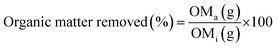Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Developing a systematic method for extraction of microplastics in soils†
Freya
Radford
 *a,
Lina M.
Zapata-Restrepo
b,
Alice A.
Horton
*a,
Lina M.
Zapata-Restrepo
b,
Alice A.
Horton
 c,
Malcolm D.
Hudson
b,
Peter J.
Shaw
b and
Ian D.
Williams
c,
Malcolm D.
Hudson
b,
Peter J.
Shaw
b and
Ian D.
Williams
 a
a
aFaculty of Engineering and Physical Sciences, University of Southampton, Highfield Campus, University Road, Southampton SO17 1BJ, UK. E-mail: f.radford@soton.ac.uk
bFaculty of Environmental and Life Sciences, University of Southampton, Highfield Campus, University Road, Southampton SO17 1BJ, UK
cNational Oceanography Centre, European Way, Southampton, SO14 SZH, UK
First published on 22nd March 2021
Abstract
Microplastics are an environmental issue of global concern. Although they have been found in a range of environments worldwide, their contamination in the terrestrial environment is poorly understood. The lack of standardised methods for their detection and quantification is a major obstacle for determining the risk they pose to soil environments. Here we present a systematic comparison of microplastic extraction methods from soils, taking into account the characteristics of the soil medium to determine the best methods for quantification. The efficiency of organic matter removal using hydrogen peroxide, potassium hydroxide and Fenton's reagent was measured. Soils with a range of particle size distribution and organic matter content were spiked with a variety of microplastic types. Density separation methods using sodium chloride, zinc chloride and canola oil were tested. Recovery efficiencies were calculated and the impact of the reagents on the microplastics was quantified using Attenuated Total Reflectance (ATR) Fourier Transform-Infrared (FTIR) spectroscopy. The optimal organic removal method was found to be hydrogen peroxide. The recovery efficiency of microplastics was variable across polymer types. Overall, canola oil was shown to be the optimal method for density separation, however, efficiency was dependent on the amount of organic matter in the soil. This outcome highlights the importance of including matrix-specific calibration in future studies considering a wide range of microplastic types, to avoid underestimation of microplastic contamination. We show here that methods for extracting microplastics from soils can be simple, cost-effective and widely applicable, which will enable the advancement of microplastic research in terrestrial environments.
Introduction
Microplastics have been found globally in a wide variety of environments.1 However only 3.8% of studies had, until recently, investigated microplastics in terrestrial soils.2 This is despite the close proximity of terrestrial environments to many potential sources, as a large proportion of plastic waste is generated and disposed of on land.3 So far, high concentrations of microplastics have been found in soils.4–6 Scheurer and Bigalke7 found evidence for microplastics in 90% of tested soils, indicating there is a high likelihood of widespread contamination. It is necessary to measure and quantify the amount of microplastics in the terrestrial environment over a wide range of spatial and temporal scales to enable the risk of adverse effects to be determined. However, studies of this sort are currently limited by the lack of suitable methods for quantifying microplastics in soils.There is in general, an absence of standard operating procedures for quantifying microplastics in the environment and this is especially the case for soils, which can be extremely complex matrices.8 Soils are heterogeneous solid mixtures comprised of minerals with a range of particle size distributions and organic matter at varying stages of decomposition.9 The complexity of organo-mineral interactions and the variability of soil media makes the collection of soil microplastic data challenging, although it is recognised as an important emerging issue.10
Initial attempts have been made to quantify microplastics in soil. Density separation methods are some of the most commonly utilised techniques to isolate microplastics from environmental matrices. These methods isolate microplastics using high density saturated salt solutions, such as sodium chloride (NaCl, 1.2 g cm−3), sodium bromide (1.4 g cm−3) and zinc chloride (ZnCl2, 1.7 g cm−3).7,11,12 Lower density solutions tend to be cheaper and less hazardous, but higher density solutions are required to extract more dense polymers such as polyvinyl chloride (PVC, 1.16–1.58 g cm−3) and polyethylene terephthalate (PET, 1.37–1.45 g cm−3).13–15 Alternatively, oil extraction methods have been developed, which use a combination of low density oil and the oleophilic property of plastic to accumulate microplastics in a layer of oil above an aqueous solution.16,17 Additional techniques such as ultrasonication18,19 and centrifugation15 may also be used to enhance these extractions.
While density separation techniques tend to target the inorganic fraction of a sample, organic matter, which has similar density to many types of plastic,9 can obscure the detection of microplastics and interfere with identification.20 To overcome this problem, digestion methods to remove the organic matter have been used. Established soil organic matter digestion techniques involve strong acids;21 however they are not recommended for microplastic studies as they are damaging to some polymers.22 Instead, potassium hydroxide (KOH), hydrogen peroxide (H2O2), and enzymatic treatments may be used with minimal impact on microplastics.23 Enzymatic treatments have been used successfully for less complex environmental samples (e.g. water and marine sediments).24 However, the complex mixture of organic matter in soil is likely to require multiple types of enzymes to fully remove organic material which may become complex and costly.25,26 Chemical digestion methods have a broader specificity and are more likely to target a larger proportion of the soil organic matter.
Hydrogen peroxide is particularly effective at removing organic material in soil13 and can be used in combination with an iron catalyst (Fenton's reagent) to accelerate the reaction. Fenton's reagent has been shown to be particularly effective in sludge and soil samples.6,25,27 Increasing temperature may also enhance organic removal,28 although this should be limited to 50 °C to remain within the heat deflection limits of most common microplastics.23,29
An additional consideration of these methods is the sample matrix characteristics. In soils, this includes chemical and physical properties such as organic matter content, particle size distribution, pH, and bulk density. It is very likely that properties such as these impact the efficiency of extraction methods, similar to the matrix effect seen in analytical chemistry techniques, where the sample matrix characteristics influence quantification and identification of contaminants.30 Some studies have started to incorporate matrix characteristics into microplastic extraction method design, for example, testing the difference between sandy and clay soils.19 It has been suggested that different methods for extracting microplastics from soil should be applied depending on the proportions of clay and organic matter.2 However, most studies lack a matrix characterisation, which is particularly important in soils due to their heterogeneous and variable nature. Method suitability must consider the impacts of reagents on microplastics31 and microplastics recovery efficiency. This is particularly important as some studies report recovery efficiencies of 85–100% (ref. 14) while others are much lower (5 to 75%).32
In this study, the aim was to systematically explore and validate methods for extracting microplastics from soils, taking sample matrix characteristics into consideration. Organic removal efficiency and density separation techniques were tested in soils with a range of organic content and particle size. These techniques were combined to establish the most effective extraction methods for a range of microplastics types. Methods were further validated for use by assessing the physical impact on, and subsequent ease of identification of, microplastics. The outcomes of these trials can inform future studies looking to quantify microplastics in soil, enabling the most suitable method to be chosen based on the sample characteristics.
