Chiral porous organic frameworks and their application in enantioseparation
Ying
Zhang
,
Xiaoning
Jin
,
Xiaofei
Ma
and
Yong
Wang
 *
*
School of Science, Tianjin Key Laboratory of Molecular Optoelectronic Science, Department of Chemistry, Collaborative Innovation Center of Chemical Science and Engineering, Tianjin University, Tianjin 300350, P. R. China. E-mail: wangyongtju@tju.edu.cn
First published on 23rd October 2020
Abstract
Porous organic frameworks (POFs) are a kind of porous material with a network structure composed of repeated monomers, which have excellent physical and chemical properties, such as a high surface area, high porosity, uniform pore sizes and structural diversity, and which have aroused broad interest among researchers. With the rapid development of materials science, increasingly more porous materials have been developed and applied, especially metal organic frameworks (MOFs) and covalent organic frameworks (COFs), which have been widely applied in the fields of luminous materials, catalytic research, adsorption and drug transport. One of the most important applications for chiral porous materials is in chiral separation and these materials have become a research hotspot in the field of chromatographic separation and analysis in recent years. In this review, from the viewpoint of enantioseparation, the synthesis of chiral porous materials and their applications in high-performance liquid chromatography (HPLC), capillary electrochromatography (CEC), and gas chromatography (GC) are reviewed. The typical applications of MOFs in solid-phase microextraction (SPME) are also discussed.
 Xiaoning Jin | Xiaoning Jin received her master's degree in 2015 from Tianjin University (TJU), and bachelor's degree at Anyang Normal University. Her research focuses on functional materials of zein microspheres and films. Email: E-mail: xiaoning_jin@tju.edu.cn. |
 Xiaofei Ma | Xiaofei Ma received his PhD in 2004 and master's degree in 2000 from Tianjin University (TJU), and dual bachelor's degree in 1997 from Xi'an Jiaotong University. He is now the Associate Dean of the National Experimental Teaching Demonstration Center of Chemistry & Chemical Engineering at Tianjin University and the National Virtual Simulation Experimental Teaching Center of Chemistry & Chemical Engineering at Tianjin University. His research interests include the preparation of nanocomposites and their application in separation and sensing. Email: E-mail: maxiaofei@tju.edu.cn. |
 Yong Wang | Yong Wang received his PhD from Singapore Nanyang Technological University (NTU) in 2011, master's degree in 2007 and dual bachelor's degree in 2004 from Tianjin University (TJU). He worked as a research fellow in NTU from Jan to Dec in 2011 and joined TJU in early 2012 as an Associate Professor. In late 2015, he was promoted to full professor and assigned as the associate director of the chemistry department. From Mar 2017 to Feb 2019, he worked in the National Natural Science Foundation of China as a project manager. He is now the Associate Dean of the School of Science in charge of graduate students and research. His research interests include high resolution chiral separation and sensing, semiconducting materials and devices. Email: E-mail: wangyongtju@tju.edu.cn. |
1 Introduction
Chirality is used to describe the structural asymmetry of matter. If a molecule does not have a center of symmetry or a plane of symmetry, we call it a chiral molecule.1 Enantiomers have almost the same physical properties (solubility, lattice, boiling point, density, etc.) as well as the same chemical properties in a nonchiral environment.2,3 However, optically active enantiomers of chiral drugs are largely different in biological activities, metabolic processes and even toxicity.4 Generally, racemic drugs have only one effective enantiomer, and the other enantiomer has no effect or even toxic side effects. According to statistics, there are more than 2000 commonly used drugs, about half of which are chiral compounds. As the demand for optically pure compounds rapidly increases, a great amount of important research efforts have been dedicated to developing highly efficient, economical and convenient chiral separation methods.5 There are three common ways to obtain a single enantiomer: (1) chiral source synthesis; (2) asymmetric synthesis; and (3) chiral separation. The process of splitting racemic compounds into optically pure isomers of a single configuration is called chiral separation or enantioseparation. Since 1848, when Pasteur first reported the separation of racemate sodium tartrate, chiral separation has developed rapidly.6 At present, classical separation methods include mechanical resolution, crystal inoculation resolution,7 kinetic resolution, membrane resolution, enzyme or microbial resolution, chemical resolution8 and chromatographic methods. Compared with other classical enantiomeric separation methods, chromatography has many obvious advantages and can meet the requirements of enantiomeric separation and determination under various conditions. Chromatography has been used for enantiomer separation since 1938, when Henderson and Rule successfully separated camphor derivative racemates on lactose.9 Subsequently, Dalgliesh successfully separated some optical isomers of amino acids by paper chromatography and proposed the three action principles hypothesis of chiral separation in 1952.10 Since then, the evolution of this techniques as well as the development of chiral stationary phases (CSPs) of high efficiency have made it a robust and strategic tool for the chemical sciences. Nowadays, various classical chiral selectors have been used to prepare CSPs for diverse chromatographic techniques, such as high-performance liquid chromatography (HPLC), gas chromatography (GC), thin layer chromatography (TLC), capillary electrochromatography (CEC), and supercritical fluid chromatography (SFC).11 Such CSPs can be classified into polysaccharides, Pirkle-type, cyclodextrin, synthetic polymers, macrocyclic glycopeptide antibiotics, proteins, crown ethers and ligand exchange-based CSPs. And many review articles have summarized the applications of these CSPs.12–20 In recent years, with the rapid development of porous materials, many researchers have made great efforts towards developing various new types of CSPs using chiral porous materials. In order to produce a timely summary and guidance for researchers, this review focuses on the use of MOFs and COFs as CSPs for chiral chromatographic separation since 2011.2 MOFs
MOFs, also known as porous coordination polymers (PCPs), are a class of microporous crystalline hybrid materials.21 MOFs are built from metal cations (or cationic clusters) bridged by multifunctional organic linkers to form one-(1-D), two-(2D), or three-dimensional (3D) coordination networks. The first MOF was introduced in 1995 by Yaghi et al.22 They prepared the robust and highly porous MOF-5, based on Zn4O(CO2)6 octahedral secondary building units (SBUs) each linked by six chelating 1,4-benzenedicarboxylate (bdc) units to give a cubic framework. Over the past twenty years, a large number of MOFs have been synthesized and reported because of the flexibility and variability of metal SBUs and organic linkers. Compared with traditional inorganic porous materials, MOFs not only have inorganic and organic properties, but also have a higher specific surface area, greater porosity and more diversified structures.23,24 Therefore, they have various promising applications, such as chromatographic separation,25,26 catalysis,27,28 gas storage,29,30 chemical sensing,31 membranes,32,33 drug delivery,34,35 proton conduction,36–38 electron conduction,39,40 and even energy conversion.41,42 As an important subclass of MOFs, chiral MOFs (CMOFs) can be generated from chiral building blocks or using achiral ligands under spontaneous resolution without any chiral sources. Ideally, they can obtain chiral functionalities through the open channels or cavities present in the MOFs. In recent years, one of the most actively emerging fields is the design and synthesis of CMOFs and exploring their applications in various fruitful research fields, including asymmetric catalysis,43,44 molecular recognition45 and enantioseparation.46,472.1 Synthetic strategies for CMOFs
The reported synthetic routes for CMOFs include: the introduction of optically pure organic ligands, the usage of chiral templates to induce chiral centers and post-synthetic modification.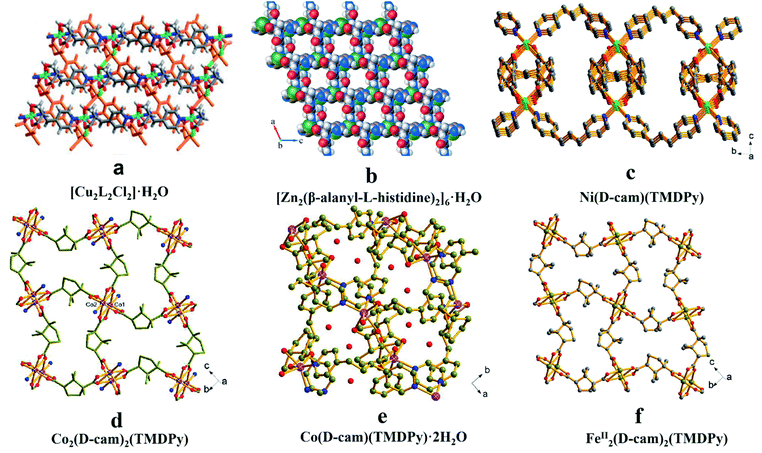 | ||
| Fig. 1 The crystal structures of MOFs: [Cu2L2Cl2]·H2O (a), [Zn2(β-alanyl-L-histidine)2]6·H2O (b), Ni(D-cam)(TMDPy) (c), Co2(D-cam)2(TMDPy) (d), Co(D-cam)(TMDPy)·2H2O (e), FeII2(D-cam)2(TMDPy) (f). Reproduced from ref. 48 with permission from The Royal Society of Chemistry. Reproduced with permission from ref. 49 and 51. | ||
2.1.2.1 Post-synthetic modification. The post-synthetic modification method involves synthesizing CMOFs by modifying ligands or metal ion clusters from the preliminary synthesis of achiral MOFs. Compared with the above two methods, the reported method can effectively avoid the racemization of chiral ligands. At the same time, due to the diversity of post-modification methods, this method greatly expands the categories of CMOFs.
For post-modification to the ligands for CMOFs, active groups such as –Br, –NH2, or –CH3 (ref. 58) are usually introduced into organic ligands to give them functional groups that react with chiral reagents. For example, Cohen and his co-workers59 modified the amino groups on IRMOF-3 containing 2-amino-1,4-benzenedicarboxylic acid (NH2-BDC) as a building block (Fig. 3a) by using (S)-(+)-2-methylbutyric anhydride and (S)-(−)-2-acetoxysuccinic anhydride, and two chiral MOFs, IRMOF-3-(S)-AM3Me and IRMOF-3-(S)-AM-SucAcO, were obtained. In 2015, Bonnefoy60 and his co-workers synthesized the first example of MOFs post.
Template molecules do not bridge with metal ions in the framework, but their structure and chemical properties will affect the structure and properties of the whole framework, which can reduce the penetration of the framework, form an open hole structure and increase chirality, etc. Therefore, a chiral template can be used to induce the synthesis of chiral MOFs.52,53 Rosseinsky and co-workers used chiral 1,2-pd as an inducer to construct two CMOFs, [Ni3(btc)2(py)6(1,2-pd)3]·11(1,2-pd)·8H2O54 (Fig. 2b) and [Ni3(btc)2(3-pic)6(1,2-pd)3]·9(1,2-pd)·11H2O55 (Fig. 2a) in 2000. In 2003, they synthesized CMOFs:56 Ni3(btc)2(py)6(1,4-bd)4(mu-1,4-bd)·2(1,4-bd) (Fig. 2c) using chiral diol as a template, where template 1,4-bd formed hydrogen bonds with the carboxylate oxygen of ligand BTC to ensure that the carboxylic acid was in a right–right direction and there were four template molecules in each structural unit. To conduct a further exploration, Lin and his co-workers57 synthesized a chiral framework compound (BMIm)2[Ni (Hbtc)2(H2O)2] (Fig. 2d) using a chiral ionic liquid as the medium. The use of L-aspartate as one component of the ionic liquid induces chirality in the resulting solid. Without the ionic liquid, no CMOF was obtained functionalized with peptides. Their microwave-assisted post-synthetic modification method yielded enantiopure peptides anchored inside MOF cavities. Al-MIL-101-NH2 (Fig. 3b), In-MIL-68-NH2 (Fig. 3c), and Zr-UiO-66-NH2 (Fig. 3d) were chosen as starting platforms. Amino acids and short peptides were modified in the MOF channel through covalent bonding. The three different MOF template molecules used in the experiment have different topological structures, 3D spatial structures and pore sizes, but they can all be modified by means of microwave assistance, which verifies the wide applicability of the method.
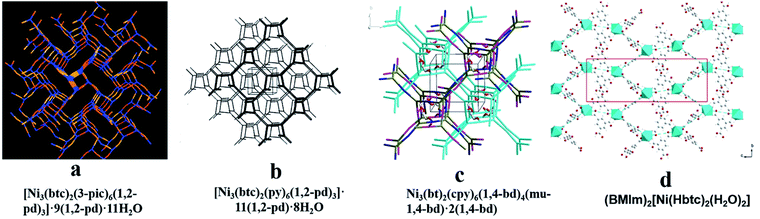 | ||
| Fig. 2 The crystal structures of MOFs: [Ni3(btc)2(3-pic)6(1,2-pd)3]·9(1,2-pd)·11H2O (a), [Ni3(btc)2(py)6(1,2-pd)3]·11(1,2-pd)·8H2O (b), Ni3(bt)2(cpy)6(1,4-bd)4(mu-1,4-bd)·2(1,4-bd) (c), (BMIm)2[Ni(Hbtc)2(H2O)2] (d). Reproduced with permission from ref. 54–57. | ||
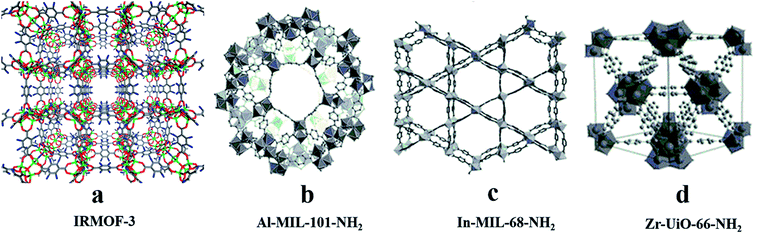 | ||
| Fig. 3 The crystal structures of MOFs: IRMOF-3 (a), Al-MIL-101-NH2 (b), In-MIL-68-NH2 (c), Zr-UiO-66-NH2 (d). Reproduced with permission from ref. 59 and 60. | ||
2.2 Enantioseparation on CMOFs
Chiral chromatography on supports based on CMOFs is becoming mature. Since 2011, ten, twenty-one, and eleven CMOFs have been used as CSPs for GC, HPLC, and CEC, respectively.In 2013,61 Yuan and co-workers synthesized InH(D-C10H14O4)2 (MOF 1) and used it as a CSP in GC for chiral separation. MOF 1 was synthesized by taking In as the metal center and D-(+)-camphoric acid as the organic ligand according to the method reported by Wang et al.62 It has a left-handed helical chain and the aperture is about 1.47 nm (Fig. 4a). In the same year, they also reported the use of Co(D-cam)1/2(bdc)1/2(tmdpy) (MOF 2) for chiral separation by GC.63 MOF 2 possesses a 3D framework containing enantiopure building blocks embedded in intrinsically chiral.
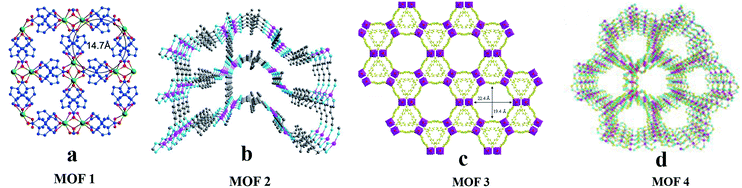 | ||
| Fig. 4 The crystal structures of MOF 1–MOF 4: MOF 1 (a), MOF 2 (b), MOF 3 (c), MOF 4 (d). Reproduced with permission from ref. 61, 63 and 64 and 66. | ||
In the same year, a CMOF [Mn3(HCOO)4·(D-cam)]n (MOF 4) with a homochiral chain was used as the stationary phase in GC.66 MOF 4 was synthesized according to a method reported in the literature.67 The MOF 4 crystal is a 3D MOF material with a single chiral chain, and its cylindrical channel diameter is 1.4 nm. Each hexagonal channel in this crystal has three single chiral chains formed by D-cam ligands, and all chiral carbons in D-cam ligands are directed toward the center of the channel, which is very suitable for chiral recognition (Fig. 4d). In 2017, they reported the use of the MOF Co-L-GG (MOF 5) as the stationary phase for separating racemates in GC68 topological nets (Fig. 4b).
