 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Evaluation of extraction methods for pharmacologically active compounds from anticonvulsant traditional Chinese medicines: Gou Teng, Tian Ma, Jiang Can using DART-TOF-MS
Kimberly N.
Karin
 *a,
Justin L.
Poklis
b and
Michelle R.
Peace
*a
*a,
Justin L.
Poklis
b and
Michelle R.
Peace
*a
aDepartment of Forensic Science, Virginia Commonwealth University Richmond, VA, USA. E-mail: mrpeace@vcu.edu
bDepartment of Pharmacology and Toxicology, Virginia Commonwealth University Richmond, VA, USA
First published on 18th January 2021
Abstract
Chinese herbal medicines (CHMs) are classified as dietary supplements. Interactions with western medications, the presence of contaminants or adulterants, or a mis-labeled or mis-used CHM may lead to toxicological emergencies that can be undetected in death investigations. Laboratories must be able to efficiently analyze cases in which CHMs are suspected. Five extractions were evaluated for their ability to extract pharmacologically active compounds from herbal matrices: water, ethanol, microwave-assisted (MAE), ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) chloroform, and acid-wash. Anticonvulsive and other pharmacologically active compounds in Gou Teng, Tian Ma, and Jiang Can purchased from Beijing, China and New York were compared in the powder and the extracts using Direct Analysis in Real Time-Mass Spectrometry (DART-MS). Approximately 0.25 g of macerated herb was used per extraction. The water and ethanol extractions were simple liquid extractions. For the MAE, powdered herb was soaked in 65% ethanol, microwaved, and concentrated. The ethanol
chloroform, and acid-wash. Anticonvulsive and other pharmacologically active compounds in Gou Teng, Tian Ma, and Jiang Can purchased from Beijing, China and New York were compared in the powder and the extracts using Direct Analysis in Real Time-Mass Spectrometry (DART-MS). Approximately 0.25 g of macerated herb was used per extraction. The water and ethanol extractions were simple liquid extractions. For the MAE, powdered herb was soaked in 65% ethanol, microwaved, and concentrated. The ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) chloroform extraction involved soaking in 1
chloroform extraction involved soaking in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ethanol
1 ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) chloroform, sonication, and concentration. In the acid-wash extraction, powdered herb was soaked in acetic acid, followed by addition of sodium hydroxide, hexane extraction, and reconstitution in ethyl acetate. The powdered herbs and extracts were analyzed using a Jeol JMS T100LC AccuTOF DART-MS in positive and negative mode. Of the evaluated methods, no single extraction worked for all active compounds from the three CHMs. The MAE extract contained the most pharmacologically active compounds, while the acid-wash contained the least for the three products. Gou Teng purchased from different sources did exhibit a difference in pharmacologically active compounds, potentially from different species.
chloroform, sonication, and concentration. In the acid-wash extraction, powdered herb was soaked in acetic acid, followed by addition of sodium hydroxide, hexane extraction, and reconstitution in ethyl acetate. The powdered herbs and extracts were analyzed using a Jeol JMS T100LC AccuTOF DART-MS in positive and negative mode. Of the evaluated methods, no single extraction worked for all active compounds from the three CHMs. The MAE extract contained the most pharmacologically active compounds, while the acid-wash contained the least for the three products. Gou Teng purchased from different sources did exhibit a difference in pharmacologically active compounds, potentially from different species.
1. Introduction
Increasing global use of Chinese Herbal Medicines (CHM) is attributed to the availability, relatively low costs, and perceived lower risk of side effects from natural products compared to pharmaceuticals.1 With the approval by the World Health Organization (WHO) of the new version of the International Statistical Classification of Diseases and Related Health Problems (ICD), which includes a chapter on traditional Chinese medicine for the first time, the worldwide usage of traditional Chinese medicine is expected to continue to increase.2 In the United States alone, the sale of herbal dietary supplements exceeded 8 billion dollars in 2017.3 Consumers use herbal products, classified by their main action, for treatment of a wide variety of ailments. Anticonvulsants, such as Gou Teng, Tian Ma, and Jiang Can, are used primarily for the treatment of muscle spasms.4Guo Teng, also known as cat's claw, is the dried stem from Uncaria rhynchophylla, U. macrophylla, U. hissata, U. sessilifructus, or U. sinensis.5 Beside the use of various parts of the plant being used to treat fever, dizziness, and spasms, they are also used as a sedative, analgesic, and antihypertensive.4,5 The main active alkaloids in Guo Teng (Table 1) include corynoxeine, isocorynoxeine, hirsuteine, hirsutine, rhynchophylline, and isorhynchophylline.4
| Compound | Chemical formula | Mass | Compound | Chemical formula | Mass |
|---|---|---|---|---|---|
| Gou Teng | |||||
| Quinolic acid5 | C7H5NO4 | 167.0219 | Corynoxeine 4,5,7 | C22H26N2O4 | 382.1893 |
| Caffeic acid5 | C9H8O4 | 180.0423 | Isocorynoxeine 5,7 | ||
| Catechin5 | C15H14O6 | 290.0790 | Corynoxine B5 | C22H28N2O4 | 384.2049 |
| Epicatechin5,6 | Isorhynchophylline 4–7 | ||||
| Angustidine5 | C19H15N3O | 301.1215 | Rhynchophylline 4,5 | ||
| Angustine5 | C20H15N3O | 313.1215 | Campesterol5 | C28H48O | 400.3705 |
| Angustoline5 | C20H17N3O2 | 331.1321 | Stigmasterol5 | C29H48O | 412.3705 |
| Vallesiachotamine5 | C21H22N2O3 | 350.1630 | β-Sitosterol5 | C29H50O | 414.3862 |
| Akuammigine5 | C21H24N2O3 | 352.1787 | Afzelin6 | C21H20O10 | 432.1056 |
| Tetrahydroalstonine5 | Macrophylline A5 | C25H32N2O5 | 440.2311 | ||
| Hirsuteine 4,5,7 | C22H26N2O3 | 366.1943 | Quercitrin6 | C21H20O11 | 448.1006 |
| Isopteropodine5 | C21H24N2O4 | 368.1736 | Ursolic acid6 | C30H48O3 | 456.3603 |
| Mitraphylline5 | Hyperin4,6 | C21H19O12 | 463.0877 | ||
| Pteropodine5 | Strictosamide5 | C26H30N2O8 | 498.2002 | ||
| Dihydrocorynantheine4,5,7 | C22H28N2O3 | 368.2100 | Vincosamide7 | ||
| Hirsutine 4,5 | 3-α-Dihydrocadambine5,7 | C27H34N2O10 | 546.2213 | ||
| Rutin5 | C27H30O16 | 610.1534 | |||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Tian Ma | |||||
| 4-Hydroxybenzaldehyde8 | C7H6O2 | 122.0368 | Vanillyl alcohol 4 | C8H10O3 | 154.0630 |
| 4-Hydroxybenzyl alcohol8,9 | C7H8O2 | 124.0524 | Vanillic acid8 | C8H8O4 | 168.0423 |
| 4-Hydroxybenzylmethylether10 | C8H10O2 | 138.0681 | Gastrodin 4 | C13H18O7 | 286.1053 |
| Vanillin 4 | C8H8O3 | 152.0473 | |||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Jiang Can | |||||
| Ammonium oxalate 11 | C2H8N2O4 | 124.0484 | Pinoresinol11 | C20H22O6 | 358.1416 |
| D-Mannitol11 | C6H14O6 | 182.0790 | Aurantiamide 11 | C25H26N2O3 | 402.1943 |
