Open Access Article
Open Access ArticleA novel method to detect hPG80 (human circulating progastrin) in the blood
Monica
Cappellini
a,
Maud
Flaceliere
a,
Veronique
Saywell
a,
Julien
Soule
a,
Emilie
Blanc
a,
Fanny
Belouin
a,
Erika
Ortiz
a,
Lucile
Canterel-Thouennon
a,
Sophie
Poupeau
a,
Sylvia
Tigrett
a,
Bérengère
Vire
a,
Pierre
Liaud
a,
Mélina
Blairvacq
a,
Dominique
Joubert
b and
Alexandre
Prieur
 *b
*b
aEurobiodev, 2040 avenue du Père Soulas, 34000, Montpellier, France
bECS-Progastrin, Chemin de la Meunière 12, 1008, Prilly, Switzerland. E-mail: a.prieur@ecs-progastrin.com
First published on 3rd September 2021
Abstract
hPG80 (human circulating progastrin) is produced and released by cancer cells. We recently reported that hPG80 is detected in the blood of patients with cancers from different origins, suggesting its potential utility for cancer detection. To accurately measure hPG80 in the blood of patients, we developed the DxPG80 test, a sandwich Enzyme-Linked Immunosorbent Assay (ELISA). This test quantifies hPG80 in EDTA plasma samples. The analytical performances of the DxPG80 test were evaluated using standard procedures and guidelines specific to ELISA technology. We showed high specificity for hPG80 with no cross-reactivity with human glycine-extended gastrin (hG17-Gly), human carboxy-amidated gastrin (hG17-NH2) or the CTFP (C-Terminus Flanking Peptide) and no interference with various endogenous or exogenous compounds. The test is linear between 0 and 50 pM hPG80 (native or recombinant). We demonstrated a trueness of measurement, an accuracy and a variability of hPG80 quantification with the DxPG80 test below the 20% relative errors as recommended in the guidelines. The limit of detection of hPG80 and the limit of quantification were calculated as 1 pM and 3.3 pM respectively. In conclusion, these results show the strong analytical performance of the DxPG80 test to measure hPG80 in blood samples.
Introduction
Progastrin is a pro-protein able to generate several peptides upon maturation (Fig. 1A).1,2 The end product is carboxy-amidated gastrin (active gastrin also named hG17-NH2), with known physiological functions such as the regulation of acid secretion or the control of proliferation of the antral mucosa.3 Aside from active gastrin, a number of other peptides have been identified, both in tissue extracts and in the plasma. Under the name of “active gastrins” are hG17-NH2, hG34-NH2 and hG71-NH2 also known as component I.4 If the main maturation pathway generates hG17-NH2 and hG34-NH2, there is a minor pathway that generates the component I. In the main maturation pathway, it is glycine-extended gastrin (hG17-Gly) that is considered as the unique immediate precursor of hG17-NH2, whereas in the minor maturation pathway, component I plays this role.4 The C-terminus flanking peptide (CTFP) has also been detected in the plasma at high concentration.5 And, although hG17-NH2 is the effective functional product of progastrin maturation, other peptides have been attributed various functions, such as for the CTFP that is able to stimulate in vitro cell proliferation and migration.5 | ||
| Fig. 1 . Overview of Gastrin maturation and antibody epitopes. (A) Processing of preprogastrin. Adapted from ref. 33. Numbers in red indicate the processing enzymes: 1 = signal peptidase, 2 = prohormone convertase 1/3, 3 = carboxypeptidase E, 4 = prohormone convertase 2, and 5 = peptidyl-alpha-amidating-monooxygenase. G34 for gastrin-34 and G17 for gastrin-17. (B) Amino acid sequence of progastrin. In blue the epitope sequence used to generate antibodies recognizing N-terminus of hPG80. In red the epitope sequence used to generate antibodies recognizing C-terminus of hPG80. | ||
However, if the complexity of pro-proteins, due to their various maturation products, is well documented in physiology, their involvement in pathology further adds a degree to this complexity. This is true in particular for progastrin.
Indeed, it has been shown in the early 90's in colorectal carcinoma extracts that progastrin maturation is incomplete in tumor tissues.6–8 The unprocessed precursors, hG17-Gly and progastrin, accumulate in the tumor where they can regulate several features of the tumor and intervene on tumorigenesis such as the disruption of cell–cell junctions,9 cell proliferation,10,11 inhibition of apoptosis,12,13 regulation of cancer stem cells,14,15 and angiogenesis.16 But they have first to be released from the tumor cells to exert their functions, which has two major consequences: (1) they can be neutralized by specific antibodies, which has been done for both precursors,15,17 and (2) they are detectable in the plasma. Although we do not want to underestimate the potential role of hG17-Gly, the data accumulated on the role of progastrin during tumorigenesis clearly indicate its dominant role over hG17-Gly. In particular, the level of progastrin in the plasma of colorectal cancer patient is known to be increased unlike that of hG17-Gly.18 And for all the above reasons, we decided to focus on progastrin, that we named hPG80 once secreted to avoid any confusion with progastrin as the physiological precursor of active gastrin.8,19
Our goal was to generate a tool readily workable for physicians. We developed a kit (DxPG80) that detects and quantifies hPG80 in human plasma. In the present study, we describe in details the analytical performance of the DxPG80 test.