Methods
Methods of extraction were tested in four stages by measuring (1) the organic matter removal efficiency of selected digestion methods, (2) the extraction efficiency of spiked microplastics with and inorganic soils using density separation techniques, (3) the efficacy of these methods when combined on organic soils and (4) validation by assessing the impact of extraction reagents on microplastic identification.Microplastics spikes
A mixed microplastic standard for spiked recovery was created. Consumer materials were used to create fragments and fibres under 5 mm in size33 of polymers representing the six main resin codes (Table 1).34 Fragments were created using a household coffee grinder and separated into small (0.25–0.5 mm) and large size fractions (0.5–1 mm), and fibres were cut to size (1–5 mm). Polymer type was identified by material labelling and confirmed using Attenuated Total Reflectance Fourier-Transform Infrared spectroscopy (ATR FT-IR) (Frontier, Perkin Elmer) with Spectrum infrared spectroscopy software (Perkin Elmer). Microplastic spikes were chosen with distinct characteristics making identification and separation from contamination sources possible. Each sample tested for recovery efficiency was spiked with five particles of each type of microplastic particle (n = 60) and shaken thoroughly prior to treatment to ensure microplastic distribution.| Resin code | Abbreviation | Shape | Size (mm) | Colour | Original product | Density (g cm−3) |
|---|---|---|---|---|---|---|
| a PET, polyethylene terephthalate; HDPE, high-density polyethylene; PVC, polyvinylchloride; LDPE, low-density polyethylene; PP polypropylene; PS, polystyrene. | ||||||
| 1 | PET | Fragment | 0.5–1 mm | Blue | Drinks bottle | 1.37–1.45 |
| Fibre | 1–5 mm | Green | Craft ribbon | |||
| 2 | HDPE | Fragment | 0.25–0.5 mm | Pink | Cleaning product bottle | 0.93–0.97 |
| 0.5–1 mm | ||||||
| 3 | PVC | Fragment | 0.25–0.5 mm | Red | Table cloth | 1.16–1.58 |
| 0.5–1 mm | ||||||
| 4 | LDPE | Fragment | 0.25–0.5 mm | Purple | Carrier bag | 0.91–0.92 |
| 0.5–1 mm | ||||||
| 5 | PP | Fragment | 0.5–1 mm | White | Storage bottle | 0.9–0.91 |
| Fibre | 1–5 mm | Purple | Carpet | |||
| 6 | PS | Fragment | 0.25–0.5 mm | White | Packaging | 0.015–0.03 |
| 0.5–1 mm | ||||||
Soil materials
Soil materials for testing were specifically created for experimental procedures. The organic fraction of soils was represented by a commercial compost (John Innes Manufacturers Association approved, no. 1 compost, sieved to 2 mm to remove large debris) and the inorganic fraction was a fine sand. The two materials were mixed in varying ratios to form representative soils with specific levels of organic matter content. For soils of purely inorganic content the focus was particle size composition. Particle size was classified according to the Wentworth Scale,35 where clay particles are <4 μm and sand particles are 0.125–2 mm. A clay material (Bentonite, Sibelco) was mixed with a fine sand (85.6% sand) in varying ratios to form six distinct soil types (see ESI, Table S1†). Organic matter content was measured in all samples using loss-on-ignition (LOI) at 550 °C and particle size distribution was analysed using the hydrometer method.36 Unless otherwise stated, soils categorised for organic matter removal experiments as ‘high organic’ had an organic matter content of 73% (±0.6 SE) and ‘low organic’ had 12% (±0.9 SE). For each sample (both organic and inorganic), 10 g of soil was used.Organic matter removal
The initial phase aimed to assess the amount of organic matter that could be removed from soil. Digestion treatments were tested on samples of low and high organic matter content to represent the extremes likely to be found in the environment,37 carried out in glass jars (330 mL capacity) and repeated three times per treatment for each sample type.Fenton's reagent (H2O2, 30% w/v + Fe2+ catalyst, Fisher Scientific), H2O2 (30% w/v, Fisher Scientific) and KOH (10% w/v, Fisher Scientific) were selected for testing based on their reported organic removal efficiency and low impact on microplastics.23,25,38 For H2O2 and KOH treatments, a 50 mL aliquot was added to each sample. Fenton's reagent digestion was carried out using 25 mL of H2O2 with 25 mL of iron catalyst (FeSO4·7H2O, 1 g L−1, Fisher Scientific) adjusted to pH 3 with concentrated sulphuric acid (H2SO4, 95% v/v, Fisher Scientific), an ice bath was used to control the maximum temperature of the reaction to 50 °C until there was no longer a visible reaction. All samples were then placed in a shaking incubator at 100 rpm at 50 °C for 24 hours or until all liquid had evaporated. The samples were then dried at 105 °C overnight, organic content was measured again in triplicate and the quantity of removed organic matter calculated. Each digestion was repeated on separate samples at 40 °C to assess the effect of temperature on digestion efficiency. Digestion treatments were additionally tested in combination with a dispersant, with the aim of dispersing the soil particles prior to digestion maximise organic removal efficiency. In separate samples, 50 mL of sodium hexametaphosphate (Na6P6O18, 1% w/v, Fisher Scientific) was used to soak samples for 24 hours prior to digestion, and each digestion process was then completed on the samples as stated above at 50 °C.
Density separation
Microplastic recovery experiments were conducted using density separation methods to assess efficiency of microplastic separation from inorganic samples only. Each method was tested on six soils with distinct particle size composition in triplicate. Three different density separation media were tested: ZnCl2 (1.7 g cm−3, APC pure), NaCl solution (1.2 g cm−3, food grade), and canola oil (food grade).For ZnCl2 and NaCl trials, 300 mL was added to the sample. The lid was tightly sealed and shaken vigorously by hand for 30 seconds to ensure full contact between the density separation medium and the sample, before leaving to settle overnight to allow dense particles to settle out. The top layer of the sample was then removed using an overflow method,39 where excess ZnCl2 or NaCl was gently added to the jar to spill the top layer of the sample into a surrounding glass crystallising dish and used to rinse the sides and inside of the lid of the jar.
