 Open Access Article
Open Access ArticlePollen grains as a low-cost, green, alternative sorbent for hydrophilic solid-phase extraction†
Jing
Li
ab,
Hae Yoon
Cho
b,
Sung Won
Kwon
b and
Seul Ji
Lee
 *b
*b
aCollege of Life Science, Shanghai Normal University, Shanghai 220234, China
bCollege of Pharmacy, Seoul National University, Seoul 08826, Republic of Korea. E-mail: dltmfwl2@snu.ac.kr
First published on 26th February 2021
Abstract
Many natural products have demonstrated functionality as novel, green sorbents for organic compounds. However, only limited reports exist on the use of such green materials as solid-phase extraction (SPE) sorbents for select organic acids. In this study, we employed pollen grains as a hydrophilic sorbent and investigated the influence of various extraction parameters using a series of experimental designs. The chemical structure and surface properties of the prepared sorbent were investigated by Fourier-transform infrared spectroscopy and scanning electron microscopy. The Plackett–Burman design was used to experimentally screen for parameters that significantly influenced the extraction performance. Three selected parameters were then statistically optimized by applying a central composite design combined with a response surface methodology. Phenolic acid residues were determined and quantified using high performance liquid chromatography with ultraviolet detection; a mass spectrometric detector in the selected ion monitoring mode was also used for identification. As a practical example, phenolic acids in the soil were successfully separated by the developed pollen sorbent. These results therefore indicate that pollen grains can be considered as a sustainable, green, and safe alternative to bare silica for extraction and separation applications.
Introduction
Green chemistry has attracted increasing attention from researchers worldwide to address various environmental issues. More specifically, chemists and engineers are tasked with designing chemicals, chemical processes, and commercial products to avoid the production of toxic substances and to minimize waste.1 Currently, three common strategies are used to reduce pollution and enhance eco-friendliness; namely, minimizing the consumption of chemical reagents,2 developing and selecting green materials and solvents to replace conventional chemicals,3–5 and establishing new chemical methodologies with low environmental impact.6 Indeed, these strategies have been used to develop many green methods for sustainable manufacturing.In the majority of studies carried out thus far, sample preparation plays an important role in obtaining reliable high-quality data by enriching the analytes and reducing matrix effects in the subsequent steps. Although liquid–liquid extraction is a core technology in this regard, conventional liquid–liquid extraction processes usually utilize solvents that are flammable, volatile, or toxic.7 Thus, since their introduction in 1978, disposable cartridges for solid-phase extraction (SPE) have become popular for a wide variety of matrices, including urine, blood, water, beverages, soil, and animal tissue.8,9 In recent years, significant efforts have been directed toward developing new, advanced sorbents with improved selectivities (i.e., improved clean-up effectiveness) and sorptive capacities (i.e., enhanced pre-concentration factors).10 The most common sorbents include surfactant-modified sorbents,11 mixed-mode polymeric sorbents,12 and molecularly imprinted sorbents,13 all of which have been synthesized using conventional chemicals.
For example, silica gel chemically bonded with various organic compounds has received considerable attention as a sorbent due to its good mechanical and thermal stabilities.14,15 However, silica is mainly obtained by mining and purifying quartz, and the chemicals used in these processes, in addition to the produced silica dust itself, can directly harm humans by causing silicosis, cancer, and an increased risk of tuberculosis.16,17 Another issue is the related, negative environmental impact from the generation and disposal of industrial wastes and emissions,18 which remains challenging to reduce despite stringent government regulations.
As a natural green material, pollen grain has been applied in drug delivery,19 microsphere technologies,20,21 and template materials.22 Its most striking structural feature is its tough outer coating (known as the exine), which is formed by an extremely stable and complex biopolymer called sporopollenin and is highly resistant to chemical attack and high temperatures.23–25 In addition, the apertural furrows and hydroxyl groups present on the pollen surface provide it with a strong adsorption ability, thereby rendering the pollen grain a promising hydrophilic SPE sorbent for miniaturized methodologies in green analytical chemistry. Moreover, the many reactive sites on the pollen surface, including the hydroxyl groups, offer opportunities for surface functionalization to enhance the compatibility between the pollen and the polymer matrix, although at present, no studies have explored this idea.26 Furthermore, pollen grains from different plants exhibit wide variations in size (5–200 μm diameters) and shapes (round, elliptical, and multifaceted), which are advantageous in the context of absorptive materials. The performances of pollen-based sorbents in extraction and separation processes can also be tailored by changing the proportions of water and acids in the activation solvent or eluting solvent. Nevertheless, few studies have reported the use of pollen grains for extraction purposes.