In 2014,64 they fabricated a 3D chiral nanoporous MOF [(CH3)2NH2] [Cd(bpdc)1.5] (MOF 3)-coated capillary column for the separation of racemates. [(CH3)2NH2][Cd(bpdc)1.5]·2DMA was synthesized according to the method of Hao et al.65 In order to release more free spaces within the framework for analyte interactions, the MOF was heated at 120 °C in vacuo for 12 h to remove the guest DMA molecules per formula unit, affording MOF 3 suitable for GC usage. It possesses a unique chiral nanotube motif built from the covalent linkage of homochiral nanotubes assembled from octuple helices. The 3D chiral nanoporous MOF possesses a large pore with a length of 22.4 Å and a width of 19.4 Å for the opening of a hexagonal nanotube and a right-handed helix (Fig. 4c).
Crystalline [Co-L-GG(H2O)] was synthesized in accordance with the method of Stylianou et al.69 MOF 5 possesses a 1-D ladder-building unit, which was constructed from the connection of octahedral Co(II) centers through L-GG linker ligands (Fig. 5a).
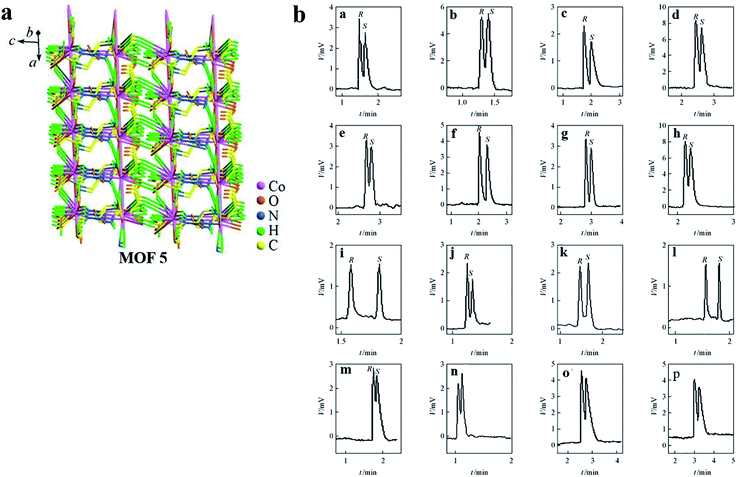 | ||
| Fig. 5 The structure of MOF 5 (a). Representative GC chromatograms obtained for the separation of racemates on a Co-L-GG coated open tubular column (b): (a) epichlorohydrin; (b) 4-chloro-1,2-epoxybutane; (c) 1,2-epoxybutane; (d) 1,2-epoxyoctane; (e) 2-butanol; (f) 3-butyn-2-ol; (g) 2-heptanol; (h) 1-phenyl-1-propanol; (i) 2-ethylhexanoic acid; (j) 2-methylbutyric acid; (k) 2-methylpentanoic acid; (l) 2-methylhexanoic acid; (m) glycidyl isopropyl ether; (n) glycidyl ether; (o) fenchone; (p) methyl phenyl sulfoxide. Reproduced with permission from ref. 68. | ||
Coated fused-silica open tubular columns with MOFs 1–5 were prepared by a dynamic coating method. The selectivity of MOFs 1–4 was checked using a wide series of racemates as test probes. It is worth noting that some chromatographic outcomes were compared with those obtained on the well-known Chirasil-L-Val (Table 1). The separation factors were higher on MOF CSPs than on Chirasil-L-Val CSP. And a chiral-coated open tubular column based on MOF 5 was used for the GC separation of sixteen racemic compounds (Fig. 5b). The chiral recognition ability of MOFs 1–5 coated open tubular columns for the separation of racemates primarily arises from the surface of the crystals in which the steric fit between the homochiral framework and the conformation of the solute molecule is the main interactive force. In addition to these interactions, other interactions, including hydrogen bonding, dipole–dipole and van der Waals forces, have also enhanced the chiral recognition and enantioselectivity in GC.
| Racemates | Separation factor (α) | ||||
|---|---|---|---|---|---|
| Chirasil-L-Val | MOF-1 CSP | MOF-2 CSP | MOF-3 CSP | MOF-4 CSP | |
| Citronellal | — | 1.13 | 1.37 | 1.25 | — |
| 1-Phenyl-1,2-ethanediol | — | 1.17 | — | — | — |
| 1-Phenyl-ethanol | 1.000 | 1.44 | 1.38 | — | — |
| 2-Amino-1-butanol | — | 1.25 | 1.10 | — | — |
| Limonene | 1.000 | 1.35 | 1.32 | — | — |
| Methionine | 1.061 | 1.10 | 1.16 | 1.40 | |
| Proline | 1.003 | 1.32 | 1.41 | — | — |
| Glutamic acid | 1.046 | 1.43 | 1.20 | — | |
| 1-Phenyl-1,2-ethanediol | — | — | 1.20 | — | — |
| Leucine | — | — | — | 1.22 | 1.21 |
| Threonine | — | — | — | 1.50 | — |
| Aspartic acid | — | — | — | 1.50 | — |
| Alanine | — | — | — | — | 1.13 |
| Phenyl-succinic acid | — | — | — | — | 1.11 |
| Ref. | 61, 63, 64 and 66 | 61 | 63 | 64 | 66 |
Recently, Yan and co-workers70 reported a post-synthetic modification (PSM) strategy to synthesize CMOFs with designed chiral recognition sites for the chiral GC separation of diverse racemates. An amino-containing MOF, MIL-101(Al)-NH2, was chosen as the parent MOF. Five chiral molecules with different chiral recognition sites were, respectively, grafted onto MIL-101(Al)-NH2via covalent bonds to obtain five chiral MOFs, MIL-101(Al)-Xs (Xs represents the specific chiral organic groups) with good stability and different chiral selectivities (Fig. 6a). Racemates including alcohols, amines, nitriles, esters and aldehydes were selected to evaluate the chiral separation abilities of MIL-101(Al)-Xs-coated capillary columns. The kinetic diameters of all the studied racemates are smaller than the pore size of MIL-101(Al)-Xs (approximately 14 Å), so they assumed that the chiral separation mainly occurred in the pores of the MOFs. The different chiral microenvironment interactions of the chiral MOFs play key roles. The resolution of the studied racemates on the prepared CMOF capillary columns is superior to that on commercial β-DEX 225 or cyclosil B chiral columns (Fig. 6b). The results reveal that the post-synthesis approach is convenient for fabricating target chiral MOFs with pre-designed functions. It can avoid the blind synthesis of chiral MOFs via direct synthesis and facilitates the evolution of CSPs in chiral chromatography.
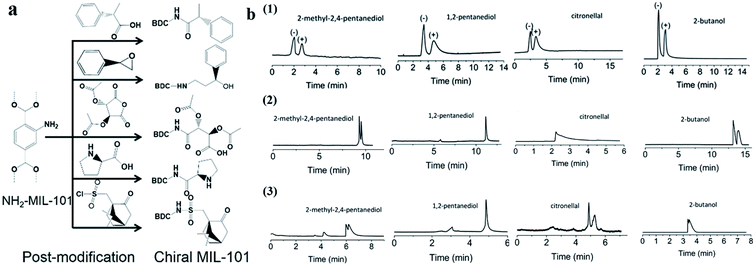 | ||
| Fig. 6 Schematic illustration of the post-synthesis of chiral MIL-101(Al)-Xs and the fabrication of their coated capillary columns for chiral GC (a). GC chromatograms on (1) MIL-101(Al)-Xs-coated columns, (2) β-DEX 225, (3) cyclosil B chiral columns for the separation of racemates (b). Reproduced from ref. 70 with permission from The Royal Society of Chemistry. | ||
The other case is that CMOFs are used to improved enantioseparations on a cyclodextrin (CD) stationary phase in GC. Yuan and co-workers71 first reported that the use of MOF 2 can improve enantioseparations on peramylated β-CD-based columns. Three capillary columns were prepared for GC: column A contained MOF 2, column B contained sodium chloride and peramylated β-CD, and column C contained MOF 2 and peramylated β-CD. Column A was prepared through a dynamic coating method. Column B was prepared with sodium chloride crystals by a dynamic method and was then coated by a static method employing a solution of peramylated β-CD and OV-17 (Silanization solution VI) in dichloromethane at 36 °C. The method for the preparation of column C was the same as that for column B. Ten racemates of 2-hexanol, linalool, citronellal, methyl L-β-hydroxyisobutyrate, limonene, rose oxide, dihydrocarvyl acetate, menthol, valine and leucine were chosen as model analytes. Column A was only able to separate four racemates. Column B decorated with peramylated β-CDs, was able to separate six chiral compounds. Column C, in which MOF 2 had been incorporated with peramylated β-CDs, was able to separate nine analytes. Fig. 7 shows GC chromatograms on column A, column B and column C for the separation of linalool, rose oxide valine and leucine. It demonstrates that incorporated MOF 2 indeed plays a significant role in enhancing the separation capabilities. The intrinsic characteristics of MOF 2, such as a high surface area, unusual integration of molecular chirality, absolute helicity and a 3D intrinsic chiral net, enabled the marriage of CMOF with β-CDs to form a superior chiral microenvironment, where the steric fit was well established between the chiral channel framework and the conformation of the racemates. Subsequently, they reported another three cases of MOF-decorated peramylated β-cyclodextrin columns: MOF 1, [Cu(sala)](n) (MOF 6),72 [Cd(LTP)2]n (MOF 7).73
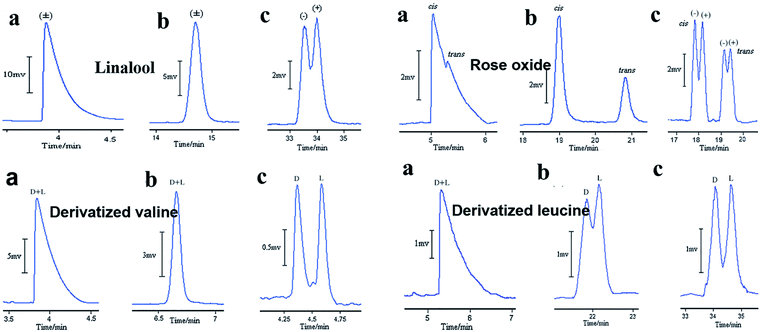 | ||
| Fig. 7 GC chromatograms on column A (a), column B (b), and column C (c) for the separation of racemates. Reproduced with permission from ref. 71. | ||
(i)CMOFs post-coated on the sodium silicate layer. In 2014, Yuan and co-workers26 first reported the utilization of [Zn2(D-cam)2(4,4′-bpy)]n (MOF 8) (Fig. 8a) as the CSP for open tubular CEC (OT-CEC) separation of chiral drugs. MOF 8 crystals were synthesized according to a procedure reported by Zhang et al.74 The left- and right-handed helices formed by alternating D-cam ligands and metal sites joined together through the common binuclear metal clusters existing in the homochiral layer. Although the ratio between the left- and right-handed helices is 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the resulting 3D framework is homochiral because of the presence of the enantiopure building block. In their work, the synthesized chiral MOF 8 was introduced into a fused silica capillary which had been coated by a sodium silicate solution layer. Baseline separations of flavanone and praziquantel were achieved on the MOF-coated column with a resolution of more than 2.1. Chiral recognition mostly depends on the best steric fit of the analytes with the 3D framework enantiopure building block and the helical homochiral layer surface of MOF 8. In addition, the authors suggest that weak hydrogen bonding between the organic ligands of MOF 8 and racemates and π–π stacking interaction between the analyte benzene ring and the pyridine rings should also contribute to the enantioselectivity. Thereafter, they75 synthesized three CMOFs via a hydrothermal method: Co2(D-cam)2(tmdpy) (MOF 9) (Fig. 8b), [Cd(D-cam)(tmdpy)]·2H2O (MOF 10) (Fig. 8c) and [Co0.5Zn0.5(L-Tyr)]n(L-tyrCo/Zn) (MOF 11) (Fig. 8d) referring to the literature.76 The three MOFs were used as CSPs in OT-CEC. MOF 9 was found to have good enantioseparation ability for six racemates: furoin, omeprazole, warfarin, benzoin, trans-1,2-diphenyloxirane and alprenolol. Among these, the resolution of furoin was the largest (Rs = 2.10). MOF 10 and MOF 11 also showed some chiral recognition ability, and three (phenylalanine, tryptophan, tyrosine) and four (2,2,2-trifluoro-1-(9-anthryl) ethanol, flavanone, hydrobenzoin and 1-(1-naphthyl)ethanol) chiral compounds were separated, respectively. Since the resolution is not very good, we can find other better methods to improve the coating effect of crystals to improve the column effect in future work. At the same time, functional groups of CMOF materials can be modified to improve the chiral recognition ability of the material.
1, the resulting 3D framework is homochiral because of the presence of the enantiopure building block. In their work, the synthesized chiral MOF 8 was introduced into a fused silica capillary which had been coated by a sodium silicate solution layer. Baseline separations of flavanone and praziquantel were achieved on the MOF-coated column with a resolution of more than 2.1. Chiral recognition mostly depends on the best steric fit of the analytes with the 3D framework enantiopure building block and the helical homochiral layer surface of MOF 8. In addition, the authors suggest that weak hydrogen bonding between the organic ligands of MOF 8 and racemates and π–π stacking interaction between the analyte benzene ring and the pyridine rings should also contribute to the enantioselectivity. Thereafter, they75 synthesized three CMOFs via a hydrothermal method: Co2(D-cam)2(tmdpy) (MOF 9) (Fig. 8b), [Cd(D-cam)(tmdpy)]·2H2O (MOF 10) (Fig. 8c) and [Co0.5Zn0.5(L-Tyr)]n(L-tyrCo/Zn) (MOF 11) (Fig. 8d) referring to the literature.76 The three MOFs were used as CSPs in OT-CEC. MOF 9 was found to have good enantioseparation ability for six racemates: furoin, omeprazole, warfarin, benzoin, trans-1,2-diphenyloxirane and alprenolol. Among these, the resolution of furoin was the largest (Rs = 2.10). MOF 10 and MOF 11 also showed some chiral recognition ability, and three (phenylalanine, tryptophan, tyrosine) and four (2,2,2-trifluoro-1-(9-anthryl) ethanol, flavanone, hydrobenzoin and 1-(1-naphthyl)ethanol) chiral compounds were separated, respectively. Since the resolution is not very good, we can find other better methods to improve the coating effect of crystals to improve the column effect in future work. At the same time, functional groups of CMOF materials can be modified to improve the chiral recognition ability of the material.
 | ||
| Fig. 8 The crystal structures of MOF 8–MOF 11: MOF 8 (a), MOF 9 (b), MOF 10 (c), MOF 11 (d). Reproduced with permission from ref. 26 and 75. | ||
In 2018, Chen and co-workers77 investigated the possibility of homochiral MOF [(Cu4I4)(dabco)2][Cu2(bbimb)]·3DMF (MOF 12) for enantioseparation in OT-CEC. MOF 12 was synthesized according to the literature.78 The coated capillary column showed good performance for the enantioseparation of monoamine neurotransmitters of epinephrine, isoprenaline and synephrine and their analogue terbutaline (Fig. 9b). Compared with other CMOF-coated capillary columns, MOF 12 exhibited better enantioseparation performance, where the Rs of epinephrine and terbutaline enantiomers were better than in some other works.79,80 The enantioseparation ability of MOF 12 was attributed to its unique DNA-like double helix.