| Citric acid11 | C6H8O7 | 192.0270 | β-Sitosterol 11 | C29H50O | 414.3862 |
| Kaempferol11 | C15H10O6 | 286.0477 | Ergost-6,22-dien-3β, 5α, 8α-triol 11 | C28H46O3 | 430.3447 |
| Quercetin11 | C15H10O7 | 302.0427 | Beauvericin 11 | C45H57N3O9 | 783.4095 |
Tian Ma, from the dried tubes of Gastrodia elata Blume, is also known as Gastrodiae Rhizoma, Chi Jian, and Gui Du You9. Tian Ma can be used in the treatment of dizziness, headache, hypertension, and chest pain, as well as neurological disorders such as epilepsy, vertigo, and tetanus.4,8 The active components in Tian Ma (Table 1) include vanillin, vanillyl alcohol, and gastrodin.4
Jiang Can is a natural product composed of silkworm larva (Bombyx mori L.) killed and stiffened through infection by Beauveria bassiana forming a white, ammonium oxalate residue on the surface of the silkworm.11,12 Uses of Jiang Can include treatment of epilepsy, convulsions, cough, asthma, headaches, and postpartum pain. The reported active components in Jiang Can (Table 1) include ammonium oxalate, aurantiamide, beauvericin, ergost-6,22-dien-3β,5α,8α-triol, pinoresinol, and β-sitosterol.4
Although consumers associate lower risks of adverse effects with natural products, CHMs can pose a legitimate risk to consumers. CHMs are classified as dietary supplements which have a low standard for quality as defined by the U.S. Food and Drug Administration (FDA) in the Dietary Supplement Health and Education Act (DSHEA) of 1994.13 Regulatory standards for dietary supplements include that they cannot be unsanitary or toxic, or pose a significant risk to consumers.13 CHMs do not have a standardized naming convention, which can lead to potentially dangerous errors.4 And, potential negative interactions between multiple herbs or between herbs and over-the-counter and/or prescribed pharmaceutical products may not be considered or known when the herbs are consumed.14
Reported adverse drug reactions from herbal product use, whether it be from the use of an herbal product or a combination of herbal products are increasing.15 Annually China's drug regulator receives reports of more than 230![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cases of adverse effects resulting from CHMs.2 Global use of CHMs is expected to continue to rise and with it potentially the occurrences of adverse reactions.2 Toxicological emergencies have been described in the literature, such as the case of a 36 year-old woman consuming an unknown herbal decoction for three days prior to admission to the hospital where she suffered cardiac arrest. Her father purchased the herbs based on the recommendation of a neighbor for the treatment of her aplastic anemia, but he did not know the name of the herbs.16 Another case involved the mistaken identity of a natural product, and a 66 year old man lost consciousness 10 minutes after consuming what he thought to be Rhizopogon roseolus.17 In cases such as these, it is critical to be able to rapidly identify pharmacologically active compounds in the herbal products. Adverse drug reactions and deaths related to consumption of herbal products are underreported because of the lack of testing. Due to the wide range of herbal products and pharmacologically active compounds in such products, a broad analytical scheme is advantageous for detection of these compounds. Current common methods for targeted identification of compounds in herbs include non-specific and/or time-consuming analytical methods such as thin layer chromatography (TLC) and high-performance liquid chromatography (HPLC).1
000 cases of adverse effects resulting from CHMs.2 Global use of CHMs is expected to continue to rise and with it potentially the occurrences of adverse reactions.2 Toxicological emergencies have been described in the literature, such as the case of a 36 year-old woman consuming an unknown herbal decoction for three days prior to admission to the hospital where she suffered cardiac arrest. Her father purchased the herbs based on the recommendation of a neighbor for the treatment of her aplastic anemia, but he did not know the name of the herbs.16 Another case involved the mistaken identity of a natural product, and a 66 year old man lost consciousness 10 minutes after consuming what he thought to be Rhizopogon roseolus.17 In cases such as these, it is critical to be able to rapidly identify pharmacologically active compounds in the herbal products. Adverse drug reactions and deaths related to consumption of herbal products are underreported because of the lack of testing. Due to the wide range of herbal products and pharmacologically active compounds in such products, a broad analytical scheme is advantageous for detection of these compounds. Current common methods for targeted identification of compounds in herbs include non-specific and/or time-consuming analytical methods such as thin layer chromatography (TLC) and high-performance liquid chromatography (HPLC).1
Extraction of the analytes of interest from herbal products can be complex and time consuming. Extraction methods can vary from liquid extraction followed by filtration or solid phase extraction to filtration with protein precipitation depending on the administration form of the CHM. A described method for Fructus corni required a 45 minute sonication in 80% methanol (v/v), dilution, and extraction using a C18 solid phase extraction column prior to ultra-fast liquid chromatography (UFLC) analysis.18 A 24 minute gradient was used to analyze the analytes of interest using UFLC. Other methods for separating the complex mixture of compounds using HPLC can take up to 65 minutes per sample.19–22
Particularly in the case of adverse drug reactions a rapid method requiring minimal sample preparation for the analysis of pharmacologically active compounds is advantageous. Direct Analysis in Real Time-Time of Flight mass spectrometry (DART-MS) is an atmospheric pressure ionization method with direct sampling to the mass spectrometer.23 DART-MS is a rapid technique with little to no sample preparation required.23 Pairing a quick, simple extraction method with DART-MS analysis could concentrate analytes of interest and remove compounds of non-interest, simplifying the mass spectra.
The aim of the study was to evaluate methods for analyte extraction paired with a rapid instrumental analysis, DART-MS, for pharmacologically active compounds in anticonvulsant herbal medicines, Gou Teng, Tian Ma, and Jiang Can. Different extraction methods were selected for evaluation based on being quick, simple methods that could be easily adopted by laboratories with the potential for extracting a wide variety of pharmacologically active compounds. The goal is for a broad extraction and analytical scheme with the ability to detect diverse pharmacologically active compounds from herbal matrices. Additionally, the herbal products were purchased from different locations with the purpose of comparing the anticonvulsive and pharmacologically active compounds in the herbs from different sources.