For the purpose of this study, we employed phenolic acids as model analytes because they are one of the most common types of organic acids. Moreover, phenolic acids bear varying numbers of hydroxyl groups on their aromatic ring, making them very polar compounds. Therefore, if the developed, hydrophilic SPE cartridges can successfully extract phenolic acids, this method will likely be effective for most polar molecules. Furthermore, phenolic acids have been found in root exudates, which have been reported to aid in amending micro biomass and cycling organic matter.27–31 In addition, it has been found that varying quantities of the different phenolic acids in the soil evokes specific microbial responses in terms of the biomass, activity, and community composition. This in turn can inhibit seed germination and plant growth.32 These chemicals are also found in significantly higher levels in soils that have been subjected to extensive cropping compared to those that have undergone normal cropping. Importantly, few studies have reported the accurate quantification of organic acids in soil through the use of sensitive, eco-friendly, and low-cost methods.
In this study, the pollen grains predominantly interact with the phenolic acid analytes via hydrogen bonding through their surface hydroxyl groups. Although a few studies have reported the application of pollen grains in SPE,33–36 the optimization of the extraction conditions was incomplete; thus, sorbent parameters that have the strongest influence on the extraction efficiency were not identified. To address these issues regarding hydrophilic interaction liquid chromatography-SPE (HILIC-SPE), we employed the Plackett–Burman factorial design to identify the significant experimental variables affecting the extraction efficiency. Thereafter, the extraction process was optimized using a central composite design (CCD) approach. Finally, the applicability of the proposed method toward phenolic acids in soil was assessed. Compared with conventional sorbents for HILIC, pollen grains are expected to be eco-friendly and inexpensive. Moreover, they offer a large selection of particle sizes (5–200 μm) and shapes for diverse applications, thereby rendering them a promising class of green, absorptive materials for use in analytical chemistry.
Materials and methods
Chemicals and materials
Dried pine pollen was obtained from Han Science Co., Ltd. (Seoul, Korea). Prior to use, it was placed in a Soxhlet extractor and refluxed in methanol for 24 h to remove any pigments and fats. Subsequently, it was washed with ACN/H2O/FA (70/27/3, ACN = acetonitrile, FA = formic acid) to eliminate carbohydrates, before drying at 60 °C for 12 h.For all experiments, analytical, reagent-grade chemicals and solvents were used. Seven of the phenolic acids (gallic acid, 4-hydroxybenzoic acid, vanillic acid, caffeic acid, p-coumaric acid, ferulic acid, and sinapic acid) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Protocatechuic acid, trans-m-coumaric acid, 1-undecanol, and 2-dodecanol were obtained from TCI (Tokyo, Japan). Acetone, water, methanol, and ACN of LC grade were obtained from Duksan Chemical Co. Ltd. (Ansan, Korea). All other reagents were purchased from Sigma-Aldrich unless otherwise noted.
The following commercial cartridges were used for SPE: Oasis HLB (3 mL) from Waters; discovery DSC-18 (3 mL) from Supelco; Strata-X 33u polymeric reversed-phase (3 mL) from Phenomenex; and silica (3 mL), C18 (3 mL), and SAX (3 mL) from Alltech.
Instrumentation and software
Fourier-transform infrared (FT-IR) spectroscopy was performed on a Nicolet iS5 FT-IR spectrometer (Thermo Fisher Scientific, Madison, UK). The microscopic morphology of the pollen grains was characterized using field emission scanning electron microscopy (FESEM) (SUPRA 55VP, Carl Zeiss, Germany).High-performance liquid chromatography (HPLC) analysis was conducted on a Flexar FX-10 system (PerkinElmer, Shelton, CT, USA) with a Flexar FX photodiode array (PDA) (PerkinElmer, USA). The detection wavelengths were 254, 280, and 310 nm. An Agilent poroshell EC-C18 column (2.1 × 150 mm) packed with 2.7 μm core–shell particles was used for chromatographic separation of the nine phenolic acids to improve the resolution with shorter retention times, higher sensitivities, and superior performances.
Design-Expert software (Version 9.0, Stat-Ease Inc., Silicon Valley, CA, USA) was used for experiment design and data analysis.