Canola oil separations were conducted based on the method developed by Crichton et al.16 100 mL of distilled water and 5 mL of canola oil was added to each sample and again, shaken for 30 seconds. An additional 200 mL of distilled water was added into the jar to create a further separation between the top layer of canola oil and the bottom of the jar. This solution was then placed in an orbital shaker for 2 hours at room temperature, 100 rpm. The solution was then left to settle overnight, after which the canola oil layer was extracted with the same overflow method using distilled water. The overflowed layer of each treatment, containing microplastics, was then vacuum filtered onto a glass fibre filter (Whatman GF/A, 1.6 μm). The overflow process was completed twice per sample, from initial shaking to vacuum filtering, to achieve maximum extraction efficiency within a reasonable timeframe (see ESI, Fig. S2† for details). All filters were inspected under a low power microscope (Nikon SMZ1000, x40) and recovered microplastics were counted, distinguishable from contamination by their chosen colours (Table 1).
Each protocol with the density separation media was separately run and tested with ultrasound to break up the soils. After each time a sample was shaken it was subjected to 5 minutes of ultrasound in an ultrasonic bath (37 Hz, Fisher Scientific: FB15055). Samples were then overflowed, filtered and analysed as above.
Method combinations
To combine extraction methods, organic removal was included as an additional step prior to density separation in samples with organic matter. Informed by the results of the organic removal efficiencies (§results, organic matter removal), H2O2 at 50 °C was chosen as the optimum digestion method and was used in combination with each of the density separation methods. Each combined method was tested on samples with a range of organic matter content, ranging from 0.2–72% (n = 18 per method).For the digestion, 50 mL of H2O2 was added to each sample and additional 50 mL once not visible reaction occurred to ensure maximum digestion. The samples were then heated and shaken (50 °C, 100 rpm) until all remaining liquid had evaporated. Using the same methods as stated previously (§methods, density separation), ZnCl2, NaCl and canola oil extractions were carried out on samples. Recovery efficiencies were calculated for each sample.
Method reagent impact on the physical and spectroscopic properties of microplastics
To complete the validation, the impact of each method on the plastics was tested. Each type of microplastic particle used as a spike was exposed separately to each treatment involved in the methods in the absence of soil. Spikes subjected to H2O2, KOH and Fenton's reagent were tested at 40 and 50 °C to evaluate the effects of the digestion methods. Each of the microplastic types were added to glass vials containing 1 mL of each of the reagents, then heated to 40 °C or 50 °C for 24 hours and removed for analysis. Similarly, the digestion methods were evaluated by exposing each of the microplastic types to 1 mL of ZnCl2, canola oil, and NaCl in glass vials. The effects of ultrasound were separately measured by adding 1 mL of distilled water to vials containing the microplastics and exposing them to the ultrasound treatment.Virgin and exposed microplastics were analysed using ATR FT-IR with a wavenumber range of 4000–600 cm−1 with spectral resolution of 4 cm−1. A library of virgin microplastics, which were not exposed to any reagents, was created including each of the 12 microplastic types used in the spiking experiments. The spectrum of microplastics exposed to each treatment (n = 3) was compared with the virgin microplastic library and assigned a hit quality index number (HQI, on a scale of 0–1)40 to determine the effect of chosen reagents.
Statistical analysis
Organic matter removal rates were measured by calculating the amount of organic matter remaining in a sample after digestion (OMa) as a percentage of the initial organic matter content (OMi).All statistical analyses were performed in RStudio (1.2.1335) software. Normal distribution of data was checked using Shapiro–Wilk tests and homogeneity of variance was checked using Levene's test. Parametric tests were applied where assumptions of normality and equal variance had been met.
Statistical analysis in the form of Kruskal–Wallis tests (non-parametric), one and two-way analysis of variance (ANOVA, parametric) were used to compare differences between groupings, and pairwise comparisons were made using the post hoc analysis of Dunn's test for non-parametric and Tukey's tests for parametric data. This analysis applied to the amount of organic matter removed from samples with different digestion methods, recovery efficiencies in inorganic and organic samples using the different extraction methods, and differences in identification hit scores for microplastics treated with the different extraction reagents.
Differences between means were tested using a T-test and Wilcoxon rank sum test for the amount of organic matter removed at 40 °C and 50 °C and with initially high and low organic content, recovery efficiencies in inorganic samples with and without the use of ultrasound and H2O2. Correlations were tested using Spearman's Rank to assess relationships between particle size distribution and recovery of plastics in inorganic samples and the relationship between percentage organic matter content and microplastic recovery across all treatments.
Results
Organic matter removal
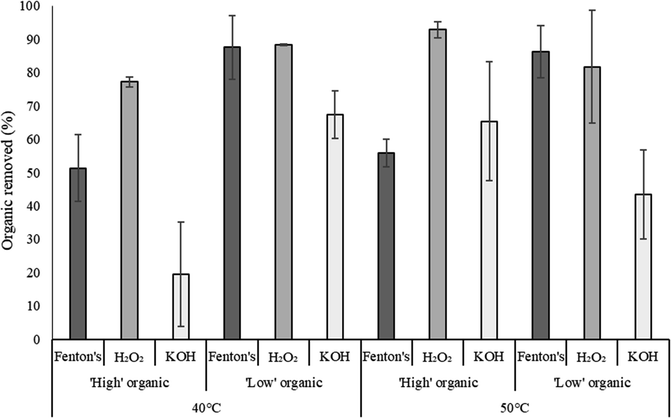
The three organic removal reagents worked at significantly different efficiencies across samples with low and high organic content (p < 0.05, Kruskal Wallis test). For samples with low organic content, removal of organic matter was similar for Fenton's and H2O2 and both these treatments were more effective than KOH (p < 0.05 for both, Dunn's test.). Samples with high organic content did not show a significant difference between the amount of organic matter removed by Fenton's and KOH (Fig. 1), but H2O2 removed more organic matter than both other treatments (p < 0.05 for both, Dunn's test). H2O2 at 50 °C removed 93% organic matter. This was significantly more than Fenton's at 40 and 50 °C, which removed 51% and 56% organic matter, respectively (p < 0.05 for both, Dunn's test). H2O2 at both 40 and 50 °C removed more organic matter than KOH at 40 °C which removed only 20% in samples with high initial organic content (p < 0.01, Dunn's test).Temperature did not affect the efficiency of organic matter removal in samples with initial low or high organic content (p > 0.05 for both, Wilcoxon rank sum). The amount of organic matter removed by H2O2 was significantly reduced by the addition of dispersant (p < 0.001, one-way ANOVA) from 93 to −1.9%. There was no difference between the amounts of organic matter removed by KOH or Fenton's reagent with or without dispersant.