Materials and methods
Antibodies
The anti-hPG80 antibodies were generated according to patents WO/2011/045080 and WO/2017/114973 and as described in Prieur et al.15 All antibodies were selected to bind hPG80 but not other products using direct ELISA.15 Specifically, wells were coated with a solution containing one of the following peptides at 50 or 250 ng: hPG80, Keyhole Limpet hemocyanin (KLH), hG17-Gly, hG17-NH2, or the CTFP. Antibodies displayed no reaction to high quantities of KLH which was coupled to the antigenic peptide used to immunize the mice or rabbit. All antibodies displayed high specificity for binding to full length hPG80 as compared to the gastrin-gene derived peptides hG17-Gly, hG17-NH2, or the CTFP for which the antibodies showed no detectable binding.Capture antibody
Briefly, a sequence residues 55 to 80 of hPG80 coupled to KLH (KLH-Cys-Ahx-Ahx-QGPWLEEEEEAYGWMDFGRRSAEDEN) was used to generate antibodies recognizing C-terminus of hPG80 (Fig. 1B). This antigenic sequence corresponds to the COOH-terminal 26 amino acid residues shown to be sufficient for the growth promoting effect of hPG80.20 We generated 23 murine monoclonal antibodies (mAbs) (SysDiag).15 Using direct ELISA and BIAcore, we showed that these mAbs exhibited high affinities for hPG80, ranging Kd from 10−7 M to 10−12 M. Targeted epitopes were characterized using Alascan and SPOT techniques.15 We then selected the antibody with the highest affinity (i.e. Kd = 6.9 × 10−12 M).15 Antibodies produced by the hybridoma are purified using Akta purifier with a protein A column, eluted with a low pH buffer and dyalised in PBS1X. The capture antibody selected is coated in excess.Detection antibody
Briefly, the N-terminus epitope corresponded to the sequence containing residues 1 to 14 of hPG80 coupled to KLH (SWKPRSQQPDAPLG-Ahx-Cys-KLH). It was used to generate antibodies recognizing the N-terminus of hPG80 (Fig. 1B). Polyclonal anti-hPG80 antibodies (pAbs) were generated by immunizing rabbits, immunopurified by using affinity column coupled to the N-terminus peptide and then eluted with a low pH buffer and dyalised in PBS1X (Eurogentec).Recombinant and native hPG80
Recombinant hPG80 (rhPG80) was produced as described in McQueen et al., with minor modifications.21 Briefly, BL21 DE3 Star bacterial cells (InVitrogen) were transformed with a vector containing the full-length human hPG80 sequence (Fig. 1B) in a PGEX-GST-TEV backbone (GE Healthcare). Bacteria were grown in LB medium containing 0.5 mM IPTG for 3 hours at 37 °C. Bacterial pellets were broken using a French Press, and both soluble and non-soluble fractions were separated by centrifugation. Thereafter, GST-tagged rhPG80 was isolated using a glutathione affinity column and rhPG80 was cleaved from GST with the Tobacco Etch Virus NIa (TEV) protease. Finally, rhPG80 was dialyzed against the final buffer (10 mM Hepes, 0.5% BSA, pH 7.4). rhPG80 was quantified using the absorbance at 280 nm and the specific absorbance calculated for the sequence of hPG80 (2585 mAU at 1 g L−1).To ensure that the DxPG80 test not only recognizes recombinant but also native hPG80 (nhPG80), including O-sulfated and phosphorylated forms,22 we stably overexpressed the GAST gene in HCT-116 cell line (human colon carcinoma cells) and showed that these cells secrete post-translationally-modified hPG80.15 nhPG80 was purified from HCT116-PG culture medium by gel-filtration. We showed that all the antibodies used in the DxPG80 test were able to detect nhPG80 as shown in.15 nhPG80 was quantified using Bradford method and by sandwich ELISA using rhPG80 to prepare the calibration samples (Fig. 2).
Specimen collection and storage
Human whole blood is collected using K2-EDTA or K3-EDTA tubes and centrifuged for 10 minutes at 1300×g at +4 °C using a refrigerated centrifuge to remove the cells and collect the plasma. Following centrifugation, the resulting supernatant is designated as plasma. It is important to immediately and carefully transfer the plasma into a clean polypropylene tube.The plasma should be maintained between +2 and +8 °C if used immediately. If the plasma is not readily analysed, the plasma should be apportioned within maximum 2 hours into aliquots (minimum volume 0.5 mL) and stored at −20 °C (±5 °C) for a maximum of one (1) month, or stored at −80 °C (±10 °C) for long term storage.
Validation range preparation
The quality control sample (CTL) is a spiked sample used to monitor the performance of a bioanalytical method and to assess the integrity and validity of the results of the unknown samples analysed in an individual batch.The calibration standard (CAL) is a matrix to which a known amount of analyte has been added or spiked. Calibration standards are used to construct calibration curves.
CAL and CTL samples are prepared using nhPG80
Depending on the experiment, we used two different sets of CAL and CTL:- Range 0–25 pM: 6 calibrators (CAL 0, 5, 10, 15, 20 and 25 pmol L−1) and 3 external controls (CTL 5, 12.5 and 22.5 pmol L−1).
- Range 0–45 pM: 6 calibrators (CAL 0, 5, 15, 25, 35, 45 pmol L−1) and 3 external controls (CTL 5, 22.5 and 35 pmol L−1).
The CAL and CTL are diluted in hPG80-negative human EDTA plasma. The 1× CAL and 1× CTL were prepared by diluting 120-fold with hPG80-negative human EDTA plasma.
