the clopidogrel tablet. The responses also show a relationship between the current and concentration; however the peak shape differs from those observed within the clopidogrel standard at higher concentrations. This may be due to interference from excipients influencing the peak shape. Fig. 2C shows calibration responses for the clopidogrel standard and tablet. There was no significant difference between the responses, suggestive that excipients did not have any influence on the current response over the range of the calibration plot. Similar studies using voltammetry have shown that clopidogrel can be determined without the influence of excipients.28,31 However, given that different formulations or other manufactured tablets may contain excipients that could interfere with and reduce the current response, the calibration response from the tablet was utilised. The sensitivity observed was 15.7 μA mg ml−1 and the limit of detection was 0.08 mg ml−1. This method was fit-for-purpose and thus is comparable to other approaches focused on the determination of clopidogrel in pharmaceutical formulations.27,28,31,32
Method development for precise measurement of clopidogrel tablets
A robust methodology was established for the assessment of method precision since this study involved the preparation of solutions and mixtures of substandard, counterfeited, or commercial tablets. Tablets were crushed into a coarse powder, dissolved in citrate buffer to make 1 mg ml−1 solution, and sonicated to ensure that maximal current was observed. Fig. 3A shows differential pulse voltammograms where different durations of sonication were utilised. Fig. 3B shows the response from multiple replicates, where there was an increase in the concentration of clopidogrel with increasing time of sonication. The accurate concentration of the tablet solution was only detected during and after 30 seconds of sonication. There was no difference in the amount of clopidogrel measured when 30 or 45 seconds of sonication was utilised. Following sonication, the solution was stable for 10 minutes of measurement without any reduction in the current response.To assess the reproducibility of the preparation for robust measurement of the clopidogrel tablet, we measured 10 tablets using the established sample preparation approaches, where the RSD was 1.6%.
Validation studies using prepared substandard and falsified clopidogrel
To assess the suitability of our established method using voltammetry for the measurement of substandard and falsified medicines, blinded samples were prepared and run using the established method. Fig. 4 shows the powdered samples that were provided and the resultant voltammogram responses in comparison to the 1 mg ml−1 clopidogrel solution. From the powdered samples provided, other than sample 5, it was not feasible to see any noticeable differences in the colour of the powder. Sample 5 was orange in colour due to the presence of sunset yellow dye as highlighted in the composition in Table 1, which is different to the off-white powder of a clopidogrel tablet. | ||
| Fig. 4 Different substandard and falsified samples and the resultant voltammograms with comparison to that of 1 mg ml−1 clopidogrel. | ||
Sample 1 had a peak at the expected anodic peak potential; however, the current value is lower than the expected value. This tablet contained 24.2 ± 2.3 mg of clopidogrel and thus the recovery matched that of the formulation highlighted in Table 1. Sample 2 had no observable response at the expected anodic peak potential and given no clopidogrel was present this was expected. However, this tablet contained paracetamol which is oxidisable and thus could have posed to be an interferent. Study has shown that when using citrate buffer at pH 3.0, paracetamol is oxidised at ∼0.5 V, and thus is at a voltage lower than our potential window.33 The tail end of this oxidation may be present at the beginning of the potential window used for the measurement of clopidogrel. Sample 3 has a peak at the expected anodic peak potential; however, the current value is lower than the expected value. This tablet contained 22.9 ± 3.1 mg of clopidogrel and thus the recovery matched that of the formulation highlighted in Table 1. This response also highlighted that other excipients did not interfere in the accurate determination of the amount of clopidogrel present. Sample 4 has an anodic peak which was slightly more positive than that of clopidogrel and was a much broader peak. Previous studies have shown that on a bare glassy carbon electrode aspirin oxidation is observed around 0.97 V, which is in the same vicinity where clopidogrel was measured.34 With 100 mg of aspirin present within this tablet, the peak amplitude was still lower and would have flagged this sample as a falsified tablet; however this response highlights that any substance that could be oxidised at this voltage with similar peak features of clopidogrel could provide a false positive result and limit the suitability of voltammetry. Finally, sample 5 had two smaller peaks, with one around 0.7 V and the other at 1.1 V, which is a similar region to that where clopidogrel was oxidised. Due to this additional peak, this measurement gave overall information that this sample was a falsified tablet although perhaps with some amount of clopidogrel present. Sample 5 was a placebo without any clopidogrel and it is plausible that this interference is due to the presence of sunset yellow dye.