2. Experimental
2.1 Materials
Gou Teng, Tian Ma, and Jiang Can were purchased from Tong Ren Tang Chinese Medicine Company (Hong Kong, People's Republic of China) in Beijing, China and Chinatown New York. Tian Ma was also purchased from C.H.T. Inc. in Chinatown New York. The Gou Teng purchased in Chinatown New York was an Herbal Doctor product (Murray Int'l Trading Co., Inc., Brooklyn, NY) labeled as a product of China. The Jiang Can purchased in Chinatown New York was a Royal King product (Kwok Shing Hong, Inc., Brooklyn, NY) labeled as a product of the People's Republic of China. The Tian Ma purchased in Chinatown New York was packaged in a clear plastic bag with no product information.Glacial acetic acid, sodium hydroxide (molecular biology grade), methanol, and n-hexane were purchased from Fisher Scientific (Hampton, NH). The ethyl acetate and dichloromethane used were from ACROS Organics (Geel, Belgium). Koptec 200 Proof Ethanol was purchased from Decon Labs Inc. (King of Prussia, PA). The chloroform used was from Pharmco-Aaper (Brookfield, CT). Polyethylene glycol (PEG) 600 was purchased from Ultra Inc. (North Kingstown, RI).
2.2 Sample preparation
The herb was ground into a powder using a Kitchen Aid blade coffee grinder (Benton Harbor, MI) and 0.25 g of powdered herbs was used for each extraction method. Five extractions: water, ethanol, microwave-assisted, ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) chloroform, and acid-wash were performed on the powdered herbs. The microwave-assisted, ethanol
chloroform, and acid-wash were performed on the powdered herbs. The microwave-assisted, ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) chloroform, and acid-wash extractions were adapted from published methods.24
chloroform, and acid-wash extractions were adapted from published methods.24
2.3 Extraction procedure
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) chloroform extraction (1
chloroform extraction (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1 EtOH
1 EtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) CHCl3).
The powdered herb was soaked in 13 mL of 1
CHCl3).
The powdered herb was soaked in 13 mL of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 EtOH
1 EtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CHCl3 overnight. The samples were sonicated for 30 min using a Fisherbrand CPX1800 Ultrasonic bath (Waltham, MA). The extract was concentrated to 1 mL using an Organomation N-Evap Analytical evaporator.
CHCl3 overnight. The samples were sonicated for 30 min using a Fisherbrand CPX1800 Ultrasonic bath (Waltham, MA). The extract was concentrated to 1 mL using an Organomation N-Evap Analytical evaporator.
2.4 DART-MS analysis
A previously validated method using a JEOL JMS T100LC Accu-TOF DART-MS (JEOL USA, Inc., Peabody, MA) was used in positive and negative mode to analyze the powdered herb and extracts.25 Briefly, orifice 1 was operated in function switching mode alternating between 20, 30, 60, and 90 V, while orifice 2 was operated at 5 V with a ring lens voltage of 3 V. The helium flow rate for the ion source was 2.0 L min−1 with a heater temperature of 350 °C. The following instrument parameters were used: discharge needle of 4000 V, detector voltage of 2000 V, and peaks voltage of 400 V (positive) or 800 V (negative). Electrode 1 and 2 were set at 150 V and 250 V, respectively. A mass range of 50 to 1500 m/z and 90 to 1500 m/z were scanned for positive and negative mode respectively.A solution of PEG 600 in methanol (positive) or in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 methanol
1 methanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) dichloromethane (negative) was used to calibrate the instrument and a positive control was used to confirm the mass values fall within ±5 mmu. For positive mode the positive control contained methamphetamine, cocaine, and nefazodone, while the negative mode control contained aspirin and furosemide.
dichloromethane (negative) was used to calibrate the instrument and a positive control was used to confirm the mass values fall within ±5 mmu. For positive mode the positive control contained methamphetamine, cocaine, and nefazodone, while the negative mode control contained aspirin and furosemide.
At the beginning of each run the calibrator and positive control were wanded, followed by a blank. For the powdered herb a blank capillary tube served as the blank, while for the extracts a solvent blank was wanded using a capillary tube. Each sample was wanded five times using a capillary tube. Before the completion of the run, the positive control was run again to ensure mass accuracy over the course of the analysis.
The background subtracted mass spectra were analyzed and compared to a compiled list of compounds referenced in literature as being contained in the herb. Data analysis was performed using T.S.S Pro version 3.0 and Mass Mountaineer. For an identification of a compound in the herb product, the mass from the spectrum was required to be within a ±5 mmu range from the monoisotopic mass of the compound.
3. Results & discussion
3.1 Gou Teng
The DART-MS spectra and the pharmacologically active compounds for the powdered Gou Teng from Tong Ren Tang in Beijing and New York were compared. Out of the three products, Gou Teng was the only product to contain a difference in pharmacologically active compounds between the products purchased from different locations. Gou Teng purchased from Beijing contained the isobaric compounds, dihydrocorynantheine/hirsutine ([M + H]+ = 369.2178), while Gou Teng purchased from New York contained the isobaric compounds, isopteropodine/mitraphylline/pteropodine ([M + H]+ = 369.1814). Differences in pharmacologically active compounds in the herbal product could result from the use of different species. Uncaria rhynchophylla, U. macrophylla, U. hissata, U. sessilifructus, or U. sinensis are all common species marketed under Gou Teng.4 The product purchased in New York was labeled as U. rhynchophylla, while the product purchased from Beijing had no indication of species on the packaging.The pharmacologically active compounds detected in the extracts from the five different extractions were compared to those detected in the powdered Gou Teng (Table 2 and Fig. 1). Of the 24 compounds with unique masses searched for in Gou Teng, 11 were detected between the powder and the extracts. For the extractions, the ethanol extract was the only one to contain all the pharmacologically active compounds detected in the powder. Additionally, campesterol was detected in the ethanol extract, but not the powdered product. The reported main pharmacologically active compounds: corynoxeine, isocorynoxeine, hirsuteine, hirsutine, isorhynchophylline, and rhynchophylline were all detected in the following extracts: water, ethanol, MAE, and ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) chloroform, in addition to the powdered product. No additional pharmacologically active compounds were detected in negative mode for the powdered product or extracts of Gou Teng.
chloroform, in addition to the powdered product. No additional pharmacologically active compounds were detected in negative mode for the powdered product or extracts of Gou Teng.