Assay procedure
Samples, CAL and CTL are tested in duplicate. Add 50 μL of Sample Dilution Buffer to all the wells that will be used from the anti-hPG80 antibody pre-coated 96 wells strips microplate included in the kit at room temperature. Transfer 50 μl of the 1× CAL, 1× CTL and samples with a multi-channel pipette (8 channels) to the pre-coated 96 wells strips microplate included in the kit at room temperature. Cover the plate with plastic paraffin film and incubate for 1 hour ± 5 min at +37 °C (±2 °C). At the end of the incubation step, discard all the liquid from the wells by inverting the plate. Proceed to a thorough washing step by adding 300 μL per well of 1× Wash solution using a multi-channel pipette. Discard the 1× Wash solution by inverting the plate and thoroughly pat dry the microtiter plate frame upside down on absorbent paper. Repeat the washing step 6 times. At the end of the washing steps, ensure the complete removal of the liquid from the wells. Add 100 μL of the 1× Conjugate (N-terminus pAb coupled to horse-raddish peroxidase, HRP) to each well using a multi-channel pipette. Cover the plate with plastic paraffin film and incubate 30 ± 3 min at +37 °C (±2 °C). At the end of the incubation step, discard all the liquid from the wells by inverting the plate. Proceed to a thorough washing step by adding 300 μL per well of 1× Wash solution using a multi-channel pipette. Discard the 1× Wash solution by inverting the plate and thoroughly pat dry the microtiter plate frame upside down on absorbent paper. Repeat the washing step 6 times. At the end of the washing steps, ensure the complete removal of the liquid from the wells. Add 100 μL of the substrate solution to each well using a multi-channel pipette. Incubate for 15 min at +37 °C (±2 °C) in the dark. Without removing the content of the wells, add 100 μl of the stop solution to each well using a multi-channel pipette in order to stop the reaction. Read and record the Optical Density (OD) at 450 nm. The OD can be corrected for TMB (3,3′,5,5′-Tetramethylbenzidine) using a second reading at 620 nm.Data analysis
Limits of the acceptance criteria were fixed according to EMEA/CHMP/EWP/192217/2009. The test is accepted when the acceptance criteria for the CAL and CTL have been met as described in Table 1. When at least one criterion is “Rejected”, the test should be performed again. CAL and CTL concentrations were established as described in the section Recombinant and native hPG80.| hPG80 concentration (pmol L−1) | Acceptable range of hPG80 concentration (pmol L−1) | % CV accepted | |
|---|---|---|---|
| CAL | 5 | 3.8–6.3 | 25 |
| 10 | 8–12 | 20 | |
| 15 | 12–18 | ||
| 20 | 16–24 | ||
| 25 | 20–30 | ||
| CTL | 5 | 3.8–6.3 | 25 |
| 12.5 | 10–15 | 20 | |
| 22.5 | 18–27 |
Standard curve calculation
A standard curve is generated for each set of specimens assayed. The mean OD values obtained from each CAL is calculated. The 6 CAL points are reported on a graph, where ≪ y ≫ corresponds to the mean OD and ≪ x ≫ corresponds to hPG80 concentrations in pmol L−1. CAL 0 is used as the anchor point. The linear regression y = ax + b is calculated, where “a” is the slope and “b” is the intercept.Calibrator acceptance criteria
CAL concentrations are calculated using the linear regression: C = (mean ODCAL − b)/a.A CAL is acceptable if the calculated value falls within or equal to the range indicated in the Table 1.
Negative control acceptance criteria
The assay negative control CAL 0 is acceptable if the mean OD (450 nM) falls within or equal to the range 0.12–0.19. This range was obtained by testing multiples hPG80-negative human EDTA plasma.Positive control acceptance criteria
CTL concentrations are calculated using the linear regression equation: C = (mean ODCTL − b)/a.A CTL is acceptable if the calculated value falls within or equal to the range indicated in the Table 1. The plate results are acceptable if all three CTL are accepted.
Calculation of plasma sample hPG80 concentration
Sample hPG80 concentration is calculated using the linear regression equation:Reagents
• Human carboxy-amidated Gastrin (hG17-NH2), (Sigma, G9020).• Human glycine-extended Gastrin (hG17-Gly), (Sigma, SCP0150).
• Human C-ter flanking peptide of gastrin (CTFP), (Auspep, CS).
• Human Recombinant Progastrin (rhPG80), (Institut Pasteur, B60).
• Monoclonal anti-hGastrin antibody (Abcam, ab88282).
• Keyhole limpet hemocyanin (KLH), (Sigma, H7007).
• Carcinoembryonic antigen (CEA), (Lee-BioSolution, 151-11).
• Prostate specific antigen (PSA), (Lee-BioSolution, 497-11).
• Cancer antigen 125 (CA-125), (Lee-BioSolution, 151-25).
• Cancer antigen 15-3 (CA-15.3), (Lee-BioSolution, 151-53).
• Triglycerides (TG), (Sigma, 17811-1AMP).
• Cholesterol, (Sigma, C8667).
• Hemoglobin, (Sigma, H7379).
• Conjugated Bilirubin, (Lee-BioSolution, 910-12).
• SN-38 (7-ethyl-10-hydroxycamptothecin), (Tocris, 2684) is an active metabolite of CPT-11 (irinotecan) that inhibits DNA topoisomerase I (IC50 values are 0.74 and 1.9 μM in P388 and Ehrlich cells respectively). Inhibits DNA and RNA synthesis (IC50 values are 0.077 and 1.3 μM respectively) but does not affect protein synthesis.
• 5-FU (5-fluorouacil), (Sigma, F6627) is an agent that affects pyrimidine synthesis by inhibiting thymidylate synthetase, thus depleting intracellular dTTP pools. It is metabolized to ribonucleotides and deoxyribonucleotides, which can be incorporated into RNA and DNA.
Plasma samples
All cancer samples were from Tissue For Research Ltd (Spectrum Health System, Grand Rapids, Michigan, USA). All patients provided consent for research on their blood samples, in line with international regulations and ICH GCP (International Conference on Harmonization-Good Clinical Practice).Results
(A) Specificity
The following gastric peptides were tested:
• Human carboxy-amidated Gastrin (hG17-NH2).