Density separation
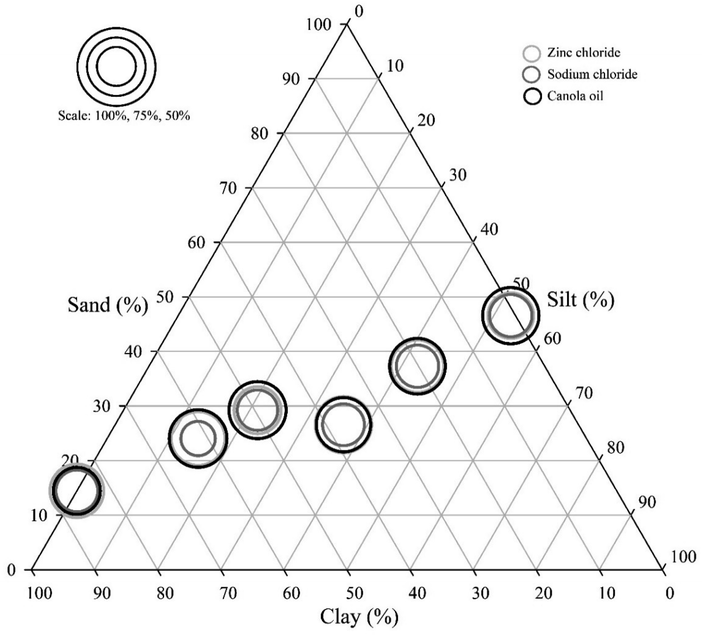
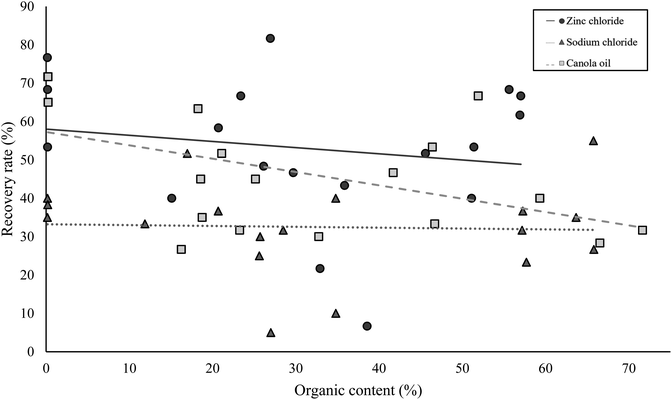
There was no significant correlation between soil particle size composition (i.e. amounts of clay in a sample) and total microplastics recovery efficiency across all methods (rs = 0.04, p > 0.05, Spearman's Rank, Fig. 3). There was no significant difference in microplastic recovery efficiency when combining ultrasound with any of the three methods; ZnCl2, NaCl or canola oil (p > 0.05 for all, t-test). Ultrasound samples were therefore not considered further. Total microplastic recovery efficiencies from inorganic samples were different between the extraction methods (p < 0.01, one-way ANOVA). Mean recovery efficiency was 59% (±1.8 SE) for NaCl, 80% (±1.7 SE) for ZnCl2 and 84% (±2.0) for canola oil extractions (Table 2). Canola oil and ZnCl2 recovered significantly more microplastics than NaCl (p < 0.01 for both, Tukey's test) but there was no difference in total microplastic recovery between canola oil and ZnCl2 extractions.| Microplastic type | Mean recovery efficiency (%) inorganic soils | Mean recovery efficiency (%) organic soils | ||||
|---|---|---|---|---|---|---|
| Sodium chloride | Zinc chloride | Canola oil | Sodium chloride | Zinc chloride | Canola oil | |
| a PET, polyethylene terephthalate; HDPE, high-density polyethylene; PVC, polyvinylchloride; LDPE, low-density polyethylene; PP polypropylene; PS, polystyrene. | ||||||
| PET fibre | 10 (±4.0) | 62 (±12) | 68 (±7.5) | 27 (±5.1) | 59 (±11) | 51 (±9.5) |
| PET fragment | 27 (±11) | 93 (±6.4) | 77 (±12) | 10 (±4.0) | 57 (±7.5) | 21 (±7.5) |
| Large HDPE | 81 (±8.9) | 89 (±7.6) | 86 (±11) | 42 (±6.8) | 46 (±7.6) | 59 (±6.8) |
| Small HDPE | 78 (±9.0) | 90 (±10) | 96 (±3.1) | 34 (±6.4) | 32 (±5.4) | 28 (±6.1) |
| Large PVC | 38 (±12) | 83 (±7.6) | 96 (±2.0) | 11 (±4.6) | 74 (±6.6) | 20 (±6.7) |
| Small PVC | 34 (±13) | 88 (±11) | 99 (±1.6) | 12 (±4.6) | 72 (±8.9) | 54 (±8.1) |
| Large LDPE | 77 (±5.3) | 80 (±14) | 77 (±6.3) | 34 (±7.4) | 50 (±7.6) | 40 (±10) |
| Small LDPE | 97 (±15) | 108 (±16) | 74 (±6.2) | 57 (±7.6) | 54 (±8.5) | 39 (±5.9) |
| PP fibre | 56 (±9.0) | 51 (±11) | 78 (±5.8) | 47 (±6.0) | 57 (±9.5) | 66 (±8.4) |
| PP fragment | 71 (±9.6) | 76 (±14) | 87 (±5.5) | 44 (±8.9) | 51 (±6.9) | 60 (±6.9) |
| Large PS | 72 (±10) | 76 (±8.0) | 84 (±8.0) | 49 (±5.9) | 50 (±8.1) | 64 (±8.3) |
| Small PS | 73 (±12) | 62 (±12) | 84 (±11) | 22 (±3.2) | 33 (±5.6) | 56 (±8.2) |
| Mean recovery | 59 (±1.8) | 80 (±1.7) | 84 (±2.0) | 33 (±2.9) | 53 (±4.4) | 47 (±3.7) |
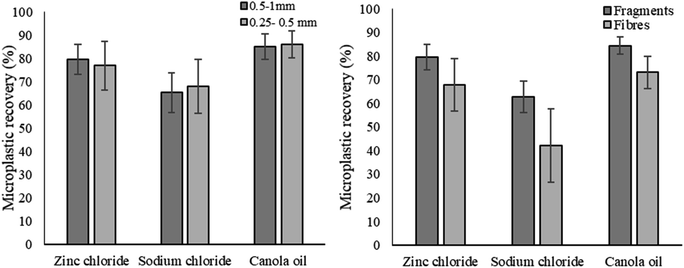
The methods recovered fragments and fibres with different efficiencies (p < 0.05, one-way ANOVA; p < 0.05, Kruskal Wallis) (Fig. 2). ZnCl2 and canola oil recovered more fragments and fibres than NaCl (p < 0.01 for all), but there were no significant differences in the recovery of fragments or fibres between ZnCl2 and canola oil methods. There were differences in recovery of large (0.5–1 mm) and small (0.25–0.5 mm) microplastics with the tested methods (p < 0.05, Kruskal Wallis test; p < 0.05 one way ANOVA). Small microplastics were better recovered with canola oil than NaCl (p < 0.01, Tukey's test) but showed no difference between canola oil and ZnCl2 or ZnCl2 and NaCl. Large microplastics had higher recovery efficiencies with canola oil and ZnCl2 than NaCl (p < 0.01 for both, Dunn's test) but there was no difference between canola oil and ZnCl2 methods.