| Gou Teng | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| # | Compound | Biological activity | Adduct | Adduct mass | Extraction method | |||||
| Powder | H2O | EtOH | MAE | EtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CHCl3 CHCl3 |
Acid wash | |||||
| 1* | Catechin | Antioxidant26 | –OH | 273.0763 | X | |||||
| Epicatechin | Antioxidant26 | –OH | ||||||||
| 1 | Catechin | Antioxidant26 | +H | 291.0869 | X | X | X | X | ||
| Epicatechin | Antioxidant26 | +H | ||||||||
| 2 | Akuammigine | Weak antagonist of pre & postsynaptic α-adrenoceptors27 | +H | 353.1865 | X | X | X | X | X | |
| Tetrahydroalstonine | Antagonist of pre α2-adrenoceptors27 | +H | ||||||||
| 3 | Hirsuteine | 5-HT3 antagonist28 | +H | 367.2022 | X | X | X | X | X | |
| 4 | Isopteropodine | Modulate function of G protein-coupled muscarinic M(1) acetylcholine & 5-HT(2) receptors29 | +H | 369.1814 | NY | NY | NY | NY | NY | |
| Mitraphylline | Anti-inflammatory30 | +H | ||||||||
| Pteropodine | Modulate function of G protein-coupled muscarinic M(1) acetylcholine & 5-HT(2) receptors29 | +H | ||||||||
| 5 | Dihydrocorynantheine | Vasodilator5 | +H | 369.2178 | B | B | B | B | B | |
| Hirsutine | 5-HT3 antagonist28 | +H | ||||||||
| 6 | Corynoxeine | 5-HT3 antagonist28 | +H | 383.1971 | X | X | X | X | X | |
| Isocorynoxeine | 5-HT3 antagonist28 | +H | ||||||||
| 7* | Campesterol | Inhibits cholesterol absorption31 | –OH | 383.3678 | X | X | X | |||
| 8 | Corynoxine B | Inhibitor of central dopamine release32 | +H | 385.2127 | X | X | X | X | X | |
| Isorhynchophylline | 5-HT3 antagonist,28 antagonist of NMDA-type ionotropic glutamate receptor33 | +H | ||||||||
| Rhynchophylline | 5-HT3 antagonist (Nakamura), antagonist of NMDA-type ionotropic glutamate receptor32 | +H | ||||||||
| 9* | Stigmasterol | Inhibits cholesterol absorption31 | –OH | 395.3678 | X | X | X | X | ||
| 10* | β-Sitosterol | Inhibits cholesterol absorption31 | –OH | 397.3834 | X | X | X | X | X | X |
| 9 | Stigmasterol | Inhibits cholesterol absorption31 | +H | 413.3783 | X | X | X | X | ||
| 11* | Ursolic acid | Anti-inflammatory, Antihyperlipidemic34 | –OH | 439.3576 | X | X | X | X | X | X |
| 11 | Ursolic acid | +H | 457.3682 | X | X | X | X | |||
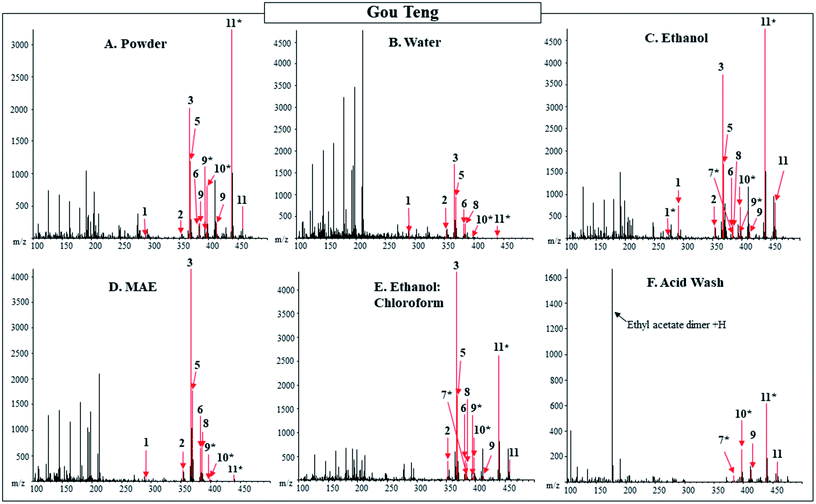 | ||
Fig. 1 DART-MS (20 V) positive ionization mode spectra of powdered and extracts of Gou Teng from Tong Ren Tang purchased in Beijing. The powdered material is shown in Panel (A) compared to water, ethanol, microwave-assisted, ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) chloroform, and acid wash extracts depicted in Panel (B–F) respectively. Labeled numbers correspond to pharmacologically active compounds found in Table 2. Gou Teng purchased from Beijing (depicted in figure) contained the isobaric compounds, dihydrocorynantheine/hirsutine ([M + H]+ = 369.2178), while Gou Teng purchased from New York contained the isobaric compounds, isopteropodine/mitraphylline/pteropodine ([M + H]+ = 369.1814). * designates M − OH adduct. chloroform, and acid wash extracts depicted in Panel (B–F) respectively. Labeled numbers correspond to pharmacologically active compounds found in Table 2. Gou Teng purchased from Beijing (depicted in figure) contained the isobaric compounds, dihydrocorynantheine/hirsutine ([M + H]+ = 369.2178), while Gou Teng purchased from New York contained the isobaric compounds, isopteropodine/mitraphylline/pteropodine ([M + H]+ = 369.1814). * designates M − OH adduct. | ||
Although not all reported pharmacologically active compounds were detected in all the products, compounds were detected in each of the products that may be responsible for producing the reported effects. The main pharmacologically active compounds: corynoxeine, isocorynoxeine, hirsuteine, hirsutine, isorhynchophylline, and rhynchophylline are reported 5-HT3 antagonists.28 Conflicting research debating whether antagonism of the 5-HT3 serotonergic receptor reduces seizures and convulsions, with reports of convulsions being decreased and other reports of increased convulsions by the administration of a 5-HT3 antagonist.35 Additionally, isorhynchophylline is a reported antagonist of N-methyl-D-aspartate (NMDA)-type ionotropic glutamate receptors.9 Antagonism of the NMDA receptors has reported anticonvulsant effects and is a therapeutic target for antiepileptic treatments.36 The reported main pharmacologically active compounds of Gou Teng detected in the powder and multiple extracts could produce the reported anticonvulsant effects.