Overall voltammetry provided the ability to clearly identify the varying samples of substandard and falsified samples. The presence of a lower concentration of clopidogrel was accurately determined. Samples where alterative compounds were present were recognised by this technique as counterfeits. However, like any analytical approach, voltammetry has limitations, where substances that can be oxidised at the same voltage as clopidogrel can cause interference and falsely indicate that clopidogrel might be present within the tablet.
Conclusions
Clopidogrel is an important anti-platelet medicine. Falsified clopidogrel tablets have been recently observed in low to middle income countries. Many of the existing methods utilised require specialised equipment and resources and thus simple effective approaches that could be used in remote locations do not exist. This study explores four different analytical approaches for the measurement and identification of substandard and falsified clopidogrel. Differential pulse voltammetry was far superior to MIR, UV/visible spectroscopy and TLC for accurate and precise measurement of clopidogrel in the presence of excipients. A voltammetry method was established where samples required sonication for 30 seconds to provide the accurate measurement of the tablet concentration. In a blinded study, voltammetry was successful in the identification of substandard and falsified formulations. Our findings clearly highlight that voltammetry is a useful and accessible technique for remote pharmaceutical analysis of substandard and falsified clopidogrel.Author contributions
The investigation was carried out by RSS and PK. The methodology was proposed by all authors. Formal analysis was carried out by RSS. The writing of the original draft was done by RSS and BAP, with review and editing by all authors. Conceptualization was done by BAP.Conflicts of interest
The authors report no conflicts of interest.References
- S. Ozawa, D. R. Evans, S. Bessias, D. G. Haynie, T. T. Yemeke, S. K. Laing and J. E. Herrington, JAMA network open, 2018, 1, e181662 CrossRef PubMed.
- D. Khurelbat, G. Dorj, B. Sunderland, T. Sanjjav, E. Bayarsaikhan, D. Damdinjav, G. Dorj, A. Jigjidsuren, O. Lkhagvasuren and B. Erdenetsetseg, BMC Public Health, 2020, 20, 1–9 CrossRef PubMed.
- W. H. Organization, A study on the public health and socioeconomic impact of substandard and falsified medical products, 2017, ISSN: 9241513438 Search PubMed.
- T. Almuzaini, H. Sammons and I. Choonara, BMJ Open, 2013, 3, e002924 CrossRef PubMed.
- J. M. Caudron, N. Ford, M. Henkens, C. Mace, R. Kiddle-Monroe and J. Pinel, Trop. Med. Int. Health, 2008, 13, 1062–1072 CrossRef PubMed.
- A. N. Giralt, B. Schiavetti, B. Meessen, C. Pouget, J. Caudron, B. Marchal, P. Massat, S. Thys and R. Ravinetto, BMJ Global Health, 2017, 2, e000172 CrossRef PubMed.
- P. C. Gøtzsche, R. Smith and D. Rennie, Deadly Medicines and Organised Crime: How Big Pharma Has Corrupted Healthcare, CRC press, 2019 Search PubMed.
- C. Edwards and C. Jeffray, in Mischief, Morality and Mobs, Routledge, 2016, pp. 165–188 Search PubMed.
- N. Sarafoff, R. A. Byrne and D. Sibbing, Curr. Pharm. Des., 2012, 18, 5224–5239 CrossRef CAS PubMed.
- N. Graham, BMJ, 2010, 340, c238 CrossRef.
- D. Świeczkowski, S. Zdanowski, P. Merks, Ł. Szarpak, R. Vaillancourt and M. J. Jaguszewski, Cardiol. J., 2020 DOI:10.5603/CJ.a2020.0168.
- A. Alghannam, Z. Aslanpour, S. Evans and F. Schifano, Integrated Pharmacy Research and Practice, 2014 Search PubMed.