Sample preparation
Soil samples were collected from Gwanak Mountain in Korea. The dried soil (2 g) (with or without spiking) was weighed, added to methanol/water (80/20, v/v, 6 mL), and sonicated for 20 min. After centrifugation (3500g for 5 min), an aliquot (4 mL) of the supernatant was transferred to a 5 mL Eppendorf tube and evaporated to dryness. Subsequently, the extract was reconstituted in ACN containing 0.1% ammonia (800 μL).A thin polyethylene frit was placed at the bottom of a 3 mL hollow SPE cartridge. The prepared pollen grain sorbent (650 mg) was accurately weighed in the laboratory and packed into the cartridge. The sorbent was then covered by another polyethylene frit and compacted at this thickness to ensure a smooth and flat surface.
The same SPE procedure was followed for all sorbents. Initially, the cartridges were preconditioned with water/ACN (20/80, v/v, 2 mL) and activated by ACN (2 mL). Thereafter, an aliquot (800 μL) of the prepared sample solution was loaded onto the pollen grain SPE cartridge. After washing the cartridge with ACN (1 mL), a mixture of water/ACN/FA (30/65.5/4.5, v/v/v, 3 mL) was used for elution. The eluate was collected in an Eppendorf tube and evaporated to dryness. The residue was dissolved in water (200 μL) and filtered through a 0.2 μm membrane filter (Whatman, Piscataway, NJ, USA) prior to injection.
Analytical conditions
Gradient elution was carried out using a binary mobile phase composed of eluent A (water with 0.1% FA) and eluent B (ACN with 0.1% FA). The linear gradient program was as follows: 0–1 min, 10% B; 1–25 min, 10–15% B; 25–35 min, 15–50% B; 35–36 min, 50–100% B; 36–46 min, 100% B. After each run, the gradient was held at 100% B for 12 min to wash the column, and the column was then equilibrated at the initial conditions for 10 min before subsequent injection. The column temperature was maintained at 30 °C, the flow rate was 0.2 mL min−1, and the injection volume was 3 μL.Experiment design
Plackett–Burman design was used to select the most significant factors that influence the SPE efficiency of the pollen grains. The following eight factors were evaluated: the sorbent amount, the activation solvent volume, the sample volume, the clean-up solvent type, the clean-up solvent volume, the proportion of FA in the eluting solvent, the proportion of H2O in the eluting solvent, and the eluting solvent volume. Each variable was examined at two levels: −1 for the low level and +1 for the high level. Table 1 lists the variables and their corresponding levels that were used in the experimental design for a total of 12 experiments. The aqueous standard solutions were prepared with a concentration of 10 μg mL−1.| Code | Factor (unit) | (−1) value | (+1) value |
|---|---|---|---|
| A | Sorbent amount (mg) | 200 | 600 |
| B | Activation solvent volume (mL) | 2 | 3 |
| C | Sample volume (mL) | 1 | 2 |
| D | Clean-up solvent type | Acetonitrile | Acetone |
| E | Clean-up solvent volume (mL) | 1 | 2 |
| F | Proportion of formic acid in eluting solvent (%) | 1 | 3 |
| G | Proportion of water in eluting solvent (%) | 30 | 40 |
| H | Eluting solvent volume (mL) | 2 | 3 |
To determine the optimal extraction conditions, three factors were performed with six star points each placed at a distance of α from the central point. Twenty experiments were required, including six central points, and they were conducted randomly. The conditions set for each experiment are listed in Table S1, ESI.† Moreover, the profile for the predicted values and the desirability option was used to optimize the extraction process.
Results and discussion
Selection of pollen type and characterization of pollen grain
For the purpose of this study, pine pollen was selected as the adsorbent material based on a number of considerations. Firstly, compared with bee pollen, the pine pollen manually collected from a single type of plant has a higher purity, a more stable composition, and is free from animal hormones. Since pines are typically pollinated by the wind, their pollen grains are small (30–40 μm), light, and abundant. These properties render them a suitable SPE sorbent.The surface structures of the pine pollen grains were visualized using SEM. Prior to surface treatment (Fig. 1a), the apertural furrows and longitudinal folds in the exine were visible. After refluxing in methanol and drying in an oven, the exine was all that remained, and its apertural furrows became more prominent (Fig. 1b). From the SEM images, the pollen grains were found to possess an average diameter of 30–50 μm.
 | ||
| Fig. 1 Scanning electron microscopy (SEM) images of the pollen samples (a) before treatment and (b) after refluxing in methanol. | ||
The diffused reflectance FT-IR spectrum of the pollen grains is shown in Fig. S2.† As indicated, a strong and broad band was visible at 3300 cm−1, which is characteristic of –OH stretching vibrations. Relatively weaker bands were also observed at 2920 and 2851 cm−1, corresponding to the –CH stretches of –CH2. Furthermore, the absorption band at 1028 cm−1 is characteristic of C–O stretching vibrations, revealing the presence of the C–OH functionality (Fig. 2).