Additionally, the 20, 30, 60, and 90 V spectra were compared for the powdered herbal products with results from Gou Teng shown as an example in Table 3 and Fig. 2. The higher orifice voltage spectra were compared to the 20 V spectra for the powdered product. As the voltage increased, the less intense [M + H]+ or [M − OH]− peaks were no longer detected, while the more intense peaks were still observed in the 30, 60, and 90 V spectra. The higher molecular weight compounds fragmented and were no longer observed in the higher voltage spectra. While fragmentation patterns of compounds would provide additional confirmation of the pharmacologically active compounds, when a technique such as DART-MS, with no separation of compounds, is used the interpretation of the fragmentation is complicated for a complex mixture. Additionally, standards for all pharmacologically active compounds in the herbal products are not available. Individual standards of the pharmacologically active compounds would supply additional evidence for the identification of the pharmacologically active compounds based on fragmentation pattern. A separation technique or standards would be required to determine the fragmentation patterns of the compounds, without either of those the 30, 60, and 90 V spectra do not provide additional information for the identification of pharmacologically active compounds present.
| Gou Teng | |||||||
|---|---|---|---|---|---|---|---|
| # | Compound | Adduct | Adduct mass | Orifice 1 voltage | |||
| 20 V | 30 V | 60 V | 90 V | ||||
| 1* | Catechin | –OH | 273.0763 | ||||
| Epicatechin | –OH | ||||||
| 1 | Catechin | +H | 291.0869 | X | |||
| Epicatechin | +H | ||||||
| 2 | Akuammigine | +H | 353.1865 | X | X | X | X |
| Tetrahydroalstonine | +H | ||||||
| 3 | Hirsuteine | +H | 367.2022 | X | X | X | X |
| 4 | Isomitraphylline | +H | 369.1814 | X | X | X | X |
| Isopteropodine | +H | ||||||
| Mitraphylline | +H | ||||||
| Pteropodine | +H | ||||||
| 5 | Dihydrocorynantheine | +H | 369.2178 | X | X | X | X |
| Hirsutine | +H | ||||||
| 6 | Corynoxeine | +H | 383.1971 | X | X | X | |
| Isocorynoxeine | +H | ||||||
| 7* | Campesterol | –OH | 383.3678 | ||||
| 8 | Corynoxine B | +H | 385.2127 | X | X | X | X |
| Isorhynchophylline | +H | ||||||
| Rhynchophylline | +H | ||||||
| 9* | Stigmasterol | –OH | 395.3678 | X | X | ||
| 10* | β-Sitosterol | –OH | 397.3834 | X | X | X | X |
| 9 | Stigmasterol | +H | 413.3783 | X | X | ||
| 11* | Ursolic acid | –OH | 439.3576 | X | X | X | |
| 11 | Ursolic acid | +H | 457.3682 | X | X | X | |
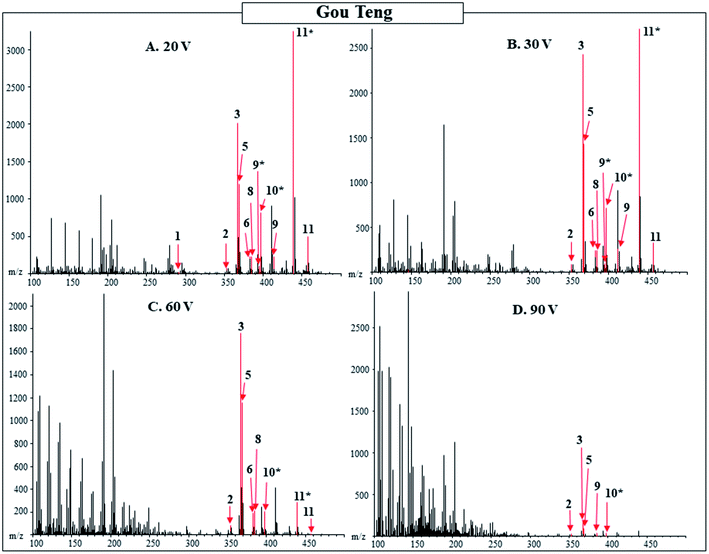 | ||
| Fig. 2 Function-switching DART-MS spectra of powdered Gou Teng from Tong Ren Tang purchased in Beijing. Orifice 1 voltage was alternated between 20, 30, 60, and 90 V and the corresponding spectra are shown in Panels (A)–(D) respectively. Labeled numbers correspond to pharmacologically active compounds found in Table 3. * designates M − OH adduct. | ||
3.2 Tian Ma
The pharmacologically active compounds detected in the extracts from the five different extractions were compared to those detected in the powdered Tian Ma (Table 4 and Fig. 3). For Tian Ma, seven pharmacologically active compounds with unique masses were searched for in the powder and the extracts. Of the seven, five unique masses were detected in the powder with no additional masses detected in the extracts. The following compounds were detected in the powdered Tian Ma: 4-hydroxybenzyl alcohol, vanillin, vanillyl alcohol, vanillic acid, and gastrodin. Vanillin and vanillic acid were only detected in negative mode. No single extract contained all the pharmacologically active compounds detected in the powder, but the MAE extract contained the most pharmacologically active compounds. The acid wash extraction was not effective in extracting any target pharmacologically active compounds from Tian Ma.| # | Compound | Biological activity | Adduct | Adduct mass | Extraction method | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Powder | H2O | EtOH | MAE | EtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CHCl3 CHCl3 |
Acid wash | |||||
| Tian Ma | ||||||||||
| 1* | 4-Hydroxybenzyl alcohol | Suppress dopaminergic & serotonergic activity37 | –OH | 107.0497 | X | X | X | X | X | |
| 2 | Vanillin | Anti-inflammatory8 | –H | 151.0395 | X | X | ||||
| 3* | Vanillyl alcohol | Suppress oxidative stress38 | –OH | 137.0603 | X | X | X | X | ||
| 4 | Vanillic acid | Inhibits inflammatory pain39 | –H | 167.0344 | X | X | ||||
| 5* | Gastrodin | Anti-depressant40 | –OH | 269.1025 | X | X | X | |||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||||
| Jiang Can | ||||||||||
| 1 | D-Mannitol | Diuretic41 | +H | 183.0869 | X | X | X | X | X | |
| 2 | Citric acid | Antioxidant42 | –H | 191.0192 | X | X | X | X | ||
| 3* | β-Sitosterol | Anticonvulsant11 | –OH | 397.3834 | X | X | ||||
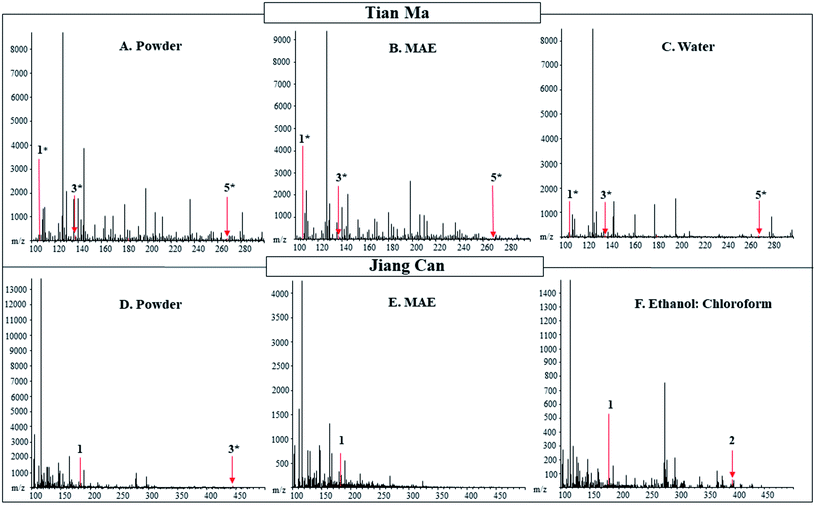 | ||
Fig. 3 DART-MS (20 V) positive ionization mode spectra of powdered and extracts of Tian Ma from C.H.T. Inc. and Jiang Can from Tong Ren Tang purchased in New York. The powdered material of Tian Ma and Jiang Can is shown in Panels (A and D), respectively. The microwave-assisted extract is shown in Panel (B) for Tian Ma and (E) for Jiang Can. In Panel (C) is the water extract of Tian Ma and in Panel (F) is the ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) chloroform extract of Jiang Can. Labeled numbers correspond to pharmacologically active compounds found in Table 4. * designates M − OH adduct. chloroform extract of Jiang Can. Labeled numbers correspond to pharmacologically active compounds found in Table 4. * designates M − OH adduct. | ||
In Tian Ma, all the reported main pharmacologically active compounds: 4-hydroxybenzaldehyde, gastrodin, vanillin, and vanillyl alcohol were detected in the powdered Tian Ma. 4-Hydroxybenzaldehyde is a reported GABAA (γ-aminobutyric acid) receptor chloride channel complex agonist.37 The reported anticonvulsant effects of Tian Ma could be a result of the 4-hydroxybenzaldehyde through agonism of the GABAA receptor, producing an inhibitory effect.43