• Human glycine-extended Gastrin (hG17-Gly).
• Human C-ter flanking peptide of gastrin (CTFP).
• Human Recombinant Progastrin (rhPG80).
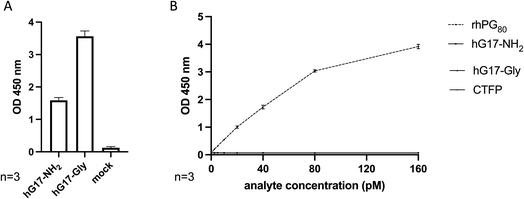
The experiments were conducted using one lot of the DxPG80 test. The concentration range of each analyte was measured on two different DxPG80 test plates. Every point was measured on 4 replicates/plate. To validate hG17-NH2 and hG17-Gly peptides, we performed a direct ELISA with an antibody that recognize all hG17. As shown Fig. 3A, both peptides are recognized by the anti-hG17 antibody.
The rhPG80 is binding specifically to DxPG80 test, whereas no binding was observed for hG17-NH2, hG17-Gly, and CTFP (Fig. 3B). Based on these results, the experiment was considered valid and specificity was good.
| hPG80 (pmol L−1) | % of recovery | ||||||
|---|---|---|---|---|---|---|---|
| CEA 20 μg mL−1 | PSA 10 mg mL−1 | KLH 2 μg mL−1 | CA-125 2000 U mL−1 | CA15-3 100 U mL−1 | hG17-NH2 2 μg mL−1 | hG17-Gly 2 μg mL−1 | |
| 50 | 100 | 103 | 103 | 106 | 103 | 104 | 106 |
| 12.5 | 98 | 100 | 102 | 104 | 104 | 102 | 105 |
| 3.13 | 94 | 95 | 108 | 101 | 103 | 98 | 101 |
| 0.78 | 101 | 99 | 120 | 102 | 102 | 101 | 102 |
| 0 | 103 | 100 | 112 | 101 | 102 | 90 | 101 |
hG17-NH2, hG17-Gly and KLH cross-reactivity were assessed using a concentration 4 times higher than the concentration used during the non-binding test during antibodies production.
CEA (carcinoembryonic antigen), PSA (prostate specific antigen), CA-125 (cancer antigen 125), and CA15-3 (cancer antigen 15-3) are cancer antigens that are used for the screening or/and follow-up of different cancers.23 Each marker was tested at a concentration considered positive for the diagnosis of cancer. Each potential cross-reactant was prepared using a specific dilution buffer (vehicle).
Fixed concentrations of each potential cross-reactant and of its vehicle (as a control) were tested using the CAL panel. Each condition was tested in triplicates. The percentage of recovery was calculated for every potential cross-reactant by using as a control the vehicle used for the preparation of its stock solution.
There is no cross-reactivity when variation in the percentage of recovery is equal or does not exceed 20%, and there is no change in the interpretation of the result.
Based on the acceptance criteria, none of the substances tested are to be considered as cross-reactants (Table 2).
| hPG80 (pmol L−1) | % of recovery | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Vehicle | Endogenous | Exogenous chemotherpay | Exogenous anti-coagulant | ||||||||
| DPBS1X | DMSO | CHCL3 | TG 0.05 mg mL−1 | Choloesterol 25 μg mL−1 | Hemoglobin 2 mg mL−1 | Conjugated bilirubin 0.5 μg mL−1 | SN-38 60 μM | 5-FU 3 mM | K2-EDTA 1.8 mg mL−1 | Sodium heparin 17 IU mL−1 | |
| 50 | 105 | 96 | 95 | 91 | 95 | 111 | 96 | 105 | 96 | 119 | 118 |
| 12.5 | 106 | 97 | 96 | 95 | 96 | 111 | 94 | 104 | 99 | 116 | 117 |
| 3.13 | 105 | 97 | 98 | 104 | 98 | 108 | 98 | 102 | 104 | 114 | 120 |
| 0.78 | 104 | 101 | 98 | 98 | 98 | 103 | 96 | 94 | 112 | 104 | 117 |
| 0 | 104 | 108 | 100 | 111 | 100 | 101 | 101 | 105 | 100 | 112 | 113 |
Fixed concentrations of each potential interfering factor and of its vehicle (as a control) were tested using the CAL panel. Each condition was tested in triplicates.
The percentage of recovery was calculated for every potential interfering factor using as a control the vehicle used for the preparation of its stock solution.
There is no interference when variation in the percentage of recovery is equal or does not exceed 20%, and there is no change in the interpretation of the result.
Based on the acceptance criteria, none of the eight substances tested showed interference according to our acceptance criteria (Table 3).
(B) Measuring range of assay
As shown in Fig. 4A, DxPG80 test is linear between nhPG80 concentrations of 0 to 50 pM in human EDTA plasma.
Hook effect was tested using nhPG80 that was produced by the HCT116-PG cell line and diluted in hPG80-negative human EDTA plasma. The concentrations of the nhPG80 were ranging from 0 to 250 pM. The experiments were conducted using one lot of DxPG80 test.
As shown in Fig. 4B, when testing DxPG80 test with concentrations of nhPG80 ranging from 0 to 250 pM, the signal begins to reach a plateau at a concentration above 60 pM.
Based on the data available we can conclude that no hook effect was observed with DxPG80 test when measuring nhPG80 ranging from 0 to 50 pM.
(C) Accuracy of measurement
The experiments were conducted using two lots of kit.
- Titration of CTLs with nhPG80 as calibrators.
In this first experiment, we titrated three controls (CTL 2.5, 12.5 and 22.5 pmol L−1) and we compared between the two different lots of DxPG80 test, using nhPG80 as calibrators (CAL 0; 1; 2.5; 5; 10; 15; 20 and 25 pmol L−1).