Different types of microplastics had different recovery efficiencies with each of the methods of extraction (Table 2). PET fibres had the lowest recovery efficiency in both NaCl and oil extractions with a mean of 10% (±4.0 SE) and 68% (±7.5 SE), respectively. PP fibres had the lowest recovery efficiency using ZnCl2 with a mean of 51% (±11 SE). The highest recovery efficiency in both ZnCl2 and NaCl extractions was with small LDPE which had mean recoveries of 108% (±16 SE) and 97% (±14 SE). Recovery efficiencies were highest for small PVC in oil extractions (99% ± 1.6 SE).
Recovery efficiencies of the densest polymers (PET fragments and fibres, and large and small PVC) were higher with ZnCl2 and canola oil than NaCl (p < 0.05, Dunn's test). Large and small HDPE and PS, large LDPE or PP fragments showed similar recovery efficiencies for all three extraction methods.
Method combinations
There was a difference in the total recovery efficiency of microplastics in organic samples when using the three density separation methods (p < 0.01, one-way ANOVA; Fig. 4); 95% confidence intervals for each treatment ranged from 8.6 to 9.0%. In combination with H2O2 as a digestion method, ZnCl2 and canola oil showed similar microplastic recovery efficiencies from organic samples and both recovered more microplastics than NaCl (p < 0.05 for both, Tukey's test). There was a significant correlation between percent organic content and total microplastic recovery efficiency using the canola oil method (r = −0.50, p < 0.05, Spearman's Rank); the higher the organic content, the lower the microplastics recovery. However, this was not the case for NaCl or ZnCl2, which both showed no correlation between percent organic content and total microplastic recovery.Microplastic fragment recovery efficiencies were different across treatments (p < 0.05, one-way ANOVA). Higher recovery efficiencies were seen for fragments using ZnCl2 compared with NaCl (p < 0.01, Tukey's test). Similarly for large microplastics, the recovery efficiencies were significantly different across treatments (p < 0.01, one-way ANOVA). ZnCl2 recovered more large microplastics than NaCl (p < 0.05, Tukey's test) but there was no difference between these treatments and canola oil. Recovery efficiencies for both fibres and small microplastics did not vary between methods across all these samples (p > 0.05 for both, Kruskal Wallis). There was no correlation between fibre recovery efficiency and organic content using any of the treatments or for small microplastics using ZnCl2 or NaCl but there was a significant but weak negative correlation for small microplastics using canola oil (r = −0.48, p < 0.05, Spearman's Rank).
Recovery efficiencies varied for each microplastic type with the different methods of extraction (Table 2). PET fragments had the lowest recovery efficiency in NaCl with a mean of 10% (±4.0). Small HDPE had the lowest recovery efficiencies in ZnCl2 (32%, ±5.4 SE) and large PVC has the lowest recoveries in canola oil (20% ±6.7 SE). Small LDPE showed the highest recovery efficiencies with NaCl (57%, ±7.6 SE), large PVC showed the highest recovered microplastic in ZnCl2 (74%, ±6.6) and PP fibres were the highest in canola oil (66%, ±8.4). There was a strong negative correlation between recovery efficiencies of PET and small LDPE fragments and organic content using the canola oil method; the higher the organic content, the fewer fragments were recovered (rs = −0.69, p < 0.01; rs = −0.55, p < 0.05, Spearman's Rank). There were no correlations between recovery efficiencies of any of the other types of microplastics with organic content or density separation technique. In combination with H2O2 digestion, multiple density separations significantly increased the number of microplastics recovered using canola oil (p < 0.05, two-way ANOVA), but not with ZnCl2 or NaCl.
Method reagent impact on the spectroscopic properties of microplastics
Organic removal treatments had minimal effects on the ease of identification with FT-IR. All microplastics subjected to organic removal treatments had good mean HQI (>0.7, as defined by Renner et al., 2019). The lowest scores were for large PVC treated with KOH at 40 °C and 50 °C, which had HQIs of 0.71, 0.77, respectively. However, there was no overall difference in HQIs for polymers with different chemical treatments or temperatures. It was not possible to measure the HQIs for PET fibres treated with H2O2 at 50 °C or KOH at 40 °C.There were some differences in microplastic identification with the different density separation treatments (p < 0.01, Kruskal Wallis). NaCl and ultrasound treatments both had significantly better identification HQI scores than canola oil (p < 0.01, for both, Dunn's test) and ZnCl2 (p < 0.01, for both, Dunn's test) for overall identification. There were significant differences between the different types of microplastic and the identification across density separation treatments (p < 0.01, Kruskal Wallis). Despite differences between treatments, all polymers subjected to density separation treatments good mean HQI (>0.7, Renner et al., 2019) with the exception of large PVC treated with canola oil, which had a HQI score of 0.49.