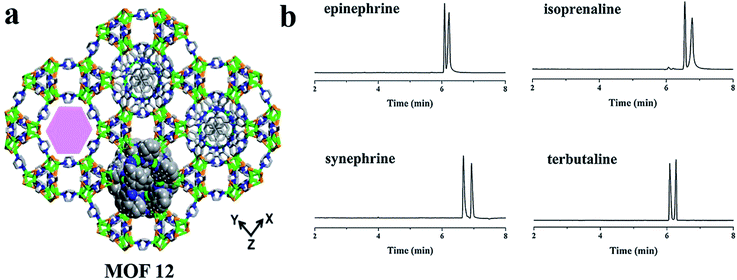 | ||
| Fig. 9 The crystal structure of MOF 12 (a). Electropherograms of the model chiral analytes (b). Reproduced with permission from ref. 77. | ||
(ii)In situ rapid preparation of CMOF-coated column. In 2015, homochiral MOF AlaZnCl (MOF 13) was in situ synthesized by Chen and co-workers80 on the inner wall of a capillary column by a layer-by-layer self-assembly approach at room temperature. The crystal structure of MOF 13 is illustrated in Fig. 10c; a homochiral helical structure framework with specific hydrophilic porous properties was formed owing to the presence of the enantiopure building block. To evaluate the enantioselective ability of a MOF-13-coated capillary column, they firstly tested the enantioseparation of some chiral amine drugs like terbutaline and carvedilol. Baseline separation was achieved and it also exhibited good separation for four monoamine neurotransmitters (epinephrine, synephrine, isoproterenol and norepinephrine) (Fig. 10d). The possible chiral recognition mechanism of MOF 13 was studied preliminarily, where the matching between the spatial chiral orientation of MOF 13 and the molecules contributes a lot to the enantioseparation. Besides, considering the existence of pyridine, –COOH and –NH2 moieties of MOF 13, the enantioseparation might also be affected by hydrophobic–hydrophobic interaction, weak hydrogen bonding and π–π interaction. Compared to other chiral columns, the chiral OT column exhibited the advantages of in situ synthesis, a controllable manner, and mild preparation conditions as well as short consumption-time in the preparation process. In 2016, they81 first reported the use of ZnO nanoparticles as efficient nucleating agents for the in situ rapid synthesis of homochiral MOF [Zn(s-nip)2]n (MOF 14) in the capillary inner wall for OT-CEC separation (Fig. 10a). The enantioseparation of monoamine neurotransmitters of isoprenaline, epinephrine and synephrine was tested on the MOF-14-coated capillary column. Enantioseparation of isoprenaline and synephrine and partial enantioseparation of structure (Fig. 9a): that is, the groove binding (through hydrogen bonding and van der Waals interactions) and intercalative binding (through π–π and hydrophobic interaction) between the analytes and the DNA-like MOF 12. This work broadened the application of homochiral MOFs with specific structures in CEC enantioseparation.
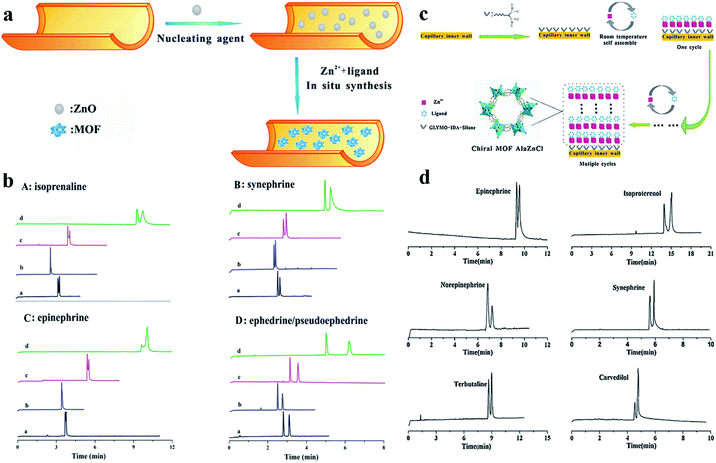 | ||
| Fig. 10 Schematic diagram for the preparation of MOF-13-coated capillary column and the crystal structure of MOF 13 (c). Electropherograms of analytes (d). Schematic diagram for the preparation process of MOF-14-coated capillary column (a). Electropherograms of the chiral analytes on the MOF-14-coated capillary columns prepared by different methods: (a): column A; (b): column B; (c): column C; (d): column D (b). Reproduced with permission from ref. 80 and 81. | ||
Epinephrine were well achieved within 4.5 min. And baseline separation of ephedrine and pseudoephedrine was also realized on MOF 14 within 3.5 min. This work would open up an avenue for the in situ fabrication of MOFs using nucleating agents in the capillary and broaden the application of chiral MOFs in OT-CEC. Finally, the enantioseparation ability of a MOF-14-coated capillary column using ZnO nanoparticles as a nucleating agent (column A) was compared with a MOF-14-coated capillary column fabricated by the layer-by-layer self-assembly approach (column B and column C) and the off-line method (direct bonding by silane coupling agent GLYMO, column D) (Fig. 10b). Column A exhibited the advantages of simple preparation process, shorter preparation time (the whole column preparation time was less than 5 h, and the preparation times of columns B, C and D were all more than 15 h) as well as shorter analysis time (less than 5 min).
Zeolite imidazole framework (ZIF) materials are an important subclass of MOF materials. ZIF-8 (Fig. 11a), one of the most favorable studied ZIFs, was synthesized by Yaghi and co-workers as a microcrystalline compound.82 In 2020, Chen83 and co-workers focused on the development of pepsin-ZIF-8 conjugate modified poly(GMA-co-EDMA) monoliths as CSPs for CEC. Poly(GMA-co-EDMA) monoliths were used as the support to grow ZIF-8 via layer-by-layer self-assembly (Fig. 11b). Then pepsin was immobilized onto the surface of the ZIF-8-poly(GMA-co-EDMA) monolithic column via Schiff base chemistry to prepare a pepsin-ZIF-8-poly(GMA-co-EDMA) column (Fig. 11c), which afforded good separation performance for hydroxychloroquine (HCQ), chloroquine (CHQ) and hydroxyzine (HXY) mixtures (Fig. 11d). In comparison with a pepsin-poly(GMA-co-EDMA) monolithic column (without ZIF-8), the resolution is strongly enhanced (HCQ: 0.34 → 2.50; CHQ: 0.45 → 1.97; HXY: 0.39 → 1.43). The results indicated that the introduction of ZIF-8 strongly enhanced the chiral resolution capacity of pepsin due to the synergistic effect of pepsin and ZIF-8. However, one disadvantage of this method is that it takes a long time to prepare a pepsin-ZIF-8-poly (GMA-co-EDMA) monolithic column by five cycles of layer-by-layer growth.
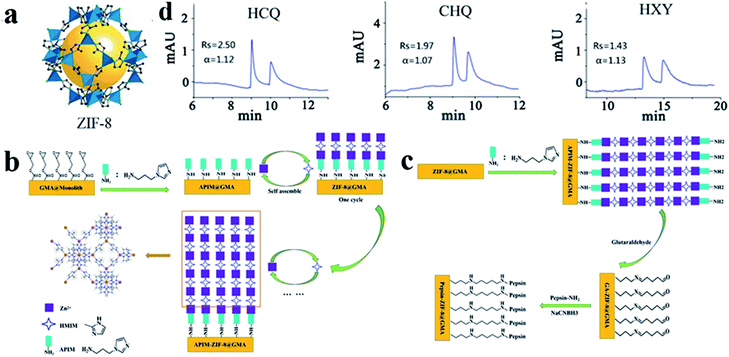 | ||
| Fig. 11 The crystal structure of ZIF-8 (a). Scheme for the preparation of ZIF-8-poly(GMA-co-EDMA) monolithic column (b) and pepsin-ZIF-8-poly(GMA-co-EDMA) monolithic column (c). Enantioseparation of basic chiral drugs on pepsin-ZIF-8-poly(GMA-co-EDMA) monolithic column by CEC (d). Reproduced with permission from ref. 83. | ||
(iii)Covalent bonding of CMOFs in capillaries. In 2016, Li and co-workers84 successfully synthesized a homochiral MOF material, [Cu(mal)(bpy)]·H2O (MOF 15), which was used to modify the capillary inner wall via covalent linkages. MOF 15 crystalline powder was prepared from L-(−)-mal, bpy, and Cu(OAc)2·H2O according to a reported procedure.85 Then, MOF 15 was modified with aminopropyl.
Homochiral MOF 8 (ref. 86) was prepared afterwards and successfully used for modifying a capillary column by forming covalent bonds in their group. First, MOF 8 crystalline powder was prepared from D-(+)-camphoric acid, 4,4′-bipyridyl and Zn(NO3)2·6H2O following a reported procedure.74 Then, APTES molecules were incorporated onto the MOF 8 particles using the same method described in the last work. Finally, the capillary columns were modified by MOF 8. DL-Phenylalanine was separated in the MOF-modified columns and DL-tyrosine, which was difficult to separate in their previous work, was also successfully resolved using this column.
In 2019,87 the HKUST-1@capillary column was prepared to enhance the enantioselectivity in chiral separation for five basic drugs. HKUST-1 (MOF 16) is a 3D square-shaped cubic MOF with a square-shaped channel system (9 × 9 Å).88,89 It possesses many unique properties, such as high micropore volume, large pore sizes and potentially valuable metal active sites90–92 and has attracted strong and broad interest among researchers for its application in chromatography as the stationary layer.93 The preparation procedure of a MOF-16-coated capillary column is shown in Fig. 13a by a simplified graphic.94 Five enantiomeric drugs were selected as the model to investigate the separation performance of the MOF-16@capillary. Compared with the bare capillary column system, better resolution and higher selectivity for all racemic drugs were achieved on the MOF-16@capillary (Fig. 13b). The improved separation performance can be ascribed to MOF 16 possessing a 3D square-shaped cube structure and hydrophobic interactions as well as π–π interactions. According to the interactions between MOF 13 phases and analytes, they speculate that an analyte of suitable size with benzene rings can be separated by this MOF stationary phase.
Triethoxysilane (APTES). Finally, the capillary wall was modified with MOF 15 modified by APTES. Utilizing the carboxyl groups prevalent in the MOF ligands, the MOF particles were introduced into capillary columns and bonded through amido bonds (Fig. 12b). This column achieved the successful separation of ephedrine–pseudoephedrine and DL-penicillamine enantiomers as well as DL-phenylalanine (Fig. 12c). This method offers a novel method for preparing MOF-modified capillary columns with the advantages of facile synthesis and versatility.
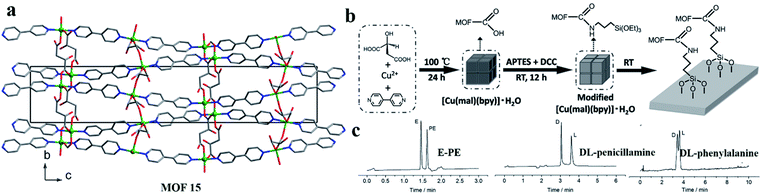 | ||
| Fig. 12 The crystal structure of MOF 15 (a). Procedure for the modification of capillary inner walls with MOF 15 crystal (b). Separation results for E-PE, DL-penicillamine, and DL-phenylalanine with a MOF-15-modified capillary column (c). Reproduced with permission from ref. 84. | ||
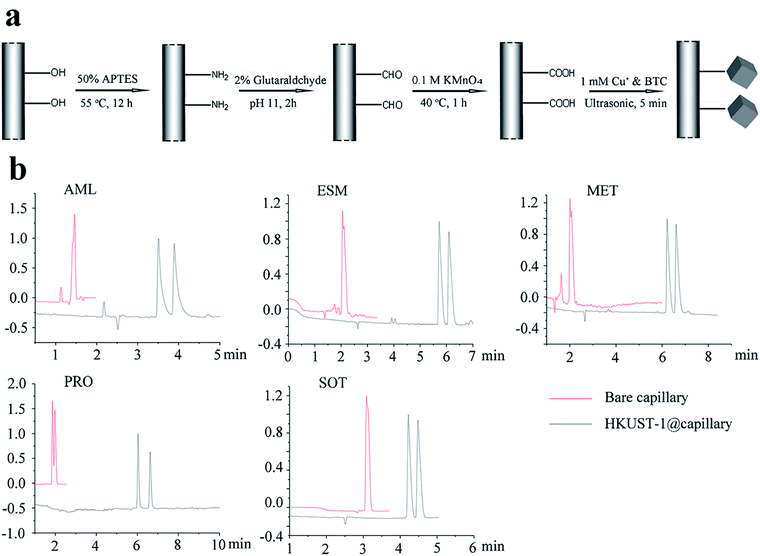 | ||
| Fig. 13 The preparation procedure of MOF 16 coated capillary column (a). Enantioseparation electropherograms of five racemic drugs in the MOF 16 column (black line) and bare column (red line) (b). Reproduced with permission from ref. 87. | ||
In 2011, Kaskel and co-workers95 converted 2-bromoterephthalic acid into dimethyl 2-bromoterephthalate, then coupled it with enantiopure chiral (S)-oxazolidinone in the presence of CuI, K2CO3 and dimethylethylene diamine in toluene. Chiral auxiliary substituted BDCs (ChirBDCs) were obtained by basic hydrolysis of the corresponding esters. The linkers were used for the synthesis of Zn4O(BTB)4/3(iPr-ChirBDC)(DEF)19(H2O)6 (MOF 17) and Zn4O(BTB)4/3(Bn-ChirBDC)(DEF)20(H2O)8 (MOF 18). The structures of both compounds consist of Zn4O6+ clusters interconnected into the 3D-network by 4 BTB and 2 substituted BDC linkers and it has been shown that in both cases chiral substituents occupy the positions in the trigonal microporous cages (Fig. 16a and b). Since the as-synthesized particles are much too large and needle-shaped, they were manually pestled. Unfortunately, the crushing process gives a broad particle size and shape distribution. This may be one reason for the low resolution. To guarantee the same packing quality, only MOF 18 was used as the stationary phase for HPLC applications. 1-Phenylethanol showed both selective and enantioselective interactions with MOF 18 (α = 1.6, Rs = 0.65). The resolution was too low to reach peak separation. In 2014, Yuan and co-workers96 reported a homochiral MOF [Cu2(D-cam)2(4,4′-bpy)]n (MOF 19) with 3D six-connected self-penetrating architectures as a promising stationary phase for enantioseparation in HPLC (Fig. 16c). Crystals of MOF 19 were obtained under hydrothermal conditions according to the method of Zhang et al.74 The following six racemates were separated in the normal-phase mode: 1-(9-anthryl)-2,2,2-trifluoroethanol, 1-(1-naphthyl)ethanol, 2-phenyl-1-propanol, 3-benzyloxy-1,2-propanediol, 1,1′-bi-2-naphthol and flavanone. Some effects, such as mobile phase composition, column temperature, and molecular size for separation on this chiral column, were also investigated. They found that adding CH3OH to the n-hexane/DCM mobile phase system did not improve the enantioselectivity. The probable reason is that alcohol solvent adsorption on MOF 19 decreased the recognition of analytic racemates. The retention time and selectivity of all the analytes were gradually reduced as the column temperature increased, which indicated that the separation processes were exothermic.