- H. Rebiere, P. Guinot, D. Chauvey and C. Brenier, J. Pharm. Biomed. Anal., 2017, 142, 286–306 CrossRef CAS PubMed.
- I. M. Bakker, D. Ohana and B. J. Venhuis, J. Pharm. Biomed. Anal., 2021, 113948 CrossRef PubMed.
- M. Bernard, W. Akrout, C. T. Van Buu, C. Metz, M. Antignac, N. Yagoubi and B. Do, J. Sep. Sci., 2015, 38, 562–570 CrossRef CAS PubMed.
- R. Martino, M. Malet-Martino, V. Gilard and S. Balayssac, Anal. Bioanal. Chem., 2010, 398, 77–92 CrossRef CAS PubMed.
- Y. Yang, D. Song, W. Jiang and B. Xiang, Anal. Lett., 2010, 43, 373–380 CrossRef CAS.
- S. Kovacs, S. E. Hawes, S. N. Maley, E. Mosites, L. Wong and A. Stergachis, PLoS One, 2014, 9, e90601 CrossRef PubMed.
- H. Yu, H. Le, S. Lumetta, B. T. Cunningham, E. Kaale and T. Layloff, IEEE Sens., 2016, 1–3 Search PubMed.
- M. D. Green, D. M. Hostetler, H. Nettey, I. Swamidoss, N. Ranieri and P. N. Newton, Am. J. Trop. Med. Hyg., 2015, 92, 8 CrossRef CAS PubMed.
- J. Sherma and F. Rabel, J. Liq. Chromatogr. Relat. Technol., 2019, 42, 367–379 CrossRef CAS.
- G. Assemat, S. Balayssac, A. Gerdova, V. Gilard, C. Caillet, D. Williamson and M. Malet-Martino, Talanta, 2019, 196, 163–173 CrossRef CAS PubMed.
- H. Rebiere, M. Martin, C. Ghyselinck, P.-A. Bonnet and C. Brenier, J. Pharm. Biomed. Anal., 2018, 148, 316–323 CrossRef CAS PubMed.
- P.-Y. Sacré, E. Deconinck, T. De Beer, P. Courselle, R. Vancauwenberghe, P. Chiap, J. Crommen and J. O. De Beer, J. Pharm. Biomed. Anal., 2010, 53, 445–453 CrossRef PubMed.
- P. Ciza, P.-Y. Sacre, C. Waffo, L. Coïc, H. Avohou, J. Mbinze, R. Ngono, R. Marini, P. Hubert and E. Ziemons, Talanta, 2019, 202, 469–478 CrossRef CAS PubMed.
- N. Zadbuke, S. Shahi, B. Gulecha, A. Padalkar and M. Thube, J. Pharm. BioAllied Sci., 2013, 5, 98–110 CrossRef PubMed.
- A. R. Mladenović, V. M. Jovanović, S. D. Petrović, D. Mijin, S. Ž. Drmanić and M. Avramov Ivić, J. Serb. Chem. Soc., 2013, 78, 2131–2140 CrossRef.
- S. Dermiş and E. Aydoğan, Pharmazie, 2010, 65, 175–181 Search PubMed.
- I. J. Tunna and B. A. Patel, Anal. Methods, 2013, 5, 2523–2528 RSC.
- A. T. Ball and B. A. Patel, Electrochim. Acta, 2012, 83, 196–201 CrossRef CAS.
- Z. R. Dizavandi, A. Aliakbar and M. Sheykhan, J. Electroanal. Chem., 2017, 805, 24–31 CrossRef CAS.
- G. Ozcelikay, S. Kurbanoglu, B. Bozal-Palabiyik, B. Uslu and S. A. Ozkan, J. Electroanal. Chem., 2018, 827, 51–57 CrossRef CAS.
- C. Engin, S. Yilmaz, G. Saglikoglu, S. Yagmur and M. Sadikoglu, Int. J. Electrochem. Sci., 2015, 10, 1916–1925 Search PubMed.
- F. Chatraei and H. R. Zare, Anal. Methods, 2012, 4, 2940–2947 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ay01602d |
| This journal is © The Royal Society of Chemistry 2021 |