Discussion
Standardised methods for quantifying microplastics in the environment, and in particular effective methods for soils, are urgently required. Here we have tested and validated several methods and found differences in the efficiency based on the reagents used and soil characteristics. The first step of testing organic matter digestions indicated that KOH is an unsuitable method for removing organic matter from soils, despite its reported success when used on biological samples.41 Pre-treatment with the dispersant sodium hexametaphosphate was deemed unsuitable as it decreased the efficiency of H2O2 and had no impact on the efficiency of KOH or Fenton's reagent; therefore, it is an unnecessary additional step.H2O2 and Fenton's reagent both resulted in considerable digestion of organic matter (>70%) indicating their suitability for removing organic matter from soils (Fig. 1). This is in line with previous studies20 which also showed that there was minimal difference between organic removal with both treatments in intertidal sediments, although this was also dependant on original organic content. We recommend here that H2O2 is the preferred method as, although both reagents removed similar amounts of organic matter overall, H2O2 removed more from soils with initially high organic content than did Fenton's reagent. Additionally, it is a simpler method to perform, requiring fewer reagents and reduced costs. The reaction can be more easily controlled, as the exothermic reaction of Fenton's reagent requires additional monitoring and control which, if not properly regulated, may result in temperatures >90 °C (ref. 31) leading to the likelihood of polymer damage.29 As there was no difference in the efficiency of reagents at the two temperatures tested (40 °C and 50 °C), we suggest that processing samples at 50 °C may be optimal to speed up processing times, allowing for larger number of samples to be processed without altering the chemical structure of polymers by remaining within the heat deflection limits of most common polymers.29 Additionally, prolonged exposure to 30% H2O2 may cause degradation to some polymer types and therefore should be shortened where possible.42
In general, density separation has been developed for aquatic sediments and different methods have been tested with good recovery rates.43–45 Here we found that density separation methods had differences in extraction efficiencies in both organic and inorganic soils (Table 2). The composition of inorganic soils had no impact on recovery rate for any of the methods tested, similar to previous studies that have shown no difference in recovery efficiency between fine, medium and coarse sediments.16 This suggests that particle size does not need to be adjusted for when applying a density separation method when within these ranges, however it may affect to the time required to effectively process a sample, as it relies on particles settling out in a solution, which according to Stoke's law denotes that the smaller the particle size, the longer it will take to settle.32 This should be considered when calculating density separation processing time as soils with higher clay content may take longer to separate fully.46 We suggest that ultrasound is not required in the density separation step of extraction. Despite its use in previous studies,13,18,19 it did not increase microplastic recovery efficiency in the present study and can therefore be excluded to simplify methods.
Of the tested density separation methods, NaCl had the lowest extraction rates from both organic and inorganic soils. The recoveries of the higher density polymers PET and PVC, which together make up over 17% of the global plastic demand,47 were particularly low. Despite this clear bias towards low-density polymers, it is a method that has been used extensively since it was first tested in 2004, predominantly due to its low cost and limited potential for harm.11,44,48 In soils with purely inorganic content, both oil and ZnCl2 had much higher recovery rates than NaCl and which extended across the different polymer types. Extraction with canola oil recovered the most small microplastics so is preferential for extractions from soils with high inorganic content, as environmental samples tend to be dominated by smaller microplastics.32,49,50 Additionally, ZnCl2 can be more expensive, more hazardous to work with, and more toxic to aquatic biota, whereas oil offers a cheap and relatively safe method.16 It is important to note that in some cases recovery rates exceeded 100%, this may be a result of fragmentation of the microplastic particles during the extraction process, or possibly due to contamination of the initial soil mixtures. Blank media are not usually analysed in spiking studies of this kind, and while the occurrence in the soils of identical microparticles to those we introduced is unlikely, this highlights the importance of including contamination measures and controls when processing environmental samples to minimise the risk of microplastic contamination.51
In soils containing organic matter, extraction efficiencies were generally much lower (Table 2). The organic fraction of soils increases the difficulty of microplastic extraction, even with the addition of a digestion step.9 In this case, canola oil and ZnCl2 showed similar overall recoveries, however the canola oil method was more obviously impacted by the presence of organic matter as extraction efficiency decreased as organic content increased, particularly for PET fragments and small LDPE. This highlights the importance of including a digestion step to reduce this effect, particularly in environmental samples where microplastics are likely to be coated or aggregated with biological material, which may further reduce efficiencies.17 Additionally, it is important to note that, even with high efficiency, there was high variability in microplastic recovery within treatments for organic soils (Table 2), this is likely due to the collection of non-plastic material which may obscure the identification and analysis of microplastic particles,24 again, suggesting the high importance of the digestion step.
We recommend here that canola oil can be used for soils with low organic content, but ZnCl2 is required to obtain sufficient extraction efficiencies in soils with higher organic matter content (>30% (ref. 52)). We anticipate that the canola oil method will be suitable for the large majority of soil types as organic matter rarely exceeds 30%.53 Only soils with high organic content, for example peats,54 will exceed this and require the use of ZnCl2. When ZnCl2 is used it must be carefully considered in terms of hazards to operators and environmental concern, and precautions must be taken to reduce its impact.12,55 Alternatively, salt solutions with densities higher than NaCl but less hazardous than ZnCl2, such as calcium chloride (1.46 g cm−3) may be explored as an intermediate between the two.16 Additionally, the oil extraction method may be further optimised by using alternative types of oil, e.g. castor oil, which may be more efficient at extracting microplastics due to their higher viscosity,17 although this may further reduce suitability for soils with high organic matter content. Furthermore, we recommend that, as simulated soils were used here, these methods may require further adjustment to account for the natural variability in environmental samples as recovery may vary depending on specific soil conditions including the type of organic and minerogenic matter present. We also suggest that the methods recommended here will require tailoring to the required sample size to ensure maximum efficiency, as particularly the canola oil method may require different ratios of reagents for larger sample volumes.56
Little impact was seen on the identification of microplastics treated with the method reagents, with the majority of HQIs above 0.7. This was expected as reagents were chosen for their previously reported low impact on plastic particles.25,31,38 PVC proved to be most susceptible to the tested reagents as it returned the lowest hit scores. This may be due to the characteristically broad C–Cl peak of PVC seen at 690 cm−1 which is at the edge of the spectral range measured (4000–650 cm−1).57 This suggests that consideration of visually matching to reference spectra when identifying PVC with FT-IR is required, and that a more conservative assessment of hit scores may be required to avoid false identification. Additionally, a decrease in HQI scores was seen for some polymers treated with canola oil and ZnCl2. This may be due to the high viscosity of both liquids and hydrophobicity of canola oil, which results in residues remaining on the particles that reduced ease of identification.40 It is therefore important that a cleaning step (e.g. alcohol rinse) to remove these residues should be further considered.16
This study is the first to compare systematically different methods for extraction of microplastics from soils, and highlights the importance of considering sample characteristics when selecting a method for extracting microplastics. Sample-dependent efficiencies should be considered and applied when quantifying microplastics in environmental samples (Table 3), similar to the principle of matrix-matched calibrations used in other areas of analytical chemistry.58
| Reagent | Suitable for use on low organic soils? (<30% organic matter) | Suitable for use on high organic soils? (>30% organic matter) | |
|---|---|---|---|
| a Rinsing with ethanol is required to minimise impacts on identification of microplastics. b To be used only on soils of lower organic content. c To be used with caution to avoid exceeding polymer heat deflection limits. | |||
| Organic removal | Hydrogen peroxide | Yes | Yes |
| Fenton's reagent | Yesc | Yesc | |
| KOH | n/a | No | |
| Density separation | Oil | Yes | Yesa,b |
| Zinc chloride | Yes | Yesa | |
| Sodium chloride | No | No | |
Microplastic recovery efficiency is dependent on the polymer type, shape and size, therefore we suggest that study-specific calibrations using a range of different polymers with different shapes and sizes similar to those used here are employed to account for this variation. It should also be considered that weathered microplastics and those smaller than the size ranges used in this study remain a challenge despite being highly likely to be found in the environment,32 therefore should be considered in future studies, particularly as their respective fragility and large surface area to volume ratios may increase susceptibility to chemical degradation. Additionally, the type of recovery microplastics should be tailored to the type of microplastics considered within a study as, if a study aims to consider smaller plastics, it should use spiking plastics within that size range. Environmental samples tend to show large compositional differences in types of polymer found,7 including different shapes and sizes5,15 making it especially important to establish methods that will account for this and avoid an underestimation of environmental microplastic concentrations.