 | ||
| Fig. 14 Molecular structures of the racemates. Reproduced with permission from ref. 97, 101 and 102. | ||
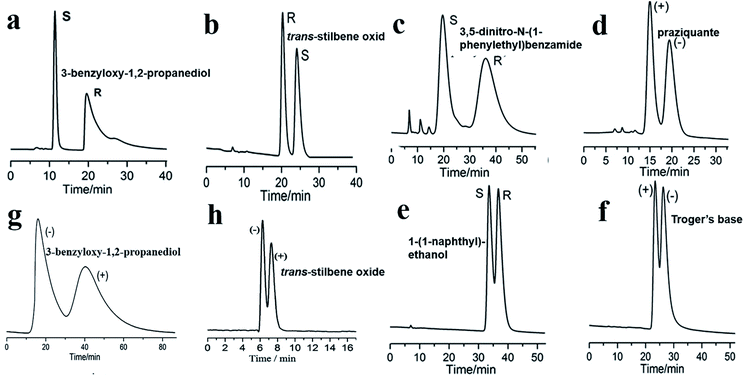 | ||
| Fig. 15 HPLC chromatograms on the packed MOF 9 column for the separation of racemates (a–f), MOF 3 column (g), MOF 8 column (h). Reproduced with permission from ref. 97. Reproduced from ref. 76 and 98 with permission from The Royal Society of Chemistry. | ||
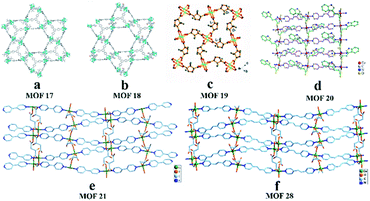 | ||
| Fig. 16 The crystal structures of MOF 17-MOF 21, MOF 28 : MOF 17 (a), MOF 18 (b), MOF 19 (c), MOF 20 (d), MOF 21 (e), MOF 28 (f). Reproduced from ref. 95 with permission from The Royal Society of Chemistry. Reproduced with permission from ref. 96, 99, 101 and 102. | ||
In 2015,97 MOF 9 was used as a CSP in HPLC. MOF 9 is based on six-connected paddlewheel [Co2(COO)4N2] building blocks, in which cobalt dimers are bridged by four bidentate carboxylate groups from four D-cam ligands. The cross-linking of adjacent dimers by D-cam ligands leads to a homochiral grid-like (4,4)-layered substructure. To investigate the chiral recognition ability of the stationary phase, the racemates of 3-benzyloxy-1,2-propanediol, trans-stilbene oxide, 3,5-dinitro-N-(1-phenylethyl)benzamide, praziquantel, 1-(1-naphthyl)ethanol, and Tröger's base were selected as test solutes. The six corresponding enantioseparation chromatograms are presented in Fig. 15, which show good.
In 2016, Yuan and co-workers99 first reported chiral MOFs with amino acid molecules as chiral ligands. In this work, L-trp and bpe as ligands were mixed under hydrothermal conditions to synthesize MOF{[Co(L-trp)(bpe)(H2O)]·H2O·NO3}n (MOF 20).100 The results of the crystal structure analysis showed that the MOF 20 crystal topology firstly formed a 1-D chiral chain by chelating coordination with the bridge ligand L-trp in cis-antimode, and then the adjacent bracelet formed a 2D chiral coordination layer structure by interconnecting the 2-bpe ligands (Fig. 16d). Moreover, there are important hydrogen-bonding forces between layers. The unique crystal structure of MOF 20 largely determines its recognition of some chiral compounds. This column achieved the successful separation of 3-benzyloxy-1,2-propanediol, 1-(9-anthryl)-2,2,2-trifluoroethanol, omeprazole, propranolol hydrochloride, alprenolol, DNB leucine, warfarin sodium, 3,5-dinitro-N-(1-phenylethyl)benzamide, pindolol, hydrobenzoin, chlorpheniramine maleate, also using a mixture of hexane/isopropanol as the mobile phase. And the MOF 20 stationary phase showed obvious complementarity with the other CSPs (MOF 3 (ref. 98) and MOF 9 (ref. 97)) (Table 2). Its chiral recognition ability may be mainly due to the stereo matching between the configuration of solute molecules and the peak shape and at least 60% valley separation. Higher separation efficiencies for 3-benzyloxy-1,2-propanediol and trans-stilbene oxide were achieved on a MOF-9 column than those on the previously reported MOF-3 (ref. 98) and MOF-8 (ref. 76) columns (Fig. 15). As for the separation mechanism, this may be due to the different pore sizes and channel shapes of the three MOFs: MOF 9 has a better steric fit with the conformation of the analyte. They think that hydrogen bonding between the active sites of MOF 9 and the hydroxyl groups of the analytes is likely to significantly enhance the chiral recognition functional sites in the 2D chiral plane of crystals and the hydrogen-bond force between the chiral planes.
| Racemate | Separation factor, α | ||
|---|---|---|---|
| MOF-20 column | MOF-3 column | MOF-9 column | |
| 3-Benzyloxy-1,2-propanediol | 4.68 | 3.72 | 2.69 |
| 3,5-Dinitro-N-(1-phenylethyl)benzamide | 1.66 | 1.49 | 2.26 |
| Propranolol hydrochloride | 1.38 | — | — |
| 1-(9-Anthryl)-2,2,2-trifluoroethanol | 1.37 | — | — |
| Pindolol | 1.47 | — | — |
| DNB leucine | 2.12 | — | — |
| Hydrobenzoin | 1.77 | 1.62 | — |
| Warfarin sodium | 1.74 | 1.35 | — |
| Omeprazole | 1.85 | — | — |
| Chlorpheniramine maleate | 1.58 | — | — |
| Alprenolol | 2.69 | — | — |
| Ref. | 99 | 98 | 97 |
In 2016101 and 2018,102 Yuan's group synthesized MOF [Cu(s-mal)(bpy)]n (MOF 21) and MOF [Cu(s-mal)(bpe)]n (MOF 28) according to a procedure by Fedin et al.85 They also used the two MOFs as stationary phase materials for HPLC. In both structures MOF 21 and MOF 28 (Fig. 16e and f), the linear N-donor spacers bpy and bpe connect the 2D chiral layers ({Cu(mal)}) into extended homochiral 3D porous networks, but they possess different chiral space groups and pore sizes. The average pore sizes were estimated to be 5 Å × 5 Å × 6 Å for MOF 21 and 5 Å × 5 Å × 9 Å for MOF 28. The results showed that MOF 21 had good chiral recognition ability towards seventeen racemic compounds (1, 2, 3, 4, 6, 7, 8, 9, 11, 12, 13, 14, 16, 17, 18, and 19) in Fig. 14. While MOF 28 can separate racemates, including 2, 3, 4, 5, 7, 12, 13, 14, 16, 20, and 25. And the MOF-28 column gave higher enantioselectivity for some racemates such as 4, 7, 12, 13, and 16 (Table 3). This is mainly ascribed to the different chiral microenvironments and pore sizes between the two materials. The order of peaks of most compounds in the MOF-21 column is R-isomer followed by S-isomer, whereas the order of peaks of 1-(1-naphthalene)-ethanol is reversed. The authors considered this may be due to the R-isomer having better orthotopic matching with MOF 21 and its retention capacity being better than that of the S-isomer.
In 2017, Yuan and co-workers103 reported six CMOFs based on amino acid as chiral ligands used as CSPs for HPLC enantioseparation. Six MOFs (Fig. 17) constructed from Zn2+ or Co2+ ions and various enantiopure amino acids (L-Tyr, L-His, L-Trp and L-Glu acid), namely [Zn(L-Tyr)]n(L-TyrZn) (MOF 22), [Zn4(btc)2(Hbtc)(L-His)2(H2O)4]·1.5H2O (MOF 23), {[Zn2(L-Trp)2(bpe)2(H2O)2]·2H2O·2NO3}n (MOF 24), [Co2(L-Trp)(INT)2(H2O)2(ClO4)] (MOF 25), [Co2(sdba)(L-Trp)2] (MOF 26) and [Co(L-Glu)(H2O)·H2O]∞ (MOF 27), were synthesized according to the methods previously reported in the literature.100,104–108 Racemates including alcohols, amines, ketones, ethers and organic acids were chosen as the analytes (Fig. 18), where many of them achieved baseline separation, indicating the good chiral resolution ability of the CSPs. MOF 22 and MOF 27 represented better chiral recognition ability than the other four. The main reason was attributed to their crystal structures. MOF 22 and MOF 27 were synthesized by only one amino acid ligand which was used as a bridging ligand to link the metal centers in the framework. The other four MOFs have two or more ligands, and the amino acid ligands coordinate to the metal centers but do not bridge the metal centers, so the chirality is likely to be imposed or modified in the structure. Therefore, their chiral recognition ability is not comparable with that of MOF 22 or MOF 27 (Table 4).
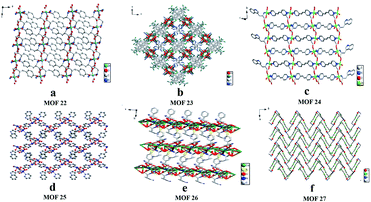 | ||
| Fig. 17 The crystal structures of MOF 22–MOF 27: MOF 22 (a), MOF 23 (b), MOF 24 (c), MOF 25 (d), MOF 26 (e), MOF 27(f). Reproduced with permission from ref. 103. | ||
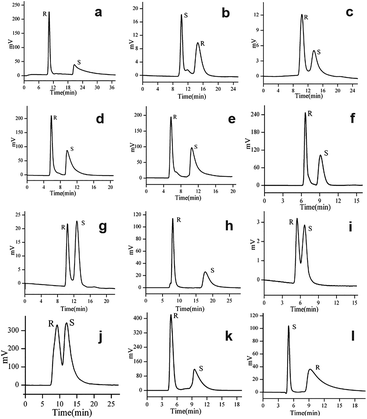 | ||
| Fig. 18 Representative chromatograms on the six MOF-based columns: chromatograms of 1,2-diphenyl-1,2-ethanediol, praziquantel and propranolol obtained on MOF-22 column, respectively (a–c), chromatograms of 1,2-diphenyl-1,2-ethanediol and omeprazole obtained on MOF-23 column (d and e), chromatograms of 1,2-diphenyl-1,2-ethanediol and N-(3,5-dinitrobenzoyl)leucine obtained on MOF-24 column (f and g), chromatograms of 1,2-diphenyl-1,2-ethanediol and ketoprofen obtained on MOF-25 column (h and i), chromatograms of benzoin obtained on MOF-26 column (j), chromatograms of 1,2-diphenyl-1,2-ethanediol and chlorprophenpyridamine obtained on MOF-27 column (k and l). Reproduced with permission from ref. 103. | ||
| MOFs CSP | Racemates | k 1 | α | R s |
|---|---|---|---|---|
| MOF-22 | 1,2-Diphenyl-1,2-ethanediol | 1.09 | 3.04 | 3.12 |
| Pindolol | 1.33 | 1.32 | 0.79 | |
| Praziquantel | 1.29 | 1.74 | 1.91 | |
| Propranolol | 1.30 | 1.55 | 1.43 | |
| 3-Benzyloxy-1,2-propanediol | 0.90 | 2.01 | 1.98 | |
| Omeprazole | 1.00 | 1.53 | 2.57 | |
| Warfarin sodium | 3.40 | 1.16 | 0.66 | |
| MOF-23 | 1,2-Diphenyl-1,2-ethanediol | 1.32 | 2.16 | 2.82 |
| Omeprazole | 1.31 | 2.44 | 3.11 | |
| N-(3,5-Dinitrobenzoyl)leucine | 1.08 | 1.87 | 2.08 | |
| 1-(4-Chlorophenyl)ethanol | 1.00 | 2.08 | 4.41 | |
| MOF-24 | 1,2-Diphenyl-1,2-ethanediol | 1.15 | 1.95 | 2.03 |
| 3-Benzyloxy-1,2-propanediol | 1.01 | 1.62 | 1.41 | |
| N-(3,5-Dinitrobenzoyl)leucine | 1.15 | 1.83 | 1.37 | |
| Omeprazole | 1.05 | 2.04 | 1.69 | |
| MOF-25 | 1,2-Diphenyl-1,2-ethanediol | 0.89 | 3.26 | 4.38 |
| Chlorprophenpyridamine | 1.03 | 1.61 | 1.45 | |
| Furoin | 1.00 | 1.41 | 1.32 | |
| Amlodipine | 1.07 | 1.34 | 1.39 | |
| Ketoprofen | 1.12 | 1.53 | 1.24 | |
| Flurbiprofen | 1.09 | 1.61 | 1.35 | |
| MOF-26 | 1,2-Diphenyl-1,2-ethanediol | 0.89 | 1.23 | 1.21 |
| 3-Benzyloxy-1,2-propanediol | 0.78 | 1.69 | 1.37 | |
| Furoin | 1.49 | 1.95 | 1.30 | |
| Benzoin | 0.86 | 1.50 | 1.33 | |
| MOF-27 | 1,2-Diphenyl-1,2-ethanediol | 1.08 | 2.21 | 3.12 |
| 3-Benzyloxy-1,2-propanediol | 1.20 | 3.23 | 4.25 | |
| Alprenolol | 0.87 | 1.35 | 0.63 | |
| Pindolol | 0.79 | 1.02 | 0.53 | |
| N-(3,5-Dinitrobenzoyl)leucine | 1.00 | 1.14 | 0.96 | |
| Warfarin sodium | 1.05 | 1.03 | 0.64 | |
| α-Methylbenzylamine | 1.03 | 2.00 | 2.35 | |
| Chlorprophenpyridamine | 1.26 | 2.17 | 2.07 |
Another method of preparing a MOF chiral column in HPLC is to synthesize a CMOF composite material with silica and then fill the stainless-steel column. Tanaka's group made a great contribution to this method. MOFs 29–35 were employed as chiral selectors for HPLC enantioseparation of sulfoxides (3–25), sec-alcohols (26–47), amide compounds (48–56) and benzoin derivatives (57–62). The structure of the compound is shown in Fig. 19.
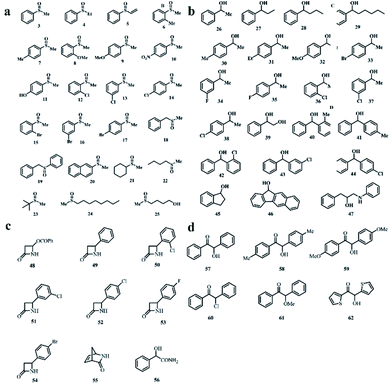 | ||
| Fig. 19 Chemical structures of selected sulfoxides (a), sec-alcohols (b), amide compounds (c), benzoin (d). Reproduced with permission from ref. 109, 110 and 112. | ||
In 2012, Tanaka and co-workers109 synthesized an (R)-CuMOF 29-silica composite from a mixture of (R)-1, Cu(NO3)2, and monodispersed spherical silica gel (Daisogel SP-120-7P; particle size, 7 μm) in DMF under solvothermal conditions. A suspension of (R)-CuMOF 29-silica composite in hexane/i-PrOH was slurry-packed into a stainless-steel column. The performance of the column packed with (R)-CuMOF 29 composite was evaluated for enantiomeric separation of various sulfoxides (2–17) by HPLC. Two eluents were examined: hexane/EtOH (eluent I) and hexane/i-PrOH (eluent II). Nine (3, 4, 5, 6, 7, 9, 13, 10, and 14) sulfoxides were completely separated into their corresponding enantiomers using eluent I as the mobile phase, while 19 and 21 were efficiently resolved using less polar eluent II. They found that the (S)-isomer of the sulfoxides was eluted first, followed by the peak for the (R)-isomer in all cases. This suggested the inclusion of the (R)-enantiomer in the cavity of (R)-MOF, which was likely to have been facilitated by intermolecular hydrogen-bonding interactions between the phenolic OH group of (R)-MOF and the O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S group of the sulfoxide (Fig. 20b). For 8 and 12, polar substituents (Cl and OMe) at the ortho-position of the phenyl group disturbed this hydrogen-bond formation and led to poor enantioselectivity.