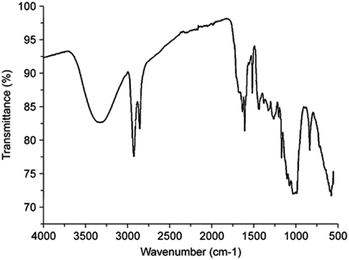 | ||
| Fig. 2 Characterization of the pollen grain surface by diffused reflectance Fourier-transform infrared (FT-IR) spectroscopy. | ||
Optimization of SPE conditions
Analysis of variance was performed to examine whether the studied experimental factors (Table 1) were significant for the performance of the proposed method. These eight factors were assessed at two levels coded as + (high) or − (low). Their effects were calculated based on the peak areas averaged over all the analytes in the chromatograms, and the significance was evaluated using Pareto charts (Fig. 3). The results were found to agree with those from the regression line plotted in the Design Expert program. For an analyte, a “probability > F′′ value < 0.05 indicates that the model and the term are significant. In the studied experimental domain, a positive sign indicates that increasing the variable will enhance the signal (i.e., the average peak areas of all analytes). Based on the obtained results, we further optimized three of the parameters (i.e., the sorbent amount, the sample volume, and the proportion of FA in the eluting solvent) using CCD, while the other five parameters (i.e., the activation solvent volume, the clean-up solvent type, the clean-up solvent volume, the proportion of H2O in the eluting solvent, and the eluting solvent volume) were kept constant.
The five fixed parameters were as follows: activation solvent volume = 2 mL, clean-up solvent type = ACN, clean-up solvent volume = 1 mL, proportion of H2O in the eluting solvent = 30%, and eluting solvent volume = 3 mL. Meanwhile, the other three parameters were varied in the following ranges during optimization using response surface methodology (RSM): sorbent amount = 200–800 mg, sample volume = 0.5–1.5 mL, and proportion of FA in the eluting solvent = 0–6%. The experimental order and the levels of the coded factors and variables are summarized in Table S1 (ESI†).
Three response surfaces were obtained from the CCD results for each phenolic acid. Using gallic acid as a typical example, the response surfaces and the corresponding contours are illustrated in Fig. 4. For each phenolic acid, a different quadratic multiple regression model was obtained for the variables and the response. The main effects, the interaction effects, and the quadratic effects were evaluated through analysis of variance at the 95% confidence level (p < 0.05). Moreover, a lack of fit, which measures the failure of the model to represent data in the experimental domain at points not included in the regression, was also checked. It was shown to be not significant relative to the pure error, indicating a good response to the model.
The models were expressed as second-order polynomial equations for the extraction yield (Y) and the coded factors as follows:
Ygallic acid = 380![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 091.56 + 64 091.56 + 64![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 525.75A − 40 525.75A − 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 654.04B + 11 654.04B + 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 337.28C + 2211.74AB + 22 337.28C + 2211.74AB + 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 655.95AC + 918.94BC − 16 655.95AC + 918.94BC − 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 929.35A2 − 43 929.35A2 − 43![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 634.08B2 + 7398.04C2 634.08B2 + 7398.04C2 |
Yprotocatechuic acid = 606![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 666.28 + 59 666.28 + 59![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 605.73A − 51 605.73A − 51![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 698.77B − 4402.98C + 1139.75AB + 26 698.77B − 4402.98C + 1139.75AB + 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 001.50AC − 10 001.50AC − 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 440.67BC − 35 440.67BC − 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 335.98A2 − 24 335.98A2 − 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 976.85B2 + 5368.52C2 976.85B2 + 5368.52C2 |
Yp-hydroxybenzoic acid = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 007 007![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 717.58 + 157 717.58 + 157![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 121.02A − 63 121.02A − 63![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 076.98B − 2695.93C + 23 076.98B − 2695.93C + 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 088.95AB + 53 088.95AB + 53![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 638.62AC − 19 638.62AC − 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 310.92BC − 16 310.92BC − 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 006.68A2 − 35 006.68A2 − 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 840.14B2 + 10 840.14B2 + 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 175.77C2 175.77C2 |
Yvanillic acid = 977![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 242.30 + 178 242.30 + 178![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 070.52A − 76 070.52A − 76![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 135.98B − 3099.81C + 30 135.98B − 3099.81C + 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 851.73AB + 59 851.73AB + 59![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 220.43AC − 10 220.43AC − 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 331.27BC − 12 331.27BC − 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 792.69A2 − 43 792.69A2 − 43![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 357.67B2 + 4657.43C2 357.67B2 + 4657.43C2 |