3.3 Jiang Can
The pharmacologically active compounds detected in the extracts from the five different extractions were compared to those detected in the powdered Jiang Can (Table 4 and Fig. 3). For Jiang Can, ten pharmacologically active compounds with uniques masses were searched for in the powder and the extract. Between the powdered Jiang Can and the extracts ammonium oxalate, D-mannitol, citric acid, and β-sitosterol were detected. The ethanol extract contained only one pharmacologically active compound from Jiang Can, while the rest of the extracts contained two pharmacologically active compounds. D-Mannitol was detected in each extract except the acid wash extract. The ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) chloroform and acid wash extracts were the only extracts to contain an anticonvulsive compound, β-sitosterol. Citric acid was only detected in negative mode and was detected in the water and MAE extracts, in addition to the powder.
chloroform and acid wash extracts were the only extracts to contain an anticonvulsive compound, β-sitosterol. Citric acid was only detected in negative mode and was detected in the water and MAE extracts, in addition to the powder.
Even though a visible white residue associated with ammonium oxalate was present on both the Jiang Can products, the parent compound was not detected. Upon heating, a proton from the ammonium can transfer to the base producing ammonia gas and the conjugate acid of the salt.44 Heat from the helium stream could cause the proton transfer from the ammonium to the oxalate producing ammonia gas and the oxalate conjugate acid. A peak with a m/z of 178.9828 in the negative spectra of Jiang Can powder could correlate to the dimer of the oxalate conjugate acid.
Of the compounds detected, ammonium oxalate and β-sitosterol have reported antiepileptic and anticonvulsant activities.11 Although not all the reported main pharmacologically active compounds were detected in the powder nor the extracts, the ammonium oxalate and β-sitosterol could produce the reported anticonvulsant effects of Jiang Can.
The MAE was the most effective extraction for pharmacologically active compounds from these anticonvulsant herbs. Despite the herbs soaking in the extraction solvents longer in the ethanol, ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) chloroform, and the acid wash extractions than in the MAE, they were less effective in extracting the pharmacologically active compounds. Shorter extraction times can result in some active compounds being below the limit of detection. Not only was the MAE most effective at extracting these structurally diverse compounds, the MAE requires the least amount of time for extraction.
chloroform, and the acid wash extractions than in the MAE, they were less effective in extracting the pharmacologically active compounds. Shorter extraction times can result in some active compounds being below the limit of detection. Not only was the MAE most effective at extracting these structurally diverse compounds, the MAE requires the least amount of time for extraction.
In general, for compounds not detected in the extracts nor the powder of these anticonvulsant products it is possible the compounds may be present below the limit of detection or the compounds may only be present in select species and not in the purchased products. Compounds detected in the extracts but not in the powder may be present below the limit of detection and were concentrated through the extraction process. Of the compounds detected in the powder, but not the extracts, many are less polar compounds with potentially poor extraction efficiency in more polar solvents used in the methods. Although not all pharmacologically active compounds were extracted, a wide variety of the structurally diverse active compounds were identified in the extracts.
With increasing popularity of natural products and the risk of toxicological emergencies and deaths from their use it is critical to be able to analyze a broad range of natural products. The two previously described cases in which the woman consumed an unknown combination of herbs and the 66 year old man consumed what he believed to be Rhizopogon roseolus when he actually ingested Schleroderma albidum, exemplify medically and forensically relevant cases in which an unknown product was consumed and an analysis is tedious and long. A rapid, inclusive extraction and analytical scheme could prove useful in identifying the products.16
4. Conclusions
Out of the five extraction methods evaluated, no single extraction worked for all the anticonvulsant and pharmacologically active compounds from the three different herbal products. The MAE extract contained the most pharmacologically active compounds, while the acid wash contained the least from the three products. While the ethanol extraction was the most effective extraction for Gou Teng, the MAE extraction was most effective for Tian Ma. For Jiang Can, no extraction clearly out-performed the others at extracting pharmacologically active compounds, but the ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) chloroform and acid wash extracts were the only extracts to contain an anticonvulsive compound. The pharmacologically active compounds, particularly the anticonvulsive compounds, from each product are structurally diverse so not one method will extract all compounds and the extraction method will determine the pharmacologically active compounds detected. Although no one method can extract all the pharmacologically active compounds, all the methods evaluated are easily adoptable, rapid extraction methods with common solvents such that products can be concurrently extracted for analysis. The DART-MS proved to be a quick and efficient analytical tool, such that multiple extractions could be easily assessed in sequence. With the growing popularity of natural products, rapid extraction and the efficient analysis of pharmacologically active compounds from natural matrices is critical for public health and public safety.