CAL on the 2 lots of DxPG80 test are shown in Fig. 5. As shown in Table 4, when we compare titration of the 3 controls (CTL) between the 2 lots of DxPG80 test, we can notice that nhPG80 relative errors are under 20% as recommended.
| CTL | OD1 | OD2 | Mean | SD | % CV | Calculated concentration (pmol L−1) | R-Bias nhPG80 lot 1 vs. lot 2 | |
|---|---|---|---|---|---|---|---|---|
| lot 1 | CTL 2.5 | 0.24 | 0.24 | 0.24 | 0.00 | 0.00 | 2.4 | 13.0 |
| CTL 12.5 | 0.68 | 0.73 | 0.70 | 0.04 | 5.54 | 13.1 | 0.7 | |
| CTL 22.5 | 1.16 | 1.15 | 1.15 | 0.01 | 0.74 | 23.6 | 6.4 | |
| lot 2 | CTL 2.5 | 0.32 | 0.31 | 0.32 | 0.00 | 1.57 | 2.7 | |
| CTL 12.5 | 0.89 | 0.86 | 0.87 | 0.02 | 2.52 | 13.1 | ||
| CTL 22.5 | 1.35 | 1.38 | 1.36 | 0.01 | 0.99 | 25.7 |
- Patients samples titration with nhPG80 as calibrators.
In this second experiment, 21 patient plasmas were titrated and compared between the two lots of DxPG80 test, using nhPG80 as calibrators (CAL 0; 2.5; 1; 5; 10; 15; 20 and 25 pmol L−1).
When we compare titration results obtained for 21 patient samples between 2 lots of DxPG80 test, we can notice that nhPG80 relative errors are under 20% for 19 of the 21 samples (Table 5).
| Sample ID | Lot 1 | Lot 2 | R-Bias nPG lot 0001 vs lot 0002 | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OD | nPG (pmol L−1) | OD | nPG (pmol L−1) | ||||||||||||||
| OD1 | OD2 | Mean | % CV | Conc DO1 | Conc DO2 | Mean Conc | % CV | OD1 | OD2 | Mean | % CV | Conc DO1 | Conc DO2 | Mean Conc | % CV | ||
| Sample 1 | 0.29 | 0.307 | 0.30 | 4.03 | 2.74 | 3.16 | 2.95 | 10.04 | 0.29 | 0.29 | 0.29 | 0.25 | 2.72 | 2.70 | 2.71 | 0.51 | 8.99 |
| Sample 2 | 0.307 | 0.307 | 0.31 | 0.00 | 3.16 | 3.16 | 3.16 | 0.00 | 0.31 | 0.32 | 0.31 | 1.13 | 3.19 | 3.29 | 3.24 | 2.15 | 2.41 |
| Sample 3 | 0.137 | 0.137 | 0.14 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.14 | 0.14 | 0.14 | 0.50 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Sample 4 | 0.266 | 0.269 | 0.27 | 0.79 | 2.38 | 2.44 | 2.41 | 1.68 | 0.29 | 0.29 | 0.28 | 2.50 | 2.76 | 2.56 | 2.66 | 5.23 | 9.40 |
| Sample 5 | 0.332 | 0.339 | 0.34 | 1.48 | 3.64 | 3.77 | 3.70 | 2.54 | 0.33 | 0.33 | 0.33 | 0.64 | 3.56 | 3.62 | 3.59 | 1.16 | 3.08 |
| Sample 6 | 0.275 | 0.287 | 0.28 | 3.02 | 2.55 | 2.78 | 2.67 | 6.06 | 0.31 | 0.32 | 0.31 | 1.58 | 3.17 | 3.31 | 3.24 | 3.01 | 17.70 |
| Sample 7 | 0.314 | 0.332 | 0.32 | 3.94 | 3.29 | 3.64 | 3.47 | 6.99 | 0.37 | 0.37 | 0.37 | 0.96 | 4.27 | 4.37 | 4.32 | 1.61 | 19.79 |
| Sample 8 | 0.337 | 0.318 | 0.33 | 4.10 | 3.73 | 3.37 | 3.55 | 7.20 | 0.33 | 0.35 | 0.34 | 4.63 | 3.48 | 3.92 | 3.70 | 8.27 | 4.05 |
| Sample 9 | 0.391 | 0.399 | 0.40 | 1.43 | 4.76 | 4.91 | 4.84 | 2.23 | 0.40 | 0.41 | 0.40 | 3.15 | 4.86 | 5.21 | 5.03 | 4.97 | 4.01 |
| Sample 10 | 0.315 | 0.328 | 0.32 | 2.86 | 3.31 | 3.56 | 3.44 | 5.09 | 0.37 | 0.38 | 0.37 | 2.47 | 4.29 | 4.55 | 4.42 | 4.09 | 22.22 |
| Sample 11 | 0.354 | 0.348 | 0.35 | 1.21 | 4.76 | 3.94 | 4.35 | 2.02 | 0.38 | 0.38 | 0.38 | 0.37 | 4.51 | 4.55 | 4.53 | 0.61 | 11.67 |
| Sample 12 | 0.71 | 0.692 | 0.70 | 1.82 | 10.83 | 10.49 | 10.66 | 2.27 | 0.73 | 0.71 | 0.72 | 2.35 | 11.51 | 11.04 | 11.27 | 2.96 | 5.40 |
| Sample 13 | 0.495 | 0.486 | 0.49 | 1.30 | 6.74 | 6.57 | 6.65 | 1.82 | 0.55 | 0.53 | 0.54 | 2.73 | 7.99 | 7.57 | 7.78 | 3.75 | 14.48 |
| Sample 14 | 0.524 | 0.543 | 0.53 | 2.52 | 7.29 | 7.65 | 7.47 | 3.42 | 0.57 | 0.60 | 0.59 | 3.14 | 8.34 | 8.85 | 8.60 | 4.21 | 13.07 |
| Sample 15 | 0.481 | 0.465 | 0.47 | 2.39 | 6.47 | 6.17 | 6.32 | 3.41 | 0.48 | 0.50 | 0.49 | 2.92 | 6.43 | 6.83 | 6.63 | 4.19 | 4.68 |
| Sample 16 | 0.304 | 0.299 | 0.30 | 1.17 | 3.1 | 3.01 | 3.06 | 2.20 | 0.31 | 0.32 | 0.32 | 1.56 | 3.25 | 3.37 | 3.32 | 2.93 | 7.88 |
| Sample 17 | 0.651 | 0.674 | 0.66 | 2.45 | 9.71 | 10.15 | 9.93 | 3.12 | 0.69 | 0.70 | 0.70 | 1.42 | 10.66 | 10.94 | 10.80 | 1.80 | 8.06 |