Ycaffeic acid = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 233 233![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 839.71 + 181 839.71 + 181![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 717.42A − 101 717.42A − 101![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 074.52B − 4372.62C + 3883.58AB + 38 074.52B − 4372.62C + 3883.58AB + 38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 878.19AC − 10 878.19AC − 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 942.45BC − 24 942.45BC − 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 389.09A2 − 43 389.09A2 − 43![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 607.82B2 + 22 607.82B2 + 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 610.24C2 610.24C2 |
Yp-coumaric acid = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 365 365![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 708.05 + 225 708.05 + 225![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 910.08A − 96 910.08A − 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 985.91B − 9030.79C + 36 985.91B − 9030.79C + 36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 243.68AB + 83 243.68AB + 83![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 802.62AC − 22 802.62AC − 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 242.40BC − 21 242.40BC − 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 850.95A2 − 48 850.95A2 − 48![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 774.70B2 + 15 774.70B2 + 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 344.42C2 344.42C2 |
Yferulic acid = 631![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 896.49 + 130 896.49 + 130![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 758.41A − 66 758.41A − 66![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 808.50B − 6093.34C + 18 808.50B − 6093.34C + 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 001.46AB + 52 001.46AB + 52![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 547.24AC − 13 547.24AC − 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 207.67BC + 739.12A2 − 22 207.67BC + 739.12A2 − 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 696.49B2 + 5359.11C2 696.49B2 + 5359.11C2 |
Ysinapic acid = 409![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100.81 + 105 100.81 + 105![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 781.70A − 53 781.70A − 53![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200.19B − 3032.62C + 3029.14AB + 37 200.19B − 3032.62C + 3029.14AB + 37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 891.51AC − 11 891.51AC − 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 450.06BC + 6776.22A2 − 19 450.06BC + 6776.22A2 − 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 446.44B2 + 2008.37C2 446.44B2 + 2008.37C2 |
Ym-coumaric acid = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 042 042![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 958.96 + 289 958.96 + 289![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 489.47A − 114 489.47A − 114![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 668.52B − 12 668.52B − 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 387.15C + 37 387.15C + 37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 388.06AB + 112 388.06AB + 112![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 152.98AC − 41 152.98AC − 41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 505.65BC − 49 505.65BC − 49![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 878.26A2 − 103 878.26A2 − 103![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 881.44B2 + 16 881.44B2 + 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 435.63C2 435.63C2 |
The regression coefficients (R2) for gallic acid, protocatechuic acid, 4-hydroxybenzoic acid, vanillic acid, caffeic acid, p-coumaric acid, ferulic acid, sinapic acid, and m-coumaric acid were determined to be 0.7376, 0.7535, 0.9201, 0.9507, 0.9313, 0.9174, 0.9327, 0.9240, and 0.9081, respectively, which are in close agreement with the experimental results. Each graph shown in Fig. 4 plots the effect of two variables, while the third variable is maintained constant at the center point. From Fig. 4a, the extraction yield (peak area) is large in the case of abundant pollen grains or when a large amount of pollen grain was used for adsorption. These observations can be explained by the fact that in the absence of pollen grains, the ratio of the sorptive area to the available analytes is low; thus, there is a lower chance for analyte removal. Fig. 4b shows the effect of the pollen grain amount and the proportion of FA in the eluting solvent. As indicated, the response increased upon increasing the FA proportion when the sample volume was fixed, and the amount of pollen grain was ≤650 mg. In HILIC-SPE elution, both the sorbent and the analytes possess positive charges under acidic conditions.38 Therefore, a more acidic solvent facilitates analyte elution. As shown in Fig. 4c, when a smaller amount of sample was introduced, the extraction efficiency increased irrespective of the FA proportion.