S group of the sulfoxide (Fig. 20b). For 8 and 12, polar substituents (Cl and OMe) at the ortho-position of the phenyl group disturbed this hydrogen-bond formation and led to poor enantioselectivity.
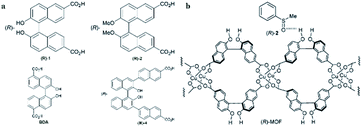 | ||
| Fig. 20 Chemical structures of (R)-1, (R)-2, BDA and (R)-4 (a). A plausible mechanism of chiral recognition (b). Reproduced from ref. 109–111 and 113 with permission from The Royal Society of Chemistry. | ||
In 2016, they110 used (R)-1 and (R)-2 as starting materials to prepare three homochiral MOFs which were further used as CSPs for HPLC. Crystals of (R)-CuMOF-30, (R)-ZnMOF-31, and (R)-CuMOF-32 were prepared using a slightly modified version of the method previously reported by Lin et al.111 Single crystal X-ray analysis revealed that (R)-ZnMOF-31 existed as [Zn[(R)-1]2(NMF)2] (Fig. 21d) and (R)-CuMOF-32 was formulated as [Cu2[(R)-2]4(H2O)2]·DMA (Fig. 21b). Then they successfully prepared a series of (R)-CuMOF-30-silica, (R)-ZnMOF-31-silica and (R)-CuMOF-32-silica composites. The packed columns for HPLC were prepared by loading the suspension of MOF-silica composite in hexane/i-PrOH into a stainless-steel column. Various sulfoxide racemates, sec-alcohols and lactams were selected as test analytes. Conversely, the (R)-CuMOF-32 column did not show any resolution, except for sulfoxide 4 (Table 5). They thought this might be due to the lack of hydrogen bonding between guest molecules and (R)-CuMOF-32, which has no hydroxyl group. For the enantiomeric separation of sec-alcohols, poor separation results were obtained on (R)-ZnMOF-31 and (R)-CuMOF-32 columns (Table 5). For the resolution of lactams (48–54), (R)-ZnMOF-31 had higher Rs values for compounds 49 and 51 than those for (R)-CuMOF-30. (R)-CuMOF-32 showed poor enantioselectivity, except for β-lactam 48 (Table 5). The poor enantioselectivity on (R)-CuMOF-32 may be because of the small entrance to the channel of (R)-CuMOF-32. In the same year, they112 reported two novel homochiral pillared MOFs: (R)-MOF-33 {[Zn(BDA)(bpe)]·2DMA} and (R)-MOF-34 {[Zn(BDA)(bpa)]·DMA}. The columns were prepared by the method mentioned above. The racemates of sulfoxides, sec-alcohols and flavanones were partially separated on both MOF 33 and MOF 34. (R)-MOF-33 exhibited more efficient chiral recognition for enantiomeric separation than (R)-MOF-34 in all cases tested. Because the crystal structure of (R)-MOF-33 possessed a large 13.7 Å by 15.0 Å pore (Fig. 21e), while that of (R)-MOF-34 was a smaller hexagonal nanotube with a diameter of 7 Å (Fig. 21f). Therefore, they think that the stereoselectivity was mostly caused by the interaction between the racemates and the inner pore space of (R)-MOF-33 and (R)-MOF-34, and that (R)-MOF-34 possessed the most appropriate size and steric fit for weak interactions such as dispersion, dipole–dipole, and hydrogen bonding.
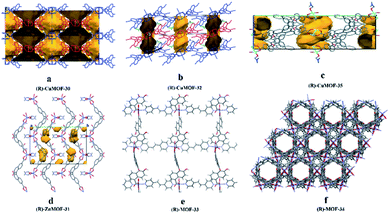 | ||
| Fig. 21 The crystal structures of MOF 30–MOF 35: (R)-CuMOF-30 (a), (R)-CuMOF-32 (b), (R)-CuMOF-35 (c), (R)-ZnMOF-31 (d), (R)-MOF-33 (e), (R)-MOF-34 (f). Reproduced from ref. 109–111 and 113 with permission from The Royal Society of Chemistry. | ||
| Compounds | (R)-CuMOF-30 | (R)-ZnMOF-31 | (R)-CuMOF-32 | |||
|---|---|---|---|---|---|---|
| α | R s | α | R s | α | R s | |
| 3 | 3.99 | 1.33 | 1.93 | 0.54 | 1.00 | — |
| 4 | 2.80 | 0.84 | 1.90 | 0.64 | 1.45 | 0.11 |
| 5 | 2.39 | 0.87 | 2.19 | 0.46 | 1.00 | — |
| 6 | 1.47 | 0.65 | 3.17 | 0.75 | 1.00 | — |
| 7 | 2.16 | 1.01 | 2.45 | 0.77 | 1.00 | — |
| 8 | 1.31 | 0.36 | 1.00 | — | 1.00 | — |
| 9 | 1.43 | 0.78 | 2.15 | 0.59 | 1.00 | — |
| 10 | 1.63 | 1.07 | 1.47 | 0.43 | 1.00 | — |
| 11 | 1.40 | 0.33 | 4.13 | 0.54 | 1.00 | — |
| 12 | 1.09 | 0.06 | 1.00 | — | 1.00 | — |
| 13 | 1.95 | 1.04 | 1.00 | — | 1.00 | — |
| 14 | 1.90 | 0.79 | 2.48 | 0.55 | 1.00 | — |
| 15 | 1.00 | — | 1.00 | — | 1.00 | — |
| 16 | 2.49 | 0.68 | 1.00 | — | 1.00 | — |
| 17 | 3.03 | 0.94 | 1.91 | 0.36 | 1.00 | — |
| 18 | 2.01 | 0.84 | 1.63 | 0.59 | 1.00 | — |
| 19 | 1.77 | 0.93 | 1.00 | — | 1.00 | — |
| 20 | 1.00 | — | 1.00 | — | 1.00 | — |
| 21 | 2.23 | 1.36 | 1.00 | — | 1.00 | — |
| 22 | 1.20 | 0.39 | 1.00 | — | 1.00 | — |
| 23 | 1.02 | 0.18 | 1.00 | — | 1.00 | — |
| 24 | 1.27 | 0.31 | 1.00 | — | 1.00 | — |
| 26 | 1.31 | 0.72 | 1.00 | — | 1.00 | — |
| 27 | 1.95 | 1.38 | 1.00 | — | 1.00 | — |
| 28 | 1.44 | 0.72 | 1.00 | — | 1.00 | — |
| 29 | 1.17 | 0.31 | 1.00 | — | 1.00 | — |
| 30 | 1.00 | — | 1.00 | — | 1.00 | — |
| 31 | 1.36 | 0.48 | 1.00 | — | 1.00 | — |
| 32 | 1.00 | — | 1.00 | — | 1.00 | — |
| 33 | 1.00 | — | 1.00 | — | 1.00 | — |
| 34 | 1.15 | 0.31 | 1.00 | — | 1.00 | — |
| 35 | 1.00 | — | 1.00 | — | 1.00 | — |
| 36 | 1.00 | — | 1.00 | — | 1.00 | — |
| 37 | 1.27 | 0.56 | 1.00 | — | 1.00 | — |
| 38 | 1.00 | — | 1.00 | — | 1.00 | — |
| 39 | 1.60 | 0.59 | 1.00 | — | 1.00 | — |
| 48 | 2.96 | 1.64 | 1.00 | — | 1.74 | 0.14 |
| 49 | 1.11 | 0.33 | 1.24 | 0.15 | 1.00 | — |
| 50 | 1.00 | — | 1.00 | — | 1.00 | — |
| 51 | 1.00 | — | 1.23 | 0.25 | 1.00 | — |
| 52 | 3.54 | 1.18 | 1.00 | — | 1.00 | — |
| 53 | 1.27 | 0.28 | 1.00 | — | 1.00 | — |
| 54 | 5.56 | 1.28 | 1.00 | — | 1.00 | — |
In 2019, Tanaka and co-workers113 reported the design and synthesis of the novel chiral (R)-CuMOF-35. It was synthesized by the solvothermal reaction of chiral organic linker (R)-4 (Fig. 20) and Cu(NO3)2·3H2O in a mixed solvent (DMF-H2O) (Fig. 21c). (R)-CuMOF-35 has a larger molecular cavity than (R)-CuMOF-30 because of the extended side chain of the chiral ligand (R)-4. A comparison of the resolving ability of the (R)-CuMOF-35 column with the (R)-CuMOF-30 column was carried out (Table 6). Only eight of the racemates could be separated on the (R)-CuMOF-30 column. Furthermore, the (R)-CuMOF-35 column gave higher enantioselectivity for all the racemates tested. The key point was a rational molecular design that provided a CMOF material with a large and favorable pore architecture (endless channels) suitable for efficient chiral recognition and separation.
| Entry | Racemates | (R)-CuMOF-30 | (R)-CuMOF-35 | ||
|---|---|---|---|---|---|
| α | R s | α | R s | ||
| 1 | 1-Phenylethanol | 1.32 | 0.65 | 2.89 | 2.03 |
| 2 | 3-Phenoxy-1,2-propanediol | 1.00 | — | 2.35 | 1.33 |
| 3 | Methyl phenyl sulfoxide | 4.40 | 1.50 | 12.8 | 1.58 |
| 4 | Ethyl phenyl sulfoxide | 1.92 | 1.03 | 11.6 | 1.40 |
| 5 | Vinyl phenyl sulfoxide | 1.65 | 0.92 | 13.2 | 1.29 |
| 6 | Styrene oxide | 1.00 | — | 1.79 | 0.95 |
| 7 | Phenyl glycidyl ether | 1.00 | — | 2.01 | 1.36 |
| 8 | N-Glycidylphthalimide | 2.18 | 0.86 | 2.34 | 1.85 |
| 9 | trans-Stilbene oxide | 2.76 | 1.03 | 2.91 | 1.00 |
| 10 | γ-Phenyl-γ-butyrolactone | 1.00 | — | 4.11 | 2.23 |
| 11 | 4-Phenyl-1,3-dioxolan-2-one | 1.13 | 0.42 | 2.15 | 2.05 |
| 12 | 4-Phenyl-2-oxazolidinone | 1.48 | 0.89 | 2.00 | 1.37 |
Silica microspheres are the preferred supporting material in HPLC. The third strategy to overcome the above-mentioned issues is to prepare uniform silica-based CMOF core–shell composites as stationary phases via the immobilization method for HPLC separations. In 2020, Yuan and co-workers114 prepared a homochiral D-His-ZIF-8@SiO2 composite by growing of D-His-ZIF-8 (MOF 36) on the carboxylic-functionalized SiO2 microspheres via a simple one-pot synthesis approach. MOF 36 and MOF-36@SiO2 core–shell microsphere composites were synthesized according to the method reported by Song et al.115 (Fig. 22). The MOF-36@SiO2-packed column exhibited low column backpressure (9–10 bar) for HPLC enantioseparation and eighteen racemic compounds, including alcohols, phenols, amines, ketones, and organic acids, were separated on this column. Its good separation performance is related to the chiral microenvironment of MOF 36. In addition, the chiral resolution process maybe mainly originates from the hydrogen-bonding interactions between chiral molecules and MOF 36. It is easy for the N or H atoms of the amino group in the MOF 36 skeleton to form hydrogen-bonding interactions due to more D-histidine groups being exposed on the MOF-36@SiO2 surface. The results indicated that the fabrication of CMOF@SiO2 core–shell microsphere composites is an effective strategy to improve their chromatographic separation performance.
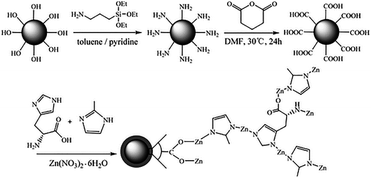 | ||
| Fig. 22 Scheme for the preparation of MOF-36@SiO2 core–shell microspheres. Reproduced with permission from ref. 115. | ||
It is worth noting that Yuan and co-workers116 immobilized a homochiral MOF on silica gel by interfacial polymerization (IP) for HPLC enantioseparation in 2018. IP is the process of dispersing two highly reactive monomers in two mutually incompatible phases (aqueous phase and organic phase) to form a polymer.117 A homochiral lanthanide porous MOF [Nd3(D-cam)8(H2O)4Cl]n (MOF 37) was synthesized (Fig. 23b) according to Liang et al.118 Then, CSP-1 (CSP-1: 1-PIP-TMC-SiO2) and CSP-2 (CSP-2: 1-MPD-TMC-SiO2) were prepared via interface polymerization (Fig. 23a). For comparison, CSP-3 and CSP-4 (CSP-3: MOF 37, CSP-4: MOF 37-SiO2) were prepared by mixing pure MOF 37 and MOF 37 with silica gel, respectively. CSP-1 was dispersed in a mixture of n-hexane/isopropanol for 5 min. The suspension was then packed downward into an empty stainless-steel column using n-hexane/isopropanol as the displacement liquid. CSP-2, CSP-3 and CSP-4 packed columns were prepared by the same procedures. In order to investigate the chiral separation ability of CSP-1 and CSP-2 for racemes, a series of chiral compounds were used to evaluate their separation performance (Fig. 24). The CSP-1 packed column showed good resolution for the separation of enantiomers. Seventeen racemates were well separated, whereas thirteen racemates were separated on the CSP-2 packed column. In contrast, only five and eight racemates were separated on CSP-3 and CSP-4, respectively (Table 7). The low column efficiency of the CSP-3 and CSP-4 packed columns may be owing to the irregular shape and nonuniform particle size of the stationary phases. CSP-1 and CSP-2 were prepared via IP to immobilize MOF 37 on the surface of silica gel to form homogeneous size particles. The results further demonstrated that IP immobilization of MOF 37 on silica gel was practicable and highly efficient for the preparation of novel stationary phases for HPLC enantioseparations.