Conclusions
We recommend that for the majority of common soils, which are likely to have low organic matter content, the preferred method for extracting microplastics from soils involves a digestion step using H2O2 at 50 °C to remove organic matter followed by a canola oil density separation. These methods proposed do not require specialized equipment, are relatively cheap and have reduced complexity to extract microplastics from soils, while minimising environmental impact and hazard to operators. This approach meets the need of the microplastics research community to allow for method harmonisation, however it is clear that method efficiency must be accounted for to prevent underestimation of microplastic concentrations and study-specific calibrations must be employed to enable high accuracy within studies. This will allow for the expansion of future research and a greater understanding of microplastic concentrations in soils.Data availability
Data supporting this study are openly available from the University of Southampton repository at: https://doi.org/10.5258/SOTON/D1455.Author contributions
Freya Radford: Conceptualization, Methodology, Formal analysis, Investigation, Writing – Original draft. Lina M. Zapata-Restrepo: Writing – Review & Editing, Methodology, Investigation. Alice A. Horton: Writing – Review & Editing, Supervision. Malcolm D. Hudson: Conceptualization, Writing – Review & Editing, Supervision, Peter J. Shaw: Conceptualization, Writing – Review & Editing, Supervision. Ian D. Williams: Conceptualization, Writing – Review & Editing, Supervision.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by Engineering and Physical Sciences Research Council funded Centre for Doctoral Training in Sustainable Infrastructure Systems (‘Managing emerging pollutants in waste water systems’, grant number EP/L01582X/1); and Southern Water. We would like to thank Peter Morgan and Dr John James Fielding for their advice and technical support in the laboratory.References
- C. M. Rochman, Science, 2018, 360, 28–29 CrossRef CAS.
- D. He, Y. Luo, S. Lu, M. Liu, Y. Song and L. Lei, TrAC, Trends Anal. Chem., 2018, 109, 163–172 CrossRef CAS.
- J. R. Jambeck, R. Geyer, C. Wilcox, T. R. Siegler, M. Perryman, A. Andrady, R. Narayan and K. L. Law, Science, 2015, 347, 768–771 CrossRef CAS PubMed.
- S. Fuller and A. Gautam, Environ. Sci. Technol., 2016, 50, 5774–5780 CrossRef CAS.
- P. van den Berg, E. Huerta-Lwanga, F. Corradini and V. Geissen, Environ. Pollut., 2020, 261, 114198 CrossRef CAS.
- J. Vollertsen and A. A. Hansen, Danish Environ. Prot. Agency. Environ. Proj., 2017, 1906, 1–55 Search PubMed.
- M. Scheurer and M. Bigalke, Environ. Sci. Technol., 2018, 52, 3591–3598 CrossRef CAS PubMed.
- J. P. da Costa, A. Paço, P. S. M. Santos, A. C. Duarte and T. Rocha-Santos, Environ. Chem., 2018, 16, 18–30 CrossRef.
- M. Bläsing and W. Amelung, Sci. Total Environ., 2018, 612, 422–435 CrossRef PubMed.
- J. N. Möller, M. G. J. Löder and C. Laforsch, Environ. Sci. Technol., 2020, 54, 2078–2090 CrossRef.
- R. C. Thompson, Y. Olsen, R. P. Mitchell, A. Davis, S. J. Rowland, A. W. G. John, D. McGonigle and A. E. Russell, Science, 2004, 304, 838 CrossRef CAS.
- B. Quinn, F. Murphy and C. Ewins, Anal. Methods, 2017, 9, 1491–1498 RSC.
- M. Liu, S. Lu, Y. Song, L. Lei, J. Hu, W. Lv, W. Zhou, C. Cao, H. Shi and X. Yang, Environ. Pollut., 2018, 242, 855–862 CrossRef CAS.
- M. Liu, Y. Song, S. Lu, R. Qiu, J. Hu, X. Li, M. Bigalke, H. Shi and D. He, Sci. Total Environ., 2019, 691, 341–347 CrossRef CAS.
- F. Corradini, P. Meza, R. Eguiluz, F. Casado, E. Huerta-Lwanga and V. Geissen, Sci. Total Environ., 2019, 671, 411–420 CrossRef CAS PubMed.
- E. M. Crichton, M. Noël, E. A. Gies and P. S. Ross, Anal. Methods, 2017, 9, 1419–1428 RSC.
- T. Mani, S. Frehland, A. Kalberer and P. Burkhardt-Holm, Anal. Methods, 2019, 11, 1788–1794 RSC.
- E. H. Lwanga, J. M. Vega, V. K. Quej, J. de los Angeles Chi, L. S. del Cid, C. Chi, G. E. Segura, H. Gertsen, T. Salánki and M. van der Ploeg, Sci. Rep., 2017, 7, 1–7 CrossRef.
- S. Zhang, X. Yang, H. Gertsen, P. Peters, T. Salánki and V. Geissen, Sci. Total Environ., 2018, 616, 1056–1065 CrossRef.
- P. Vermeiren, C. Muñoz and K. Ikejima, Environ. Pollut., 2020, 114298 CrossRef CAS PubMed.
- Z.-Y. Hseu, Bioresour. Technol., 2004, 95, 53–59 CrossRef CAS PubMed.
- A. Karami, A. Golieskardi, C. K. Choo, N. Romano, Y. Bin Ho and B. Salamatinia, Sci. Total Environ., 2017, 578, 485–494 CrossRef CAS.
- T. Hamm, C. Lorenz and S. Piehl, in YOUMARES 8–Oceans Across Boundaries: Learning from each other, Springer, 2018, pp. 179–195 Search PubMed.
- O. Setälä, M. Granberg, M. Hassellöv, T. Karlsson, M. Lehtiniemi, K. Mattsson, J. Strand, J. Talvitie and K. Magnusson, Monitoring of microplastics in the marine environment: Changing directions towards quality controlled tailored solutions, Nordic Council of Ministers, 2019 Search PubMed.