chloroform and acid wash extracts were the only extracts to contain an anticonvulsive compound. The pharmacologically active compounds, particularly the anticonvulsive compounds, from each product are structurally diverse so not one method will extract all compounds and the extraction method will determine the pharmacologically active compounds detected. Although no one method can extract all the pharmacologically active compounds, all the methods evaluated are easily adoptable, rapid extraction methods with common solvents such that products can be concurrently extracted for analysis. The DART-MS proved to be a quick and efficient analytical tool, such that multiple extractions could be easily assessed in sequence. With the growing popularity of natural products, rapid extraction and the efficient analysis of pharmacologically active compounds from natural matrices is critical for public health and public safety.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This project was supported in part by Award No. NIJ 2016-DN-BX-0150, awarded by the National Institute of Justice, Office of Justice Programs, U.S. Department of Justice and the National Institutes of Health Award No. P30DA033934. The opinions, findings, and conclusions or recommendations expressed in this publication/program/exhibition are those of the author(s) and do not necessarily reflect those of the Department of Justice. We thank Dr Ruiqin Yang of the School of Forensic Science at the Peoples' Public Security University of China for her assistance in acquiring the CHM. Dr James Hutchings for his assistance and expertise throughout the project.Notes and references
- Y. Wang, C. Li, L. Huang, L. Liu, Y. Guo, L. Ma and S. Liu, Rapid Identification of traditional Chinese herbal medicine by direct analysis in real time (DART) mass spectrometry, Anal. Chim. Acta, 2014, 845, 70–76 CrossRef CAS.
- D. Cyranoski, Why Chinese medicine is heading for clinics around the world, Nature, 2018, 561, 448–450 CrossRef CAS.
- T. Smith, K. Kawa, V. Eckl, C. Morton and R. Stredney, Herbal Supplement Sales in US Increased 8.5% in 2017, Topping $8 Billion, HerbalGram, 2018, vol. 119, pp. 62–71 Search PubMed.
- K. C. Huang, The Pharmacology of Chinese Herbs, CRC Press, Boca Raton, FL, 2nd edn, 1999 Search PubMed.
- A. Ndagijimana, X. Wang, G. Pan, F. Zhang, H. Feng and O. Olaleye, A review on indole alkaloids isolated from Uncaria rhynchophylla and their pharmacological studies, Fitoterapia, 2013, 86, 35–47 CrossRef CAS.
- A. S. Ravipati, N. Reddy and S. R. Koyyalamudi, Biologically Active Compounds from the Genus Uncaria (Rubiaceae), Stud. Nat. Prod. Chem., 2014, 43, 381–408 CAS.
- H. Pan, W. Yang, Y. Zhang, M. Yang, R. Feng, W. Wu and D. Guo, An integrated strategy for the systematic characterization and discovery of new indole alkaloids from Uncaria rhynchophylla by UHPLC/DAD/LTQ-Orbitrap-MS, Anal. Bioanal. Chem., 2015, 407, 6057–6070 CrossRef CAS.
- J. H. Jang, Y. Son, S. S. Kang, C. S. Bae, J. C. Kim, S. H. Kim, T. Shin and C. Moon, Neuropharmacological Potential of Gastrodia elata Blume and Its Components, J. Evidence-Based Complementary Altern. Med., 2015, 2015, 1–14 Search PubMed.
- P. Chen and L. Shee, Gastrodiae Rhizoma (tian ma): a review of biological activity and antidepressant mechanisms, J. Tradit. Complement. Med., 2011, 1, 31–40 CrossRef.
- M. K. Pyo, J. L. Jin, Y. K. Koo and H. S. Yun-Choi, Phenolic and Furan Type Compounds Isolated from Gastrodia elata and their Anti-Platelet Effects, Arch. Pharmacal Res., 2004, 27, 381–385 CrossRef CAS.
- M. Hu, Z. Yu, J. Wang, W. Fan, Y. Liu, J. Li, H. Xiao, Y. Li, W. Peng and C. Wu, Traditional Uses, Origins, Chemistry and Pharmacology of Bombyx batryticatus: A Review, Molecules, 2017, 22, 1–24 Search PubMed.
- V. Kumar, S. K. Tewari and A. K. Awasthi, Surface ultrastructure of Beauveria bassiana infecting silkworm Bombyx mori Linn, Curr. Sci., 1994, 67, 546–548 Search PubMed.
- M. Wang, S. Sang, L. S. Hwang and C. Ho, Herbs: Challenges in Chemistry and Biology (ACS Symposium Series 925), Oxford University Press, Washington, DC, 2006 Search PubMed.
- A. Singh and K. Zhao, Herb-Drug Interactions of Commonly Used Chinese Medicinal Herbs, Int. Rev. Neurobiol., 2017, 135, 197–232 CrossRef.
- Z. P. Zeng and J. G. Jiang, Analysis of the adverse reactions induced by natural product-derived drugs, Br. J. Pharmacol., 2010, 159, 1374–1391 CrossRef CAS.
- S. Han, H. Lim and H. Noh, Intravenous lipid emulsion therapy for cardiac arrest and refractory ventricular tachycardia due to multiple herb intoxication, Clin. Exp. Emerg. Med., 2019, 6, 366–369 CrossRef.
- Y. Sato, H. Tomonari, Y. Kaneko and K. Yo, Mushroom poisoning with Scleroderma albidum: a case report with review of the literature, Acute Med. Surg., 2020, 7, 1–3 Search PubMed.
- G. Cao, C. Zhang, Y. Zhang, X. Cong, H. Cai, B. Cai, X. Li and J. Yao, Global detection and identification of components from crude and processed traditional Chinese medicine by liquid chromatography connected with hybrid ion trap and time-of-flight-mass spectrometry, J. Sep. Sci., 2011, 34, 1845–1852 CrossRef CAS.
- M. H. Liu, X. Tong, J. X. Wang, W. Zou, H. Cao and W. W. Su, Rapid separation and identification of multiple constituents in traditional Chinese medicine formula Shenqi Fuzheng Injection by ultra-fast liquid chromatography combined with quadrupole-time-of-flight mass spectrometry, J. Pharm. Biomed. Anal., 2013, 74, 141–155 CrossRef CAS.
- X. Zheng, P. Shi, Y. Cheng and H. Qu, Rapid analysis of a Chinese herbal prescription by liquid chromatography-time-of-flight tandem mass spectrometry, J. Chromatogr. A, 2008, 1206, 140–146 CrossRef CAS.
- L. Sun, H. Wei, F. Zhang, S. Gao, Q. Zeng, W. Lu, W. Chen and Y. Chai, Qualitative analysis and quality control of Traditional Chinese Medicine preparation Tanreqing injection by LC-TOF/MS and HPLC-DAD-ELSD, Anal. Methods, 2013, 22, 6431–6440 RSC.