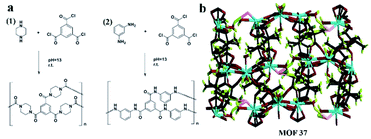 | ||
| Fig. 23 Schematic demonstration of interfacial polymerization: (1) PIP and TMC, (2) MPD and TMC (a). The 3D structure of MOF 37 (b). Reproduced with permission from ref. 116. | ||
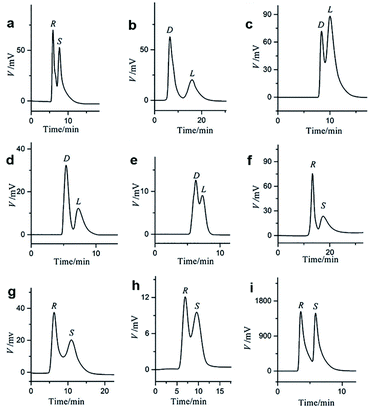 | ||
| Fig. 24 HPLC chromatograms on the four CSP packed columns for the separation of racemates of furoin (a), threonine (b), glutamic acid (c), leucine (d) on CSP-1; proline (e) and mandelic acid (f) on CSP-2; warfarin (g) and naproxen (h) on CSP-3; Tröger's (i) base on CSP-4. Reproduced with permission from ref. 116. | ||
| Racemates | CSP-1 | CSP-2 | CSP-3 | CSP-4 | ||||
|---|---|---|---|---|---|---|---|---|
| α | R s | α | R s | α | R s | α | R s | |
| Ofloxacin | 1.78 | 2.36 | — | — | 1.71 | 0.40 | — | — |
| Warfarin | 1.53 | 1.26 | 1.37 | 0.49 | 2.22 | 1.42 | 1.14 | 0.02 |
| Mandelic acid | — | — | 2.29 | 1.17 | 1.67 | 0.62 | 3.40 | 1.01 |
| Ketoprofen | — | — | 1.54 | 0.56 | 2.12 | 1.22 | — | — |
| Naproxen | 1.60 | 1.04 | 1.57 | 0.41 | 1.92 | 1.67 | — | — |
| Hydrobenzoin | — | — | — | — | — | — | 1.53 | 0.72 |
| Tröger's base | — | — | — | — | — | — | 2.40 | 1.28 |
| Ibuprofen | — | — | — | — | — | — | 3.54 | 2.03 |
| Furoin | 1.55 | 1.35 | — | — | — | — | — | — |
| Benzoin | 1.63 | 0.57 | — | — | — | — | — | — |
| DNB-leucine | — | — | 1.15 | 1.04 | — | — | — | — |
| Flurbiprofen | — | — | 1.12 | 0.41 | — | — | — | — |
| Alprenolol | — | — | 1.58 | 0.55 | — | — | — | — |
| Phenylalanine | 2.24 | 1.73 | — | — | — | — | 1.53 | 0.39 |
| Glutamic acid | 1.90 | 1.77 | — | — | — | — | 1.70 | 0.78 |
| Tryptophan | — | — | — | — | — | — | 1.72 | 0.79 |
| Threonine | 1.68 | 2.08 | — | — | — | — | — | — |
| Tyrosine | 2.16 | 1.64 | — | — | — | — | — | — |
| Alanine | 0.80 | 2.54 | 2.09 | 1.64 | — | — | — | — |
| Cysteine | 2.46 | 1.91 | 2.34 | 2.33 | — | — | — | — |
| Aspartic acid | 1.83 | 1.04 | 1.27 | 0.25 | — | — | — | — |
| Valine | 1.30 | 0.29 | 2.08 | 1.43 | — | — | — | — |
| Histidine | 1.56 | 0.42 | 2.11 | 1.74 | — | — | — | — |
| Leucine | 1.88 | 0.99 | — | — | — | — | — | — |
| Serine | 1.80 | 1.03 | — | — | — | — | — | — |
| Isoleucine | 2.28 | 0.89 | — | — | — | — | — | — |
| Proline | — | — | 1.87 | 2.40 | — | — | — | — |
Ideally, the synthetic MOF has a uniform particle size and can be slurry-packed into an empty stainless-steel column without manual grinding to obtain a MOF column. In 2019, Nieves Corella-Ochoa and co-workers119 reported the first success of this strategy by using the ligand S-HTA derived from L-histidine. It self-assembles with Cu(II) cations in water to build a porous 3D homochiral MOF [Cu(H2O)2(s-TA)2]·6H2O (MOF 38) (Fig. 25a). This novel MOF 38 is highly porous and stable upon complete solvent loss, as well as in water and polar or nonpolar solvents thanks to its robust porous structure with helicoidal 1-nm-wide channels running in three directions (cubic structure). They successfully achieved HPLC separation of a variety of racemic mixtures, including trans-2,3-diphenyloxirane, 1-phenylethan-1-ol, benzoin or flavanone with either polar (acetonitrile, alcohols) or nonpolar (hexanes) solvent conditions. These results ultimately demonstrate the potential versatility of MOF 38 to optimize chromatographic separation conditions for the direct HPLC separation of structurally diverse chiral small molecules.
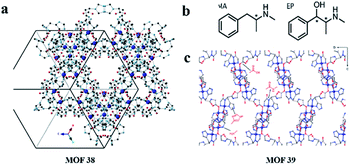 | ||
| Fig. 25 Structures of MOF 38, MOF 39 (a and c), MA and EP (b). Reproduced with permission from ref. 119 and 125. | ||
SPE is a method of sample preconcentration technique that can concentrate and purify analytes from complex samples. Owing to its advantages of simplicity, flexibility, economy, high speed, wide separation profile, low organic solvent consumption and environmental friendliness,120 SPE has been widely applied in the analysis of heavy metal ions,121 herbicides,122 and pesticides.123 Since the adsorbent plays an important role in SPE, the development of novel adsorbents for SPE is of great importance to achieve high extraction efficiency and selectivity. MOF is an adsorbent that can be used for SPE.
In 2016, Yuan and co-workers124 first reported on the exploration of MOFs as sorbents in SPE used for the fast analysis of the ee of chiral compounds. They presented a new method for high-throughput screening of the ee values of (±)-1,1′-bi-2-naphthol using SPE. The as-synthesized crystals of MOF 8 were milled due to their large size and selected with the help of an ethanol suspension followed by packing into each empty cartridge. Under optimal conditions, the ee can be rapidly quantified using a standard curve. This method was successfully applied to the analysis of (±)-1,1′-bi-2-naphthol with a 5% error. In 2017, Martí-Gastaldo and co-workers125 reported the separation ability of a chiral Cu(II) 3D MOF based on the tripeptide Gly-L-His-Gly (GHG). Cu(GHG) (MOF 39) was synthesized by slow diffusion of glycyl-L-histidylglycine and Cu(II) acetate to produce prismatic, micrometric blue crystals by following a synthetic procedure.126 MOF 39 crystallizes to produce an open 3D framework, built from the interconnection of 4-fold helicoidal Cu–peptide–Cu chains by 2-carboxylate bridges in C-terminal Gly (Fig. 25c). They firstly simulated the adsorption of the two enantiomers of metamphetamine (MA) and ephedrine (EP) by Monte Carlo (MC) simulations. MC simulations suggest that MOF 39 might display stereoselectivity, in particular for EP enantiomers, associated with the control of non-covalent interactions over their binding geometries. With promising results from the simulation, they attempted actual separation experiments from solutions of the racemates. They studied the ability of MOF 39 to selectively recognize enantiomers from solution by soaking fresh solid MOF materials in racemic mixtures of MA and EP. Enantioselective recognition was evaluated chromatographically as a function of contact time. As a result, 30 ± 3% of (+)-MA and 37 ± 3% of (+)-EP were preferentially adsorbed after 4 and 2 h, respectively, and the adsorption of (−)-MA and (−)-EP was negligible. Experimental results agreed well with the MC simulations. And they supported the preferential interaction of (+)-enantiomers in the chiral pocket linked to specific conformations that enable H-bond formation with the histidine side chain. And MOF 39 was capable of separating >50% of (+)-ephedrine from a racemic mixture within only 4 min when used as a chiral SPE cartridge.
Graphene-oxide (GO) can improve the physico-chemical properties of MOFs, such as their structure, morphology and size by increasing the dispersion forces within the MOFs and inhibiting their aggregation. Based on the synergistic effect of MOFs and GO, GO@MOFs exhibit superior dispersibility, thermal stability, solvent resistance and so on. MGO (magnetic graphene-oxide)-MOF functional materials combine the superior properties of each component and make up for the deficiencies of each single component. Therefore, they show excellent physicochemical properties and broad application prospects.
In 2018, Zhang and co-workers127 prepared a novel magnetic graphene oxide-MOF [Zn2(BTC)(NO3)(DMA)3]n (MOF 40) as a sorbent for the fast enantioselective capture of chiral drug intermediates. First, they prepared Fe3O4@SiO2-NH2-GO (MGO). Then an aliquot of MGO was added according to the yield of ZnBND and treated to obtain the MGO–MOF 40 composite. Enantioseparation of 2,2′-furoin on MGO–MOF 40 was evaluated by dispersive magnetic nanoparticle solid phase extraction (d-MNSPE) and the enantiomeric composition of the separated enantiomer was analyzed by HPLC. Under optimal conditions, for 2,2′-furoin, the whole enantioselective adsorption process could be completed in no more than 4 min with an ee value as high as 85%, which was much more efficient than conventional approaches that require a very long time. Yuan and co-workers also reported that MOF 3 and MOF 8 acted as CSPs for LC enantioseparation of 2,2′-furoin. However, complexity and a lengthy procedure time were found with their methods. An ee value of 66% was achieved for benzoin within 6 min. In spite of being reused six times, the chiral selection performance and stability of the sorbents remained comparatively steady. At present, research on the detailed recognition mechanism is in progress.
3 COFs
A COF material is a kind of regular structured crystalline polymer material formed by organic building units through covalent bonds.128 Benefitting from high crystallinity,129 low density and high specific surface area,130 COFs have broad application prospects in gas storage,131 separation, catalysis,132,133 sensing,134 optoelectronics,135 drug delivery136 and energy storage.137 COF materials can be divided into two categories: 2D COFs and 3D COFs, according to the extended dimensions of the framework. At present, most COFs reported in the literature are 2D materials, whose topological structures include hcb (honeycomb) and sql (square lattice). Currently, there are very few examples of 3D COFs, and their topology is mainly dia (diamond).In recent years, chiral porous materials, especially chiral COF materials (CCOFs), have attracted the interest of chemists due to their excellent properties. However, the main obstacle to the development and application of CCOF materials lies in how to construct chiral centers in materials while realizing high crystallinity and porosity. So far, only a few reports have successfully realized the design and synthesis of CCOFs. Different COF construction primitives have been designed and synthesized. Then various COF materials have been constructed by chemical bonds, including B–O, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, C
N, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N(Ar), C
N(Ar), C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, C–N and B
C, C–N and B![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N,138–140 between different elements. So the structural diversity of COF materials comes from the fact that they can not only arbitrarily regulate the construction primitives, but also adopt a variety of connection modes. The current synthesis methods of CCOF materials can be divided into two categories: bottom-up strategy and post-synthetic modification. The bottom-up strategy is the design and synthesis of COF monomers: functional groups are introduced into the construction primitives and then the synthesis of COF materials is carried out. The post-synthetic modification is to first synthesize the basic framework of the COF material, and then load the functional metal particles or organic small molecules into the COF material framework. Most of the CCOF materials are synthesized through a bottom-up strategy.
N,138–140 between different elements. So the structural diversity of COF materials comes from the fact that they can not only arbitrarily regulate the construction primitives, but also adopt a variety of connection modes. The current synthesis methods of CCOF materials can be divided into two categories: bottom-up strategy and post-synthetic modification. The bottom-up strategy is the design and synthesis of COF monomers: functional groups are introduced into the construction primitives and then the synthesis of COF materials is carried out. The post-synthetic modification is to first synthesize the basic framework of the COF material, and then load the functional metal particles or organic small molecules into the COF material framework. Most of the CCOF materials are synthesized through a bottom-up strategy.
An important application of CCOF materials is chiral separation. An efficient CSP has the basic properties of good porosity permeability, low mass transfer resistance, good stability and reproducibility, etc. CCOF materials have all the corresponding properties mentioned above, such as large specific surface area, neat structure and high pore volume, good solvent and thermal stability. Therefore, CCOF is a promising CSP for chromatography.
3.1 Enantioseparation on CCOFs
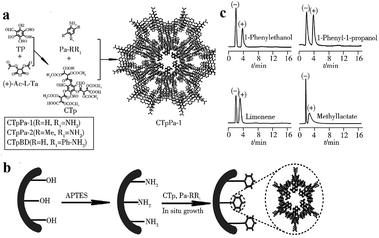 | ||
| Fig. 26 Synthesis of chiral CCOFs (a), preparation process of chiral CCOFs capillary column (b) and separation chromatogram of chiral isomers on CTpPa-1 column (c). Reproduced with permission from ref. 141. | ||
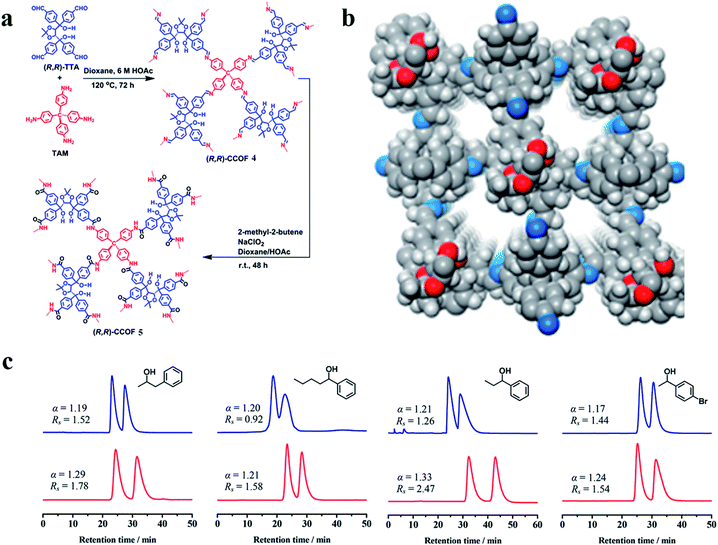 | ||
| Fig. 27 Synthesis of the CCOFs (a). Structural representations of COF 4 (b). HPLC separation of racemic 1-phenyl-2-propanol, 1-phenyl-1-pentanol, 1-phenyl-1-propanol and 1-(4-bromophenyl)ethanol on the COF 4 (blue line) and 5 (red line) packed columns, respectively (c). Reproduced with permission from ref. 142. | ||
In the same year, Chen and co-workers143 obtained CCOFs (COF 6) by immobilizing a series of biomolecules with chiral centers (lysozyme, peptide, lysine) into the achiral polyimide COFs by covalent bonding (Fig. 28a and b). This new COF 6 material can not only inherit the inherent chirality and activity of biomolecules, but also provide protection and dispersion for biomolecules. COF 6 can be used as a new type of HPLC CSP directly filling in the empty string. The afforded COF 6 exhibited versatile and high chiral separation efficiency and demonstrated good reusability and reproducibility due to the protection of achiral polyimide COFs (Fig. 28c). Surface-enhanced Raman scattering studies unveiled that the different interactions between the lysozyme secondary structure and with ∼5 μm of silicon dioxide into a stainless-steel hollow column to prepare an HPLC column. Various racemates, such as alcohols, sulfoxides, carboxylic acids and esters, can be baseline or partially resolved on the two COF-based CSPs. COF 5 showed improved resolution ability compared to COF 4 under similar conditions (Fig. 27c). They inferred that the resolution abilities of the CCOFs are likely to result from a combination of the chiral channels with the amphiphilic channel interior lined with chiral hydroxyl groups, which may govern host–alcohol interactions, hence favoring the enantioselectivity during the adsorption process racemates account for the observed chiral separation capacity.
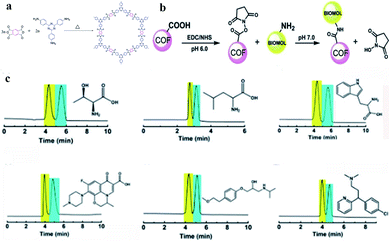 | ||
| Fig. 28 Synthesis reaction of COF 6 (a). Illustration of the covalent strategy to bond various biomolecules with COFs (b). Enantiomeric separation chromatograms (c). Reproduced with permission from ref. 143. | ||
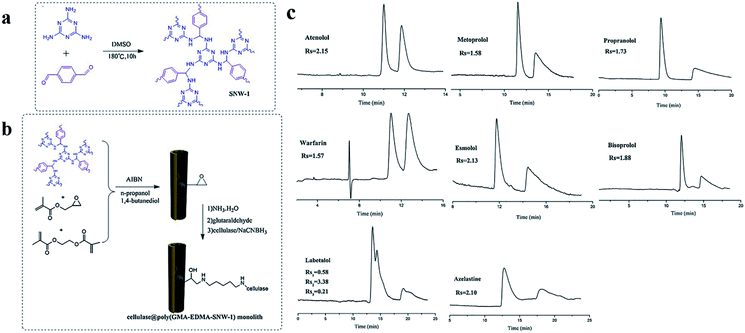 | ||
| Fig. 29 General scheme for the preparation of COF 7 (a) and fabrication of cellulase@poly(GMA-EDMA-COF 7) capillary monolithic column (b). Resolution of eight pairs of enantiomers on the cellulase@poly(GMA-EDMA-COF 7) monolith (c). Reproduced with permission from ref. 144. | ||
β-Cyclodextrin (β-CD) and its derivatives have been widely applied for molecular specific recognition. The integration of CD units into a 3D crystalline structure has encouraged a broad interest. In 2018, Feng146 and co-workers synthesized β-CD COF (COF 8) through acetic acid catalyzed polycondensation between heptakis(6-amino-6-deoxy)-β-CD (Am7CD) and terephthalaldehyde (TPA) in green solvents for the first time. Ji147 and co-workers prepared a β-CD COF modified open-tubular column by a photopolymerization method for CEC enantioseparation (Fig. 30b). Baseline separation for six pharmaceutical racemates was achieved on the β-CD COF-GMA@capillary (Fig. 30c). The results suggested that the formation of the COF structure contributes a lot to the remarkable separation performance. In addition, the hydrophobic interaction between analytes and stationary phases is the primary force for separation.