| Sample 18 | 0.339 | 0.356 | 0.35 | 3.46 | 3.77 | 4.09 | 3.93 | 5.82 | 0.49 | 0.46 | 0.47 | 4.48 | 6.71 | 6.12 | 6.41 | 6.50 | 38.70 |
| Sample 19 | 0.605 | 0.599 | 0.60 | 0.70 | 8.83 | 8.72 | 8.78 | 0.92 | 0.66 | 0.66 | 0.66 | 0.00 | 10.15 | 10.15 | 10.15 | 0.00 | 13.53 |
| Sample 20 | 1.163 | 1.202 | 1.18 | 2.33 | 19.46 | 20.20 | 19.83 | 2.65 | 1.22 | 1.28 | 1.25 | 3.17 | 21.08 | 22.18 | 21.63 | 3.60 | 8.34 |
| Sample 21 | 0.464 | 0.445 | 0.45 | 2.96 | 6.15 | 5.79 | 5.97 | 4.29 | 0.52 | 0.52 | 0.52 | 0.55 | 7.22 | 7.30 | 7.26 | 0.77 | 17.78 |
In conclusion nhPG80 can be used as analyte in calibrators for titration of controls or patients' samples.
| % = (mean measured [nhPG80] − nominal [nhPG80])/nominal [nhPG80] × 100 |
The experiments were conducted using two lots of DxPG80kit.
Accuracy results for hPG80 titration in controls using DxPG80 test are shown in Table 6.
| CTL (pmol L−1) | hPG80 Measured Mean (pmol L−1) | % relative error |
|---|---|---|
| 5 | 4.5 | −9.5 |
| 12.5 | 11.3 | −9.3 |
| 22.5 | 22.2 | −1.3 |
The Accuracy of the three controls 5, 12.5 and 22.5 pM is considered acceptable as each relative error is ≤20% (and 25% for LLoQ, lower limit of quantification). With DxPG80 test, the relative error is below 10% therefore in the acceptable range of the guideline.
The % CV obtained on DxPG80 test are shown in Table 7.
| Control panel | % CV | |
|---|---|---|
| Within-run precision | CTL 5 | 8.8 |
| CTL 12.5 | 6.0 | |
| CTL 22.5 | 6.6 | |
| Inter-run precision | CTL 5 | 3.1 |
| CTL 12.5 | 8.9 | |
| CTL 22.5 | 6.9 | |
| Inter-operator precision | CTL 2.5 | 4.0 |
| CTL 12.5 | 5.4 | |
| CTL 22.5 | 4.8 |
The mean within-run variability ranges from 6.0 to 8.8% and is hence found acceptable.
The inter-run variability was evaluated on two lots of DxPG80 kit, using the CAL panel.
For the inter-run variability, a total mean concentration was calculated for each CTL using mean concentrations from all the experiments used for the study. The inter-run variability is considered as acceptable when ≤20% (and 25% for low nhPG80 concentrations).
The % CV obtained on DxPG80 test are shown in Table 7.
The mean inter-run variability ranges from 3.1 to 8.9% and is hence found acceptable.
The inter-operator % CV was calculated over sixteen experiments performed by four different operators, on one lot of DxPG80 test. Each operator measured the nhPG80 from:
- n = 2 (duplicates) of all calibrators.
- n = 16 (replicates) of three controls (CTL 2.5, CTL 12.5 and CTL 22.5 pmol L−1).
Mean hPG80 concentrations were calculated for each CTL sample per plate. The inter-operator variability is considered acceptable when ≤20% (and 25% for low nhPG80 concentration).
The % CV obtained on DxPG80 test are shown in Table 7.
The mean inter-operator variability ranges from 4.0 to 5.4% and is hence found acceptable.
• LoD = 3 × SD.
• LLoQ = 10 × SD.
The experiments were conducted using three lots of DxPG80 test.
hPG80 concentrations in pmol L−1 were calculated using the standard curve equation of the nhPG80 calibrators prepared in hPG80-negative human EDTA plasmas.
The analytical sensitivity obtained for the DxPG80 is a LoD of 1 pM and a LLoQ of 3.3 pM. Of note, the calculation is slightly different from the LoD and LLoQ described in24,25 to follow the exact guideline EMEA/CHMP/EWP/192217/2009.
The experiments were conducted using three lots of DxPG80 test.
The Total errors obtained for the DxPG80 kit are shown in Table 8.
| CTL panel | % precision | % accuracy | % total error |
|---|---|---|---|
| CTL 5 | 3.1 | 9.5 | 12.6 |
| CTL 12.5 | 8.9 | 9.3 | 18.2 |
| CTL 22.5 | 6.9 | 1.3 | 8.2 |
The total error ranges from 8.2 to 18.2% and is hence found acceptable.