- S. Zeng, L. Wang, T. Chen, Y. Wang, H. Mo and H. Qu, Direct analysis in real time mass spectrometry and multivariate data analysis: a novel approach to rapid identification of analytical markers for quality control of traditional Chinese medicine preparation, Anal. Chim. Acta, 2012, 733, 38–47 CrossRef CAS.
- R. B. Cody, J. A. Laramee and H. D. Durst, Versatile New Ion Source for the Analysis of Materials in Open Air under Ambient Conditions, Anal. Chem., 2005, 77, 2297–2302 CrossRef CAS.
- T. Aniszewski, Alkaloids: Chemistry, Biology, Ecology, and Applications, Elsevier, Amsterdam, Netherlands, 2015 Search PubMed.
- J. L. Poklis, S. A. Raso, K. N. Alford, A. Poklis and M. R. Peace, Analysis of 25I-NBOMe, 25B-NBOMe, 25C-NBOMe and Other Dimethoxyphenyl-N-[(2-Methoxyphenyl) Methyl]Ethanamine Derivatives on Blotter Paper, J. Anal. Toxicol., 2015, 39, 617–623 CrossRef CAS.
- J. Shay, H. A. Elbaz, I. Lee, S. P. Zielske, M. H. Malek and M. Huttemann, Molecular Mechanisms and Therapeutic Effects of (−)-Epicatechin and Other Polyphenols in Cancer, Inflammation, Diabetes, and Neurodegeneration, Oxid. Med. Cell. Longevity, 2015, 2015, 1–13 CrossRef.
- P. Demichel and J. Roquebert, Effects of raubasine stereoisomers on pre- and postsynaptic α-adrenoceptors in the rat vas deferens, Br. J. Pharmacol., 1984, 83, 505–510 CrossRef CAS.
- Y. Nakamura, Y. Ishida, M. Kondo and S. Shimada, Yokukansan contains compounds that antagonize the 5-HT3 receptor, Phytomedicine, 2018, 43, 120–125 CrossRef CAS.
- T. H. Kang, K. Matsumoto, M. Tohda, Y. Murakami, H. Takayama, M. Kitajima, N. Aimi and H. Watanabe, Pteropodine and isopteropodine positively modulates the function of rat muscarinic M(1) and 5-HT(2) receptors expressed in Xenopus oocyte, Eur. J. Pharmacol., 2002, 444, 39–45 CrossRef CAS.
- S. Montserrat-de la Paz, R. de la Puerta, A. Fernandez-Arche, A. M. Quilez, F. J. Muriana, M. D. Garcia-Gimenez and B. Bermudez, Pharmacological effects of mitraphylline from Uncaria tomentosa in primary human monocytes: skew toward M2 macrophages, J. Ethnopharmacol., 2015, 170, 128–135 CrossRef CAS.
- R. Brauner, C. Johannes, F. Ploessl, F. Bracher and R. L. Lorenz, Phytosterols Reduce Cholesterol Absorption by Inhibition of 27-Hydroxycholesterol Generation, Liver X Receptor α Activation, and Expression of the Basolateral Sterol Exporter ATP-Binding Cassette A1 in Caco-2 Enterocytes, J. Nutr., 2012, 142, 981–989 CrossRef CAS.
- I. Sakakibara, S. Terabayashi, M. Kubo, M. Higuchi, Y. Komatsu, M. Okada, K. Taki and J. Kamei, Effect on locomotion of indole alkaloids from the hooks of Uncaria plants, Phytomedicine, 1999, 6, 163–168 CrossRef CAS.
- T. H. Kang, Y. Murakami, H. Takayama, M. Kitajima, N. Aimi, H. Watanabe and K. Matsumoto, Protective effect of rhynchophylline and isorhynchophylline on in vitro ischemia-induced neuronal damage in the hippocampus: putative neurotransmitter receptors involved in their action, Life Sci., 2004, 76, 331–343 CrossRef CAS.
- J. Liu, Pharmacology of oleanolic acid and ursolic acid, J. Ethnopharmacol., 1995, 49, 57–68 CrossRef CAS.
- H. Zhao, Y. Lin, S. Chen, X. Li and H. Huo, 5-HT3 Receptors: A Potential Therapeutic Target for Epilepsy, Curr. Neuropharmacol., 2018, 16, 29–36 CAS.
- W. Loscher, Pharmacology of glutamate receptor antagonists in the kindling model of epilepsy, Prog. Neurobiol., 1998, 54, 721–741 CrossRef CAS.
- J. W. Jung, B. H. Yoon, H. R. Oh, J. H. Ahn, S. Y. Kim, S. Y. Park and J. H. Ryu, Anxiolytic-Like Effects of Gastrodia elata and Its Phenolic Constituents in Mice, Biol. Pharm. Bull., 2006, 29, 261–265 CrossRef CAS.
- I. S. Kim, D. K. Choi and H. J. Jung, Neuroprotective Effects of Vanillyl Alcohol in Gastrodia elata Blume Through Suppression of Oxidative Stress and Anti-Apoptotic Activity in Toxin-Induced Dopaminergic MN9D Cells, Molecules, 2011, 16, 5349–5361 CrossRef CAS.
- C. Calixto-Campos, T. T. Carvalho, M. S. Hohmann, F. A. Pinho-Ribeiro, V. Fattori, M. F. Manchope, A. C. Zarpelon, M. M. Baracat, S. R. Georgetti, R. Casagrande and W. A. Verri J, Vanillic Acid Inhibits Inflammatory Pain by Inhibiting Neutrophil Recruitment, Oxidative Stress, CytokineProduction, and NFκB Activation in Mice, J. Nat. Prod., 2015, 78, 1799–1808 CrossRef CAS.
- W. C. Chen, Y. S. Lai, S. H. Lin, K. H. Lu, Y. E. Lin, S. Panyod, C. T. Ho and L. Y. Sheen, Anti-depressant effects of Gastrodia elata Blume and its compound gastrodin and 4-hydroxybenzyl alcohol, via the monoaminergic system and neuronal cytoskeletal remodeling, J. Ethnopharmacol., 2016, 182, 190–199 CrossRef CAS.
- O. Mathisen, M. Raeder and F. Kiil, Mechanism of osmotic diuresis, Kidney Int., 1981, 19, 431–437 CrossRef CAS.
- O. M. E. Abdel-Salam, N. M. Shaffie, E. A. Omara and N. N. Yassen, Citric Acid an Antioxidant in Liver, The Liver, 2018, 2018, 183–198 Search PubMed.
- A. S. Galanopoulou, GABAA Receptors in Normal Development and Seizures: Friends or Foes?, Curr. Neuropharmacol., 2008, 6, 1–20 CrossRef CAS.
- M. Olszak-Humienik, On the thermal stability of some ammonium salts, Thermochim. Acta, 2001, 378, 107–112 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2021 |