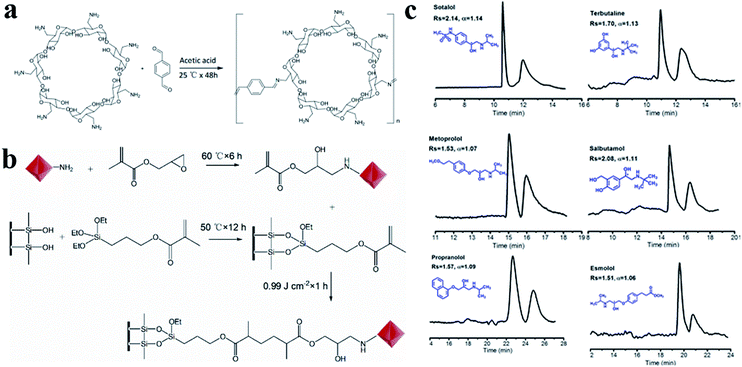 | ||
| Fig. 30 Schematic diagram of preparation of β-CD COF (a), β-CD-COF-modified open-tubular column (b). Enantiomeric separation chromatograms on β-CD-COF-GMA@capillary (c). Reproduced with permission from ref. 147. | ||
In 2020, Wang148 and co-workers reported a bottom-up strategy for the construction of MDI-β-CD modified TpPa-1 COF (COF 9) using MDI-β-CD as the chiral selector and imine-based TpPa-1 COF as the matrix (Fig. 31a). A coated OT column was prepared using COF 9 as a CSP by an in situ growth approach (Fig. 31b). The enantiomers of four amino acids (tryptophan, tyrosine, arginine, and lysine) and four β-blockers (atenolol, labetalol, sotalol, and celiprolol) were separated (Fig. 31c). The molecular stereo configuration was found to affect the resolution, where small steric hindrance and strong π–π interactions resulted in high resolution.
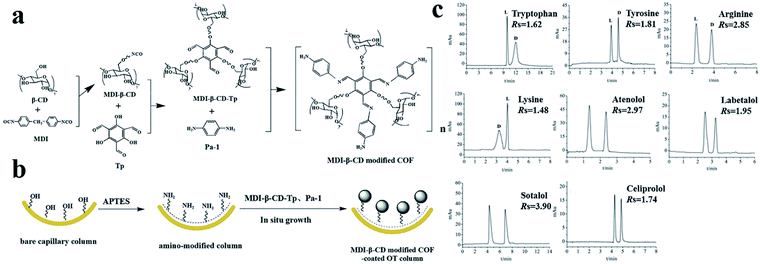 | ||
| Fig. 31 Schematic diagram of preparation of COF 9 (a), COF-9-coated OT column (b). Chiral separation chromatograms of analytes (c). Reproduced with permission from ref. 148. | ||
4 The mechanism of chiral recognition
From a mechanistic point of view, the separation performance of POFs mostly depends on their pore size, channel shape and molecular interactions. The perfect match in size and shape as well as the possible interactions between the analyte and the chiral porous organic frameworks (CPOFs) channels are the main interactive forces. In a study by Tang and co-workers,149 the minimum kinetic diameters of the guest molecules separated were much smaller than that of the CMOF aperture size (approximately 9.8 Å). For larger molecules like ketoprofen (9.4 Å) or naproxen (9.7 Å), the enantiomer pairs were co-eluted with poorer retention than those resolved enantiomers, indicating they did not enter the chiral channels. A similar situation was also observed in Yan's study,70 which indicates that chiral separation mainly occurs inside the pores of CPOFs. Poor enantioseparation results may be ascribed to a size mismatch between the analytes and CPOF pores.101,102,110,112,113 The second important factor affecting the separation is the shape or crystal structure of the CPOFs. For example, the unique DNA-like double helix structure of MOF 12,77 the 3D square-shaped cube structure of MOF 16 (ref. 87) and the 2D chiral coordination layer structure of MOF 20 (ref. 99) largely determine their enantiorecognition ability and some computational results also supported this point of view.150–152 In addition, diverse molecular interactions such as dipole–dipole, hydrogen bonding, π–π interaction, hydrophobic–hydrophobic interaction and weak ligand coordination coming from the CSPs or mobile phase may facilitate chiral recognition and enantioselectivity. To comprehensively elucidate the host–guest interaction mechanisms between chiral molecules and CPOFs, more powerful tools like synchrotron radiation X-ray diffraction may be employed, except for traditional analytical techniques and computational simulation.5 Conclusions and perspectives
In addition to the advantages of large specific surface area, high stability and adjustable aperture, CPOFs can also be functionalized or combined with other materials to enlarge the separation profile,71–73,83,147,148 which endows them with great potential for chiral separation. However, to achieve greater success, there are still some essential challenges to overcome.Regarding the synthesis strategy, only a few ligands have been used by far, such as tartaric acid, camphor acid, and natural amino acids. CCOFs are still in the initial development stage, and the harsh synthesis conditions limit their rapid development. Even if CPOFs have been applied in chiral chromatography, most of them have unsatisfactory separation efficiency. One possible reason is that the number of CPOFs used for chiral separation is much smaller than the synthesized CPOF families. Many recent reported CPOFs could be evaluated to look for more efficient CPOF CSPs. Another reason may lie in their particle size and shape distribution. Spherical particles with a narrow size distribution are important requirements for chromatographic separation. The CPOFs prepared via a traditional manual pestle crushing process afford poor reproducibility with a broad particle size and shape distribution and the eddy diffusion resulting from crystals' irregular shapes and the wide size distribution of CPOFs greatly lowered the column efficiency. Therefore, CPOFs with uniform particle size and spherical shape could be ideal CSP materials. In addition, since the separation mechanism is still not very clear, researchers should devote more effort to this issue combining experimental and theoretical simulation. Anyway, although CPOFs are still in their infancy for enantiomeric separation, the future opens out a bright prospect for this newly emerging materials system.
Abbreviations
| L | (S)-3-Hydroxy-2-(pyridin-4-ylmethylamino)propanoic acid |
| 1,2-pd | 1,2-Propanediol |
| H3btc | 1,3,5-Isotribenzoic acid |
| py | Pyridine |
| 3-pic | 3-Picoline |
| 1,4-bd | 1,4-Butanediol |
| BMIm | 1-Butyl-3-methylimidazole |
| D-cam | D-Camphoric acid |
| bdc | 1,4-Benzenedicarboxylate |
| tmdpy | 4,4′-Trimethylenedipyridine |
| bpdc | 4,4′-Biphenyldicarboxylate |
| DMA | N,N-dimethylacetamide |
| L-GG | Dipeptide H-Gly-L-Glu |
| H2sala | N-(2-Hydroxybenzyl)-L-alanine |
| LTP | L(−)-Thiazolidine-4-carboxylic acid |
| 4,4′-bpy | 4,4′-Bipyridine |
| L-Tyr | L-Tyrosine acid |
| dabco | 1,4-Dia-zabicyclo[2,2,2]-octane |
| H2bbimb | 1,3-Bis(2-benzimidazol)-benzene |
| DMF | N,N-Dimethylformamide |
| s-nip | (S)-2-(1,8-Naphthalimido)-3-(4-imidazole) propanoate |
| mal | Malic acid |
| BDC | 1,4-Benzenedicarboxylate |
| BTB | Benzene-1,3,5-tribenzoate |
| L-Trp | L-tryptophan acid |
| bpe | 1,2-Bis(4-pyridinyl) ethylene |
| L-His | L-Histidine acid |
| L-Glu | L-Glutamic acid |
| INT | Isonicotinate |
| sdba | 4,4′-Sulfonyldibenzoate |
| DEF | N,N′-Diethylformamide |
| NMF | N-Methylformamide |
| DCM | Dichloromethane |
| (R)-1 | (R)-2,2′-Dihydroxy-1,1′-binaphthalene-6,6′-dicarboxylic acid |
| (R)-2 | (R)-2,2′-Dimethoxy-1,1′-binaphthalene-6,6′-dicarboxylic acid |
| bpa | Transbis(4-pyridyl)ethane |
| H2BDA | (R)-1,1′-Binaphthyl-2,2′-dihydroxy-5,5′-dicarboxylic acid |
| (R)-4 | (R)-3,3′-Bis(6-carboxy-2-naphthyl)-2,2′-dihydroxy-1,1′-binaphthyl |
| S-HTA | (S)-3-(1H-Imidazol-5-yl)-2-(4H-1,2,4-triazol-4-yl)propanoic acid |
| GLYMO | 3-Glycidoxypropyltrimethoxysilane |
| DNB | 2,4-Dinitrobenzene |
| PIP | Piperazine |
| TMC | Trimesoyl chloride |
| MPD | m-Phenylenediamine |
| Tp | 1,3,5-Triformylphloroglucinol |
| (+)-Ac-L-Ta | (+)-Diacetyl-L-tartaric anhydride |
| TAM | Tetra(4-anilyl)methane |
| TADDOL | 1,3-Dioxolane-4,5-dimethanols |
| Pa-1 | 1,4-Phenylenediamine |
| Pa-2 | 2,5-Dimethyl-p-phenylenediamine |
| BD | Benzidine |
| TPA | Terephthalaldehyde |
| Am7CD | Heptakis(6-amino-6-deoxy)-β-CD |
| GMA | Glycidyl methacrylate |
| EDMA | Ethylene dimethacrylate |
| MDI | 4,4′-Diphenylmethane diisocyanate |
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially funded by the National Natural Science Foundation of China (No. 21922409, 21976131) and the Tianjin Research Program of Application Foundation and Advanced Technology (18JCZDJC37500, 17JCYBJC20500).References
- J. Gal, in Differentiation of Enantiomers I, ed. V. Schurig, 2013, vol. 340, pp. 1–20 Search PubMed.
- E. R. Francotte, J. Chromatogr. A, 2001, 906, 379–397 CrossRef CAS.
- H. Lorenz and A. Seidel-Morgenstern, Angew. Chem., Int. Ed., 2014, 53, 1218–1250 CrossRef CAS.
- H. W. Liu, Anal. Bioanal. Chem., 2014, 406, 11–12 CrossRef CAS.
- S. M. Xie and L. M. Yuan, J. Sep. Sci., 2017, 40, 124–137 CrossRef CAS.
- C. Fernandes, M. E. Tiritan and M. Pinto, Chromatographia, 2013, 76, 871–897 CrossRef CAS.
- K. Tanaka, in Comprehensive Chirality, ed. E. M. Carreira and H. Yamamoto, Elsevier, Amsterdam, 2012, pp. 63–90 Search PubMed.
- S. Yamada, C. Hongo, R. Yoshioka and I. Chibata, Agric. Biol. Chem., 1979, 43, 395–396 CAS.
- G. M. Henderson and H. G. Rule, Nature, 1938, 141, 917–918 CrossRef CAS.
- C. E. Dalgliesh, J. Chem. Soc., 1952, 3940–3942 RSC.
- A. Cavazzini, L. Pasti, A. Massi, N. Marchetti and F. Dondi, Anal. Chim. Acta, 2011, 706, 205–222 CrossRef CAS.
- B. Chankvetadze, J. Chromatogr. A, 2012, 1269, 26–51 CrossRef CAS.
- J. Haginaka, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2008, 875, 12–19 CrossRef CAS.
- M. L. Tang, J. Zhang, S. L. Zhuang and W. P. Liu, TrAC, Trends Anal. Chem., 2012, 39, 180–194 CrossRef CAS.
- J. Shen and Y. Okamoto, Chem. Rev., 2016, 116, 1094–1138 CrossRef CAS.
- Y. Okamoto and T. Ikai, Chem. Soc. Rev., 2008, 37, 2593–2608 RSC.
- T. J. Ward and K. D. Ward, Anal. Chem., 2012, 84, 626–635 CrossRef CAS.
- I. Ilisz, R. Berkecz and A. Peter, J. Sep. Sci., 2006, 29, 1305–1321 CrossRef CAS.
- Z. Z. Wang, O. Y. Jin and W. R. G. Baeyens, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2008, 862, 1–14 CrossRef CAS.
- D. A. Tsioupi, R. I. Stefan-van Staden and C. P. Kapnissi-Christodoulou, Electrophoresis, 2013, 34, 178–204 CrossRef CAS.
- J. R. Li, J. Sculley and H. C. Zhou, Chem. Rev., 2012, 112, 869–932 CrossRef CAS.
- O. M. Yaghi and H. L. Li, J. Am. Chem. Soc., 1995, 117, 10401–10402 CrossRef CAS.
- G. Ferey, Chem. Soc. Rev., 2008, 37, 191–214 RSC.
- G. Ferey, in From Zeolites To Porous MOF Materials: The 40th Anniversary of International Zeolite Conference, Proceedings the 15th International Zeolite Conference, ed. R. Xu, Z. Gao, J. Chen and W. Yan, 2007, vol. 170, pp. 66–84 Search PubMed.
- K. Yusuf, A. Aqel and Z. Alothman, J. Chromatogr. A, 2014, 1348, 1–16 CrossRef CAS.
- Z. X. Fei, M. Zhang, J. H. Zhang and L. M. Yuan, Anal. Chim. Acta, 2014, 830, 49–55 CrossRef CAS.
- A. Dhakshinamoorthy and H. Garcia, Chem. Soc. Rev., 2014, 43, 5750–5765 RSC.
- K. Tanaka, K. Sakuragi, H. Ozaki and Y. Takada, Chem. Commun., 2018, 54, 6328–6331 RSC.
- Y. B. He, W. Zhou, G. D. Qian and B. L. Chen, Chem. Soc. Rev., 2014, 43, 5657–5678 RSC.
- M. P. Suh, H. J. Park, T. K. Prasad and D. W. Lim, Chem. Rev., 2012, 112, 782–835 CrossRef CAS.
- L. E. Kreno, K. Leong, O. K. Farha, M. Allendorf, R. P. Van Duyne and J. T. Hupp, Chem. Rev., 2012, 112, 1105–1125 CrossRef CAS.
- S. L. Qiu, M. Xue and G. S. Zhu, Chem. Soc. Rev., 2014, 43, 6116–6140 RSC.
- T. Duerinck and J. F. M. Denayer, Chem. Eng. Sci., 2015, 124, 179–187 CrossRef CAS.
- P. Horcajada, R. Gref, T. Baati, P. K. Allan, G. Maurin, P. Couvreur, G. Ferey, R. E. Morris and C. Serre, Chem. Rev., 2012, 112, 1232–1268 CrossRef CAS.
- J. Q. Sha, X. H. Zhong, L. H. Wu, G. D. Liu and N. Sheng, RSC Adv., 2016, 6, 82977–82983 RSC.