Discussion
Before discussing the technology we developed to detect hPG80 in the plasma, it is important to describe how progastrin and its processed peptides were first detected. Several laboratories played prominent roles over the years. They all generated antibodies able to recognize active gastrins, hG17-Gly or unmaturated progastrin. This allowed to switch from chromatography to radio-immunoassays and to ELISA technologies.7,26–28 Interestingly, Rehfeld has developed a technology able to quantify total peptide gene expression: the PIA for “Processing Independent Assay”, based on the detection of a sequence of 10 amino acid residues of the precursor protein that is neither modified nor cleaved during cellular processing.29,30 An antibody is raised against this sequence, and after trypsin digestion of the sample to be assayed, a radioimmunoassay is performed. However, this technology has some limitations, in particular in terms of analytical variance and labor-intensiveness of the measurements. The variability in clearance of the different precursors and bioactive end-products is also an issue.However, due to the fact that hPG80 is now recognized as a new cancer target, it was important to develop a test that could detect hPG80 in the blood with 100% specificity. We thus choose to develop a sandwich ELISA, that fulfills this criteria.
The challenge was to generate antibodies that were able to detect hPG80 and not active gastrins, hG17-Gly or the CTFP. The capture antibody is a monoclonal antibody generated against the C-Terminus of hPG80 and the detection antibody is a polyclonal antibody generated against the N-terminus.
This sandwich ELISA test thus ensures a high specific recognition of hPG80. It has a good sensitivity (LoD = 1 pM), with a linearity from 0 to 50 pM. It recognizes recombinant hPG80 and native hPG80, which is very important as hPG80 bears postmaturation modifications that may have induced differences in the recognition of hPG80 present in human.31,32 The DxPG80 test fulfills the EMEA/CHMP/EWP/192217/2009 guidelines for method validation. It is CE IVD marked and can therefore be used in the clinical environment by professionals.
The DxPG80 kit has been used to detect hPG80 in a number of cancer patients and in different situations. Before the development of this kit, only colorectal cancer patients were known to accumulate hPG80 in their blood. Now, we know that 83% of the cancer patients have detectable levels of hPG80 in the blood. Indeed You et al. showed that hPG80 was present in the 11 tumor types tested.25 hPG80 was detected in the blood of patients (n = 1546) at significantly higher concentration than in healthy blood donors (n = 557) with a median hPG80 of 4.88 pM versus 1.05 pM (p <0.0001), respectively. The presence of hPG80 in the blood reflects the variations in the tumor: (1) plasma levels correlate with mRNA expression (lung cancer; Spearman r = 0.8; p = 0.0023); (2) plasma levels significantly decrease upon surgery (peritoneal carcinomatosis decrease from 5.36 pM (before surgery) to 3.00 pM (post surgery), p <0.0001 and upon remission (hepatocellular cancer, decrease from 11.54 pM to 1.99 pM (p < 0.0001); (3) the level of hPG80 at diagnostic is a prognostic factor in metastatic renal cell carcinoma (mRCC) patients: Furthermore, mRCC patients with high hPG80 levels (>4.5 pM) had significantly lower OS (overall survival) compared to patients with low hPG80 levels (<4.5 pM) (12 versus 31.2 months, respectively; p = 0.0031); (4) efficacy of treatments correlates with hPG80 level kinetic variations and recurrence is associated with an increase in hPG80 level (hepatocellular cancer).25 All these data re-inforce the value of hPG80 as a new cancer target and prone to the usefulness of the detection of hPG80 in the blood.
Funding
This research received no external funding.Author contributions
AP, DJ principle investigators. AP, DJ wrote the manuscript. BV and MC provided edits to the first draft in manuscript writing. AP, DJ, MC, MF, VS, JS, EB, FB, EO, LT and PL analysed the data. MC, MF, VS, JS, EB, FB, EO, LT and PL performed the hPG80 assays. SP and ST provided quality management. MB contributed to the recruitment of patients. All authors have read and agreed to the published version of the manuscript.Conflicts of interest
DJ is a co-founder of the company and a senior scientific advisor. AP is a co-founder of the company and the Chief Scientific Officer of the company. All other authors: no conflict of interest.References
- J. F. Rehfeld, Scand. J. Clin. Lab. Invest., 2008, 68, 353–361 CrossRef CAS PubMed.
- A. Varro, S. Voronina and G. J. Dockray, Journal, 1995, 95, 1642–1649 CAS.
- L. R. Johnson, Scand. J. Clin. Lab. Invest., Suppl., 1982, 74, 89–92 CAS.
- J. F. Rehfeld and A. H. Johnsen, Eur. J. Biochem., 1994, 223, 765–773 CrossRef CAS PubMed.
- K. A. Smith, O. Patel, S. Lachal, I. Jennings, B. Kemp, J. Burgess, G. S. Baldwin and A. Shulkes, Journal, 2006, 131, 1463–1474 CAS.
- L. Bardram, Gastroenterology, 1990, 98, 1420–1426 CrossRef CAS.
- G. D. Ciccotosto, A. McLeish, K. J. Hardy and A. Shulkes, Gastroenterology, 1995, 109, 1142–1153 CrossRef CAS.
- W. W. Van Solinge, F. C. Nielsen, L. Friis-Hansen, U. G. Falkmer and J. F. Rehfeld, Gastroenterology, 1993, 104, 1099–1107 CrossRef CAS.
- F. Hollande, D. J. Lee, A. Choquet, S. Roche and G. S. Baldwin, Journal, 2003, 116, 1187–1197 CAS.