Design Expert software was then used to maximize the average response of the target analytes, and the optimized model variables were determined to be as follows: sorbent amount = 650 mg, sample volume = 0.795 mL, and proportion of FA in the eluting solvent = 4.5%. All subsequent experiments were performed using these conditions.
Analytical performance and method validation
Two different detectors were used to evaluate the analytical performance of the described method, namely a diode array detector (DAD) for quantitative analysis. The DAD monitored UV absorption at wavelengths of 254, 280, and 310 nm. Calibration curves were established for the nine phenolic acids in log–log plots of the standard concentrations versus the peak areas. For validation purposes, duplicate assenting experiments were conducted using the optimized conditions.The linear ranges were 10–40 μg mL−1 for gallic acid, 0.4–40 μg mL−1 for protocatechuic acid, 10–40 μg mL−1 for 4-hydroxybenzoic acid, 10–40 μg mL−1 for vanillic acid, 10–40 μg mL−1 for caffeic acid, 0.2–20 μg mL−1 for p-coumaric acid, 0.2–20 μg mL−1 for ferulic acid, 0.4–40 μg mL−1 for sinapic acid, and 0.2–20 μg mL−1 for m-coumaric acid. The method precision was below 11.57% RSD, and the relative recovery was between 77.58% and 118.98% in all concentration ranges (Tables S2 and S3, ESI†).
Comparison with other commercial SPE sorbents
The possible influences of different parameters on the extraction process were examined, and the established optimal conditions for the absolute recovery were used for comparison with other commercial SPE sorbents (i.e., six kinds of SPE cartridges obtained from the market).To isolate the phenolic acids, the C18 column relies on van der Waals forces, hydrogen bonds, or dipole–dipole interactions. Polymeric sorbents are stable over a broader pH range and possess a larger surface area, and are advantageous because of the selective π–π interactions with aromatic-containing analytes. The recoveries of the various phenolic acids achieved using the pollen grain SPE and the commercial SPE sorbents are shown in Fig. 3. As indicated, the pollen grain sorbent was found to be appropriate for the isolation and preconcentration of polar compounds. As an example, gallic acid is more polar than the other studied compounds, and so its recovery was ∼75% for pollen grain but <40% for the C18 sorbent. Although some of the polymeric sorbents displayed higher recoveries than the pollen grain sorbent, the eco-friendliness and low cost of the latter still render pollen grains a promising sorbent (Fig. 5).
SPE cartridge bed capacity
When a solute (sample) is continually applied to a cartridge (sorbent), some of the analytes will eventually appear in the effluent. Such breakthrough occurs once the capacity of the sorbent has been reached. The efficiency of an SPE cartridge is therefore determined by the bed capacity of the extraction sorbent. In other words, the analyte concentration and the sample volume are limited by the sorbent capacity.To measure the bed capacity, aliquots (800 μL) of the nine standard solutions (10–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 μg mL−1) were loaded onto the SPE sorbent (650 mg) conditioned with water/ACN (20/80, v/v, 2 mL) and ACN (2 mL). The cartridge was then washed with ACN (1 mL), and the analytes were eluted using water/ACN/FA (30/65.5/4.5, v/v/v, 3 mL). The eluate was collected and evaporated to dryness under a stream of nitrogen. Finally, the residue was dissolved in methanol (200 μL) and analyzed by HPLC-UV.
000 μg mL−1) were loaded onto the SPE sorbent (650 mg) conditioned with water/ACN (20/80, v/v, 2 mL) and ACN (2 mL). The cartridge was then washed with ACN (1 mL), and the analytes were eluted using water/ACN/FA (30/65.5/4.5, v/v/v, 3 mL). The eluate was collected and evaporated to dryness under a stream of nitrogen. Finally, the residue was dissolved in methanol (200 μL) and analyzed by HPLC-UV.