- R. R. Hurley, A. L. Lusher, M. Olsen and L. Nizzetto, Environ. Sci. Technol., 2018, 52, 7409–7417 CrossRef CAS PubMed.
- M. G. J. Löder, H. K. Imhof, M. Ladehoff, L. A. Löschel, C. Lorenz, S. Mintenig, S. Piehl, S. Primpke, I. Schrank and C. Laforsch, Environ. Sci. Technol., 2017, 51, 14283–14292 CrossRef.
- G. S. Zhang and Y. F. Liu, Sci. Total Environ., 2018, 642, 12–20 CrossRef CAS PubMed.
- J. C. Prata, J. P. da Costa, A. C. Duarte and T. Rocha-Santos, TrAC, Trends Anal. Chem., 2019, 110, 150–159 CrossRef CAS.
- Q. Qiu, Z. Tan, J. Wang, J. Peng, M. Li and Z. Zhan, Estuarine, Coastal Shelf Sci., 2016, 176, 102–109 CrossRef CAS.
- W. Zhou, S. Yang and P. G. Wang, Bioanalysis, 2017, 9(23), 1839–1844 CrossRef CAS.
- K. Munno, P. A. Helm, D. A. Jackson, C. Rochman and A. Sims, Environ. Toxicol. Chem., 2018, 37, 91–98 CrossRef CAS PubMed.
- Z. Wang, S. E. Taylor, P. Sharma and M. Flury, PLoS One, 2018, 13, e0208009 CrossRef.
- ISO/TR 21960, ISO/TR 21960:2020(en) Plastics — Environmental aspects — State of knowledge and methodologies Table, 2020.
- ASTM, D7611/D7611M-20, Standard Practice for Coding Plastic Manufactured Articles for Resin Identification, West Conshohocken, PA, 2020 Search PubMed.
- C. K. Wentworth, J. Geol., 1922, 30, 377–392 CrossRef.
- B. H. Sheldrick and C. Wang, Soil Sampling and Methods of Analysis, Lewis Publishers, 1993, pp. 499–511 Search PubMed.
- B. B. K. Huat, A. Asadi and S. Kazemian, Am. J. Eng. Appl. Sci., 2009, 2, 184–188 CrossRef.
- A. S. Tagg, M. Sapp, J. P. Harrison and J. J. Ojeda, Anal. Chem., 2015, 87, 6032–6040 CrossRef CAS.
- A. A. Horton, C. Svendsen, R. J. Williams, D. J. Spurgeon and E. Lahive, Mar. Pollut. Bull., 2017, 114, 218–226 CrossRef CAS.
- G. Renner, A. Nellessen, A. Schwiers, M. Wenzel, T. C. Schmidt and J. Schram, TrAC, Trends Anal. Chem., 2019, 111, 229–238 CrossRef CAS.
- C. J. Thiele, M. D. Hudson and A. E. Russell, Mar. Pollut. Bull., 2019, 142, 384–393 CrossRef CAS.
- M.-T. Nuelle, J. H. Dekiff, D. Remy and E. Fries, Environ. Pollut., 2014, 184, 161–169 CrossRef CAS.
- M. Claessens, L. Van Cauwenberghe, M. B. Vandegehuchte and C. R. Janssen, Mar. Pollut. Bull., 2013, 70, 227–233 CrossRef CAS.
- R. L. Coppock, M. Cole, P. K. Lindeque, A. M. Queirós and T. S. Galloway, Environ. Pollut., 2017, 230, 829–837 CrossRef CAS.
- H. K. Imhof, J. Schmid, R. Niessner, N. P. Ivleva and C. Laforsch, Limnol. Oceanogr.: Methods, 2012, 10, 524–537 CrossRef CAS.
- R. R. Filgueira, L. L. Fournier, C. I. Cerisola, P. Gelati and M. G. García, Geoderma, 2006, 134, 327–334 CrossRef.
- Plastics Europe, Plastics the facts 2019, https://www.plasticseurope.org/application/files/9715/7129/9584/FINAL_web_version_Plastics_the_facts2019_14102019.pdf, accessed 18 May 2020.
- E. Pagter, J. Frias and R. Nash, Mar. Pollut. Bull., 2018, 135, 932–940 CrossRef CAS PubMed.
- M. Haave, C. Lorenz, S. Primpke and G. Gerdts, Mar. Pollut. Bull., 2019, 141, 501–513 CrossRef CAS PubMed.
- Y. Chen, Y. Leng, X. Liu and J. Wang, Environ. Pollut., 2020, 257, 113449 CrossRef CAS PubMed.
- J. C. Prata, V. Reis, J. P. da Costa, C. Mouneyrac, A. C. Duarte and T. Rocha-Santos, J. Hazard. Mater., 2021, 403, 123660 CrossRef CAS PubMed.
- P.-T. Huang, M. Patel, M. C. Santagata and A. Bobet, JTRP Technical Reports, 2009, FHWA/IN/JTRP-2008/02, DOI:10.5703/1288284314328.
- M. M. Pulleman, J. Bouma, E. A. Van Essen and E. W. Meijles, Soil Sci. Soc. Am. J., 2000, 64, 689–693 CrossRef CAS.
- F. Rezanezhad, J. S. Price, W. L. Quinton, B. Lennartz, T. Milojevic and P. Van Cappellen, Chem. Geol., 2016, 429, 75–84 CrossRef CAS.
- M. O. Rodrigues, A. M. M. Gonçalves, F. J. M. Gonçalves and N. Abrantes, MethodsX, 2020, 100785 CrossRef CAS.
- W. Courtene-Jones, B. Quinn, C. Ewins, S. F. Gary and B. E. Narayanaswamy, Mar. Pollut. Bull., 2020, 154, 111092 CrossRef CAS PubMed.
- A. Käppler, D. Fischer, S. Oberbeckmann, G. Schernewski, M. Labrenz, K.-J. Eichhorn and B. Voit, Anal. Bioanal. Chem., 2016, 408, 8377–8391 CrossRef PubMed.
- L. Cuadros-Rodríguez, M. G. Bagur-González, M. Sánchez-Vinas, A. González-Casado and A. M. Gómez-Sáez, J. Chromatogr. A, 2007, 1158, 33–46 CrossRef.
- X. Han, X. Lu and R. D. Vogt, Environ. Pollut., 2019, 254, 113009 CrossRef CAS.
- C. B. Alvim, J. A. Mendoza-Roca and A. Bes-Piá, J. Environ. Manage., 2020, 255, 109739 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ay02086a |
| This journal is © The Royal Society of Chemistry 2021 |