- S. Horike, D. Umeyama and S. Kitagawa, Acc. Chem. Res., 2013, 46, 2376–2384 CrossRef CAS.
- P. Ramaswamy, N. E. Wong and G. K. H. Shimizu, Chem. Soc. Rev., 2014, 43, 5913–5932 RSC.
- M. Yoon, K. Suh, S. Natarajan and K. Kim, Angew. Chem., Int. Ed., 2013, 52, 2688–2700 CrossRef CAS.
- L. Sun, M. G. Campbell and M. Dinca, Angew. Chem., Int. Ed., 2016, 55, 3566–3579 CrossRef CAS.
- V. Stavila, A. A. Talin and M. D. Allendorf, Chem. Soc. Rev., 2014, 43, 5994–6010 RSC.
- H. L. Wang, Q. L. Zhu, R. Q. Zou and Q. Xu, Chem, 2017, 2, 52–80 CAS.
- W. Xia, A. Mahmood, R. Q. Zou and Q. Xu, Energy Environ. Sci., 2015, 8, 1837–1866 RSC.
- M. Yoon, R. Srirambalaji and K. Kim, Chem. Rev., 2012, 112, 1196–1231 CrossRef CAS.
- F. J. Song, C. Wang and W. B. Lin, Chem. Commun., 2011, 47, 8256–8258 RSC.
- S. C. Liu, S. Shinde, J. M. Pan, Y. Ma, Y. S. Yan and G. Q. Pan, Chem. Eng. J., 2017, 324, 216–227 CrossRef CAS.
- X. J. Li, C. L. Chang, X. Wang, Y. Bai and H. W. Liu, Electrophoresis, 2014, 35, 2733–2743 CrossRef CAS.
- P. Peluso, V. Mamane and S. Cossu, J. Chromatogr. A, 2014, 1363, 11–26 CrossRef CAS.
- M. Wang, M. H. Xie, C. D. Wu and Y. G. Wang, Chem. Commun., 2009, 2396–2398 RSC.
- A. P. Katsoulidis, K. S. Park, D. Antypov, C. Marti-Gastaldo, G. J. Miller, J. E. Warren, C. M. Robertson, F. Blanc, G. R. Darling, N. G. Berry, J. A. Purton, D. J. Adams and M. J. Rosseinsky, Angew. Chem., Int. Ed., 2014, 53, 193–198 CrossRef CAS.
- A. Mantion, L. Massuger, P. Rabu, C. Palivan, L. B. McCusker and A. Taubert, J. Am. Chem. Soc., 2008, 130, 2517–2526 CrossRef CAS.
- J. Zhang, E. Chew, S. Chen, J. T. H. Pham and X. Bu, Inorg. Chem., 2008, 47, 3495–3497 CrossRef CAS.
- J. Zhang, R. Liu, P. Y. Feng and X. H. Bu, Angew. Chem., Int. Ed., 2007, 46, 8388–8391 CrossRef CAS.
- H. Y. An, E. B. Wang, D. R. Xiao, Y. G. Li, Z. M. Su and L. Xu, Angew. Chem., Int. Ed., 2006, 45, 904–908 CrossRef CAS.
- C. J. Kepert, T. J. Prior and M. J. Rosseinsky, J. Am. Chem. Soc., 2000, 122, 5158–5168 CrossRef CAS.
- D. Bradshaw, T. J. Prior, E. J. Cussen, J. B. Claridge and M. J. Rosseinsky, J. Am. Chem. Soc., 2004, 126, 6106–6114 CrossRef CAS.
- T. J. Prior and M. J. Rosseinsky, Inorg. Chem., 2003, 42, 1564–1575 CrossRef CAS.
- Z. J. Lin, A. M. Z. Slawin and R. E. Morris, J. Am. Chem. Soc., 2007, 129, 4880–4881 CrossRef CAS.
- K. K. Tanabe and S. M. Cohen, Chem. Soc. Rev., 2011, 40, 498–519 RSC.
- S. J. Garibay, Z. Q. Wang, K. K. Tanabe and S. M. Cohen, Inorg. Chem., 2009, 48, 7341–7349 CrossRef CAS.
- J. Bonnefoy, A. Legrand, E. A. Quadrelli, J. Canivet and D. Farrusseng, J. Am. Chem. Soc., 2015, 137, 9409–9416 CrossRef CAS.
- S. M. Xie, X. H. Zhang, Z. J. Zhang and L. M. Yuan, Anal. Lett., 2013, 46, 753–763 CrossRef CAS.
- L. P. Wang, T. Y. Song, L. L. Huang, J. N. Xu, C. O. Li, C. X. Ji, L. Shan and L. Wang, CrystEngComm, 2011, 13, 4005–4009 RSC.
- S. M. Xie, X. H. Zhang, Z. J. Zhang, M. Zhang, J. Jia and L. M. Yuan, Anal. Bioanal. Chem., 2013, 405, 3407–3412 CrossRef CAS.
- S. M. Xie, X. H. Zhang, B. J. Wang, M. Zhang, J. H. Zhang and L. M. Yuan, Chromatographia, 2014, 77, 1359–1365 CrossRef CAS.
- X. R. Hao, X. L. Wang, C. Qin, Z. M. Su, E. B. Wang, Y. Q. Lan and K. Z. Shao, Chem. Commun., 2007, 4620–4622 RSC.
- S. M. Xie, Z. J. Zhang and L. M. Yuan, Chem. Res. Chin. Univ., 2014, 35, 1652–1657 CAS.
- J. Zhang, S. M. Chen, H. Valle, M. Wong, C. Austria, M. Cruz and X. H. Bu, J. Am. Chem. Soc., 2007, 129, 14168–14169 CrossRef CAS.
- L. Li, S. M. Xie, J. H. Zhang, L. Chen, P. J. Zhu and L. M. Yuan, Chem. Res. Chin. Univ., 2017, 33, 24–30 CrossRef CAS.
- K. C. Stylianou, L. Gomez, I. Imaz, C. Verdugo-Escamilla, X. Ribas and D. Maspoch, Chemistry, 2015, 21, 9964–9969 CrossRef CAS.
- W. T. Kou, C. X. Yang and X. P. Yan, J. Mater. Chem. A, 2018, 6, 17861–17866 RSC.
- H. Liu, S. M. Xie, P. Ai, J. H. Zhang, M. Zhang and L. M. Yuan, ChemPlusChem, 2014, 79, 1103–1108 CrossRef CAS.
- S. M. Xie, H. Liu, J. R. Yang, P. Ai and L. M. Yuan, Chin. J. Chromatogr., 2016, 34, 113–118 CrossRef CAS.
- J. R. Yang, S. M. Xie, J. H. Zhang, L. Chen, R. Y. Nong and L. M. Yuan, J. Chromatogr. Sci., 2016, 54, 1467–1474 CAS.
- J. Zhang, Y. G. Yao and X. H. Bu, Chem. Mater., 2007, 19, 5083–5089 CrossRef CAS.
- P. J. Zhu, Y. Tao, J. H. Zhang, M. Zi and L. M. Yuan, Chin. J. Chromatogr., 2016, 34, 1219–1227 CrossRef CAS.
- M. Zhang, X. D. Xue, J. H. Zhang, S. M. Xie, Y. Zhang and L. M. Yuan, Anal. Methods, 2014, 6, 341–346 RSC.
- C. J. Pan, W. J. Lv, X. Y. Niu, G. X. Wang, H. L. Chen and X. G. Chen, J. Chromatogr. A, 2018, 1541, 31–38 CrossRef CAS.
- X. L. Luo, Y. Cao, T. Wang, G. H. Li, J. G. Yon, Y. G. Yang, Z. X. Xu, J. Zhang, Q. S. Huo, Y. L. Liu and M. Eddaoudi, J. Am. Chem. Soc., 2016, 138, 786–789 CrossRef CAS.
- Z. X. Fei, M. Zhang, S. M. Xie and L. M. Yuan, Electrophoresis, 2014, 35, 3541–3548 CrossRef CAS.
- C. J. Pan, W. F. Wang, H. G. Zhang, L. F. Xu and X. G. Chen, J. Chromatogr. A, 2015, 1388, 207–216 CrossRef CAS.
- C. J. Pan, W. F. Wang and X. G. Chen, J. Chromatogr. A, 2016, 1427, 125–133 CrossRef CAS.
- K. S. Park, Z. Ni, A. P. Cote, J. Y. Choi, R. Huang, F. J. Uribe-Romo, H. K. Chae, M. O'Keeffe and O. M. Yaghi, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 10186–10191 CrossRef CAS.
- W. Ding, T. Yu, Y. Du, X. Sun, Z. Feng, S. Zhao, X. Ma, M. Ma and C. Chen, Microchim. Acta, 2020, 187, 51 CrossRef CAS.
- J. C. Ma, N. S. Ye and J. Li, Electrophoresis, 2016, 37, 601–608 CrossRef CAS.
- M. S. Zavakhina, D. G. Samsonenko, A. V. Virovets, D. N. Dybtsev and V. P. Fedin, J. Solid State Chem., 2014, 210, 125–129 CrossRef CAS.
- N. S. Ye, J. C. Ma, J. X. An, J. Li, Z. M. Cai and H. Zong, RSC Adv., 2016, 6, 41587–41593 RSC.
- X. D. Sun, Y. Tao, Y. X. Du, W. Ding, C. Chen and X. F. Ma, Microchim. Acta, 2019, 186, 462 CrossRef.
- S. C. Yang, F. G. Ye, Q. H. Lv, C. Zhang, S. F. Shen and S. L. Zhao, J. Chromatogr. A, 2014, 1360, 143–149 CrossRef CAS.
- S. El-Hankari, J. Huo, A. Ahmed, H. F. Zhang and D. Bradshaw, J. Mater. Chem. A, 2014, 2, 13479–13485 RSC.
- A. S. Munch and F. Mertens, J. Mater. Chem., 2012, 22, 10228–10234 RSC.
- A. Ahmed, M. Forster, R. Clowes, D. Bradshaw, P. Myers and H. F. Zhang, J. Mater. Chem. A, 2013, 1, 3276–3286 RSC.
- T. Bao, J. Zhang, W. P. Zhang and Z. L. Chen, J. Chromatogr. A, 2015, 1381, 239–246 CrossRef CAS.
- C. Prestipino, L. Regli, J. G. Vitillo, F. Bonino, A. Damin, C. Lamberti, A. Zecchina, P. L. Solari, K. O. Kongshaug and S. Bordiga, Chem. Mater., 2006, 18, 1337–1346 CrossRef CAS.
- Q. S. Qu, Y. Si, H. Xuan, K. H. Zhang, X. M. Chen, Y. Ding, S. J. Feng and H. Q. Yu, Microchim. Acta, 2017, 184, 4099–4106 CrossRef CAS.
- M. Padmanaban, P. Muller, C. Lieder, K. Gedrich, R. Grunker, V. Bon, I. Senkovska, S. Baumgartner, S. Opelt, S. Paasch, E. Brunner, F. Glorius, E. Klemm and S. Kaskel, Chem. Commun., 2011, 47, 12089–12091 RSC.
- M. Zhang, J. H. Zhang, Y. Zhang, B. J. Wang, S. M. Xie and L. M. Yuan, J. Chromatogr. A, 2014, 1325, 163–170 CrossRef CAS.
- J. Kong, M. Zhang, A. H. Duan, J. H. Zhang, R. Yang and L. M. Yuan, J. Sep. Sci., 2015, 38, 556–561 CrossRef CAS.
- M. Zhang, Z. J. Pu, X. L. Chen, X. L. Gong, A. X. Zhu and L. M. Yuan, Chem. Commun., 2013, 49, 5201–5203 RSC.
- R. Y. Nong, J. Kong, J. H. Zhang, L. Chen, B. Tang, S. M. Xie and L. M. Yuan, Chem. Res. Chin. Univ., 2016, 37, 19–25 CAS.
- S. Mendiratta, M. Usman, T. T. Luo, B. C. Chang, S. F. Lee, Y. C. Lin and K. L. Lu, Cryst. Growth Des., 2014, 14, 1572–1579 CrossRef CAS.
- C. Hu, L. Li, N. Yang, Z. H. Zhang, S. M. Xie and L. M. Yuan, Acta Chim. Sin., 2016, 74, 819–824 CrossRef CAS.
- S. M. Xie, C. Hu, L. Li, J. H. Zhang, N. Fu, B. J. Wang and L. M. Yuan, Microchem. J., 2018, 139, 487–491 CrossRef CAS.
- J. H. Zhang, R. Y. Nong, S. M. Xie, B. J. Wang, P. Ai and L. M. Yuan, Electrophoresis, 2017, 38, 2513–2520 CrossRef CAS.
- B. Zhou, N. J. O. Silva, F. N. Shi, F. Palacio, L. Mafra and J. Rocha, Eur. J. Inorg. Chem., 2012, 32, 5259–5268 CrossRef.
- J. H. He, G. J. Zhang, D. R. Xiao, H. Y. Chen, S. W. Yan, X. Wang, J. Yang and E. B. Wang, CrystEngComm, 2012, 14, 3609–3614 RSC.
- Y. Xie, Y. Yan, H. H. Wu, G. P. Yong, Y. Cui, Z. Y. Wang, L. Pan, J. Li, R. Fan, R. P. Li, Y. C. Tian, G. Q. Pan, L. S. Sheng and X. Li, Inorg. Chim. Acta, 2007, 360, 1669–1677 CrossRef CAS.
- D. R. Xiao, G. J. Zhang, J. L. Liu, L. L. Fan, R. Yuan and M. L. Tong, Dalton Trans., 2011, 40, 5680–5683 RSC.
- Y. G. Zhang, M. K. Saha and I. Bernal, CrystEngComm, 2003, 34–37 RSC.
- K. Tanaka, T. Muraoka, D. Hirayama and A. Ohnish, Chem. Commun., 2012, 48, 8577–8579 RSC.
- K. Tanaka, N. Hotta, S. Nagase and K. Yoza, New J. Chem., 2016, 40, 4891–4894 RSC.
- Y. Cui, H. L. Ngo, P. S. White and W. B. Lin, Chem. Commun., 2003, 994–995 RSC.
- K. Tanaka, T. Muraoka, Y. Otubo, H. Takahashi and A. Ohnishi, RSC Adv., 2016, 6, 21293–21301 RSC.
- K. Tanaka, T. Kawakita, M. Morawiak and Z. Urbanczyk-Lipkowska, CrystEngComm, 2019, 21, 487–493 RSC.
- Y. Yu, N. Xu, J. Zhang, B. Wang, S. Xie and L. Yuan, ACS Appl. Mater. Interfaces, 2020, 12, 16903–16911 CrossRef CAS.
- X. Wang, Y. Zhu, J. Liu, C. Liu, C. Cao and W. Song, Chem.–Asian J., 2018, 13, 1535–1538 CrossRef CAS.
- P. Zhang, L. Wang, J. H. Zhang, Y. J. He, Q. Li, L. Luo, M. Zhang and L. M. Yuan, J. Liq. Chromatogr. Relat. Technol., 2018, 41, 903–909 CrossRef CAS.
- R. G. Beaman, P. W. Morgan, C. R. Koller, E. L. Wittbecker and E. E. Magat, J. Polym. Sci., 1959, 40, 329–336 CrossRef CAS.
- X. Q. Liang, T. Wu and Z. L. Fan, Chin. J. Struct. Chem., 2016, 35, 1736–1744 CAS.
- N. Corella-Ochoa, J. B. Tapia, H. N. Rubin, V. Lillo, J. Gonzalez-Cobos, J. L. Nunez-Rico, S. R. G. Balestra