Recovery is commonly determined as the mass ratio between the analyte in the eluent and that introduced to the sorbent bed. Here, we represent the bed capacity by the absolute recovery at different analyte concentrations (Fig. 6). We observed that the bed capability is related to the molecular polarity of the sorbent, and the recovery is enhanced when the polarity is increased. In addition, we found that the bed capability decreased considerably when the analyte concentration was in the range of 0–1000 μg mL−1, and then remained stable up to 5000 μg mL−1.
 | ||
| Fig. 6 Bed capacity curves for pollen grain sorbents, determined by the absolute recovery and analyte concentration. | ||
Application to environmental samples
The applicability and usefulness of the developed methodology were tested in the extraction and preconcentration of phenolic acids in soil samples. Spiked samples were also obtained using the standard addition method, and the recoveries were used to evaluate the ability of the developed system to determine the organic acids present in complex matrices. Indeed, there was a good correlation between the added and extracted amounts of analytes in these matrices. The recoveries of the added standards exceeded 77.6% in all cases, thereby confirming the accuracy of the procedure and its independence from matrix effects. According to the results presented in Table S4 (ESI†), the pollen-grain-based sorbent successfully extracted phenolic acids from soil samples.Conclusions
Pollen is a natural, non-toxic material consisting of uniform, micron-sized, mesoporous spheres. In this study, we showed that pollen grains can act as an efficient green SPE sorbent for HILIC, and demonstrated its success in extracting organic acids from soil samples. For the first time, the effects of eight extraction parameters were considered together by applying a Plackett–Burman design. Subsequent optimization using CCD combined with response surface morphology further improved the extraction efficiency. Due to the fact that the surfaces of the pollen grains are non-ionic and rich in hydroxyl groups, the adsorption during HILIC was mainly determined by the pH of the eluting solution, with nonpolar compounds generally showing low retentions. Our results therefore suggest that the hydrophilic pollen-grain particles can replace silica as an SPE sorbent in HILIC for pharmaceutical, chemical, and biological applications.Author contributions
Jing Li: conceptualization, methodology, formal analysis, writing – original draft. Hae Yoon Cho: formal analysis, validation. Sung Won Kwon: data curation, project administration, funding acquisition. Seul Ji Lee: writing – review & editing, funding acquisition.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Bio-Synergy Research Project of the Ministry of Science, ICT and Future Planning through the National Research Foundation [NRF-2012M3A9C4048796]; and the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) [NRF-2020R1C1C1006137].References
- I. T. Horvath and P. T. Anastas, Chem. Rev., 2007, 107, 2169 CrossRef CAS.
- S. J. Haswell and P. Watts, Green Chem., 2003, 5, 240 RSC.
- J. W. Comerford, I. D. V. Ingram, M. North and X. Wu, Green Chem., 2015, 17, 1966 RSC.
- P. G. Jessop, Green Chem., 2011, 13, 1391 RSC.
- P. C. Marr and A. C. Marr, Green Chem., 2016, 18, 105 RSC.
- M. W. Nam, J. Zhao, M. S. Lee, J. H. Jeong and J. Lee, Green Chem., 2015, 17, 1718 RSC.
- M. H. Zhu, J. M. Zhao, Y. B. Li, N. Mehio, Y. R. Qi, H. Z. Liu and S. Dai, Green Chem., 2015, 17, 2981 RSC.
- M. C. Hennion, J. Chromatogr. A, 1999, 856, 3 CrossRef CAS.
- F. Augusto, L. W. Hantao, N. G. S. Mogollon and S. C. G. N. Braga, TrAC, Trends Anal. Chem., 2013, 43, 14 CrossRef CAS.
- N. Fontanals, N. Miralles, N. Abdullah, A. Davies, N. Gilart and P. A. G. Cormack, J. Chromatogr. A, 2014, 1343, 55 CrossRef CAS.
- Q. Xie, J. Xie, L. N. Chi, C. J. Li, D. Y. Wu, Z. J. Zhang and H. N. Kong, Sci. China: Technol. Sci., 2013, 56, 1749 CrossRef CAS.
- N. Fontanals, P. A. G. Cormack, R. M. Marce and F. Borrull, TrAC, Trends Anal. Chem., 2010, 29, 765 CrossRef CAS.
- H. H. Yang, W. H. Zhou, X. C. Guo, F. R. Chen, H. Q. Zhao, L. M. Lin and X. R. Wang, Talanta, 2009, 80, 821 CrossRef CAS.
- Y. Wang, S. L. Ji, F. Zhang, F. F. Zhang, B. C. Yang and X. M. Liang, J. Chromatogr. A, 2015, 1403, 32 CrossRef CAS.
- M. Ghaedi, K. Niknam, S. Zamani, H. A. Larki, M. Roosta and M. Soylak, Mater. Sci. Eng., C, 2013, 33, 3180 CrossRef CAS.
- R. A. C. Cohen, A. Patel and F. H. Y. Green, Semin. Respir. Crit. Care Med., 2008, 29, 651 CrossRef.