- C. Seva, C. J. Dickinson and T. Yamada, Science, 1994, 265, 410–412 CrossRef CAS PubMed.
- P. Singh, A. Owlia, A. Varro, B. Dai, S. Rajaraman and T. Wood, Cancer Res., 1996, 56, 4111–4115 CAS.
- J. Pannequin, N. Delaunay, M. Buchert, F. Surrel, J. F. Bourgaux, J. Ryan, S. Boireau, J. Coelho, A. Pélegrin, P. Singh, A. Shulkes, M. Yim, G. S. Baldwin, C. Pignodel, G. Lambeau, P. Jay, D. Joubert and F. Hollande, Journal, 2007, 133, 1554–1568 CAS.
- H. Wu, A. Owlia and P. Singh, Am. J. Physiol.: Gastrointest. Liver Physiol., 2003, 285, G1097–G1110 CrossRef CAS PubMed.
- J. Giraud, L. M. Failla, J. M. Pascussi, E. L. Lagerqvist, J. Ollier, P. Finetti, F. Bertucci, C. Ya, I. Gasmi, J. F. Bourgaux, M. Prudhomme, T. Mazard, I. Ait-Arsa, L. Houhou, D. Birnbaum, A. Pelegrin, C. Vincent, J. G. Ryall, D. Joubert, J. Pannequin and F. Hollande, Cancer Res., 2016, 76, 3618–3628 CrossRef CAS PubMed.
- A. Prieur, M. Cappellini, G. Habif, M. P. Lefranc, T. Mazard, E. Morency, J. M. Pascussi, M. Flaceliere, N. Cahuzac, B. Vire, B. Dubuc, A. Durochat, P. Liaud, J. Ollier, C. Pfeiffer, S. Poupeau, V. Saywell, C. Planque, E. Assenat, F. Bibeau, J. F. Bourgaux, P. Pujol, A. Sezeur, M. Ychou and D. Joubert, Clin. Cancer Res., 2017, 23, 5267–5280 CrossRef CAS PubMed.
- S. Najib, A. Kowalski-Chauvel, C. Do, S. Roche, E. Cohen-Jonathan-Moyal and C. Seva, Oncogene, 2015, 34, 3120–3130 CrossRef CAS PubMed.
- S. Khajeh, M. R. Tohidkia, A. Aghanejad, T. Mehdipour, F. Fathi and Y. Omidi, Artif. Cells, Nanomed., Biotechnol., 2018, 46, 1082–1090 CrossRef CAS PubMed.
- R. K. Siddheshwar, J. C. Gray and S. B. Kelly, Gut, 2001, 48, 47–52 CrossRef CAS PubMed.
- T. J. Koh and D. Chen, Regul. Pept., 2000, 93, 37–44 CrossRef CAS PubMed.
- P. D. Ottewell, A. Varro, G. J. Dockray, C. M. Kirton, A. J. Watson, T. C. Wang, R. Dimaline and D. M. Pritchard, Am. J. Physiol.: Gastrointest. Liver Physiol., 2005, 288, G541–G549 CrossRef CAS PubMed.
- K. McQueen, S. Kovac, P. K. Ho, K. Rorison, J. Pannequin, G. Neumann, A. Shulkes and G. S. Baldwin, J. Protein Chem., 2002, 21, 465–471 CrossRef CAS PubMed.
- J. R. Bundgaard, J. Vuust and J. F. Rehfeld, J. Biol. Chem., 1997, 272, 21700–21705 CrossRef CAS PubMed.
- S. Holdenrieder, L. Pagliaro, D. Morgenstern and F. Dayyani, BioMed Res. Int., 2016, 2016, 9795269 Search PubMed.
- M. Kohli, W. Tan, B. Vire, P. Liaud, M. Blairvacq, F. Berthier, D. Rouison, G. Garnier, L. Payen, T. Cousin, D. Joubert and A. Prieur, Cancers, 2021, 13 Search PubMed.
- B. You, F. Mercier, E. Assenat, C. Langlois-Jacques, O. Glehen, J. Soule, L. Payen, V. Kepenekian, M. Dupuy, F. Belouin, E. Morency, V. Saywell, M. Flaceliere, P. Elies, P. Liaud, T. Mazard, D. Maucort-Boulch, W. Tan, B. Vire, L. Villeneuve, M. Ychou, M. Kohli, D. Joubert and A. Prieur, EBioMedicine, 2019, 51, 102574 CrossRef PubMed.
- L. Bardram, L. Hilsted and R. JF, Journal, 1990, 1–5 Search PubMed.
- M. L. Kochman, J. DelValle, C. J. Dickinson and C. R. Boland, Biochem. Biophys. Res. Commun., 1992, 189, 1165–1169 CrossRef CAS PubMed.
- J. Nemeth, B. Taylor, S. Pauwels, A. Varro and G. J. Dockray, Gut, 1993, 34, 90–95 CrossRef CAS PubMed.
- J. F. Rehfeld and J. P. Goetze, Peptides, 2021, 135, 170427 CrossRef CAS PubMed.
- N. R. Jorgensen, J. F. Rehfeld, L. Bardram and L. Hilsted, Scand. J. Gastroenterol., 1998, 33, 379–385 CrossRef CAS PubMed.
- A. Varro, H. Desmond, S. Pauwels, H. Gregory, J. Young and G. J. Dockray, Biochem. J., 1988, 256, 951–957 CrossRef CAS PubMed.
- S. J. Brand, J. Klarlund, T. W. Schwartz and J. F. Rehfeld, J. Biol. Chem., 1984, 259, 13246–13252 CrossRef CAS.
- J. Copps, R. F. Murphy and S. Lovas, Protein Pept. Lett., 2009, 16, 1504–1518 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2021 |