- T. Kootbodien, S. Iyaloo, K. Wilson, N. Naicker, S. Kgalamono, T. Haman, A. Mathee and D. Rees, Int. J. Environ. Res. Public Health, 2019, 16, 1867 CrossRef.
- F. Golbabaei, A. Gholami, G. Teimori-Boghsani, M. Yaseri and M. Kianmehr, Open Environ. Res. J., 2019, 12, 1–6 CrossRef.
- A. Diego-Taboada, S. T. Beckett, S. L. Atkin and G. Mackenzie, Pharmaceutics, 2014, 6, 80 CrossRef.
- H. S. Lin, M. C. Allen, J. Wu, B. M. deGlee, D. Shin, Y. Cai, K. H. Sandhage, D. D. Deheyn and J. C. Meredith, Chem. Mater., 2015, 27, 7321 CrossRef CAS.
- T. Zhang, Q. R. Li, H. Y. Xiao, Z. Y. Mei, H. X. Lu and Y. M. Zhou, Appl. Clay Sci., 2013, 72, 117 CrossRef CAS.
- W. J. Zhu, H. Huang, W. K. Zhang, X. Y. Tao, Y. P. Gan, Y. Xia, H. Yang and X. Z. Guo, Electrochim. Acta, 2015, 152, 286 CrossRef CAS.
- S. R. Hall, H. Bolger and S. Mann, Chem. Commun., 2003, 2784 RSC.
- S. R. Hall, V. M. Swinerd, F. N. Newby, A. M. Collins and S. Mann, Chem. Mater., 2006, 18, 598 CrossRef CAS.
- M. Jenik, A. Seifner, P. Lieberzeit and F. L. Dickert, Anal. Bioanal. Chem., 2009, 394, 523 CrossRef CAS.
- O. O. Fadiran and J. C. Meredith, J. Mater. Chem. A, 2014, 2, 17031 RSC.
- H. P. Bais, S. W. Park, T. L. Weir, R. M. Callaway and J. M. Vivanco, Trends Plant Sci., 2004, 9, 26 CrossRef CAS.
- D. E. Pataki, D. S. Ellsworth, R. D. Evans, M. Gonzalez-Meler, J. King, S. W. Leavitt, G. H. Lin, R. Matamala, E. Pendall, R. Siegwolf, C. Van Kessel and J. R. Ehleringer, BioScience, 2003, 53, 805 CrossRef.
- A. G. O'Donnell, M. Seasman, A. Macrae, I. Waite and J. T. Davies, Plant Soil, 2001, 232, 135 CrossRef.
- H. Kato-Noguchi and F. A. Macias, J. Plant Physiol., 2005, 162, 1304 CrossRef CAS.
- S. F. Ye, Y. H. Zhou, Y. Sun, L. Y. Zou and J. Q. Yu, Environ. Exp. Bot., 2006, 56, 255 CrossRef CAS.
- X. H. Qu and J. G. Wang, Appl. Soil Ecol., 2008, 39, 172–179 CrossRef.
- Q. Lu, J. H. Wu, Q. W. Yu and Y. Q. Feng, J. Chromatogr. A, 2014, 1367, 39 CrossRef CAS.
- Q. Lu, Q. Zhao, Q. W. Yu and Y. Q. Feng, J. Agric. Food Chem., 2015, 63, 4771 CrossRef CAS.
- Q. Lu, Q. Zhao, Q. W. Yu and Y. Q. Feng, J. Agric. Food Chem., 2015, 63, 4771 CrossRef CAS.
- G. Mafra, M. T. García-Valverde, J. Millán Santiago, E. Carasek, R. Lucena and S. Cárdenas, Separations, 2020, 7, 2 CrossRef CAS.
- B. Buszewski and S. Noga, Anal. Bioanal. Chem., 2012, 402, 231 CrossRef CAS.
- J. X. Wang, S. Kong, J. Y. Yan, G. W. Jin, Z. M. Guo, A. J. Shen, J. Y. Xu, X. L. Zhang, L. J. Zou and X. M. Liang, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2014, 960, 214 CrossRef CAS.
- D. Prat, J. Hayler and A. Wells, Green Chem., 2014, 16, 4546 RSC.
- M. A. Bezerra, R. E. Santelli, E. P. Oliveira, L. S. Villar and L. A. Escaleira, Talanta, 2008, 76, 965 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ay00044f |
| This journal is © The Royal Society of Chemistry 2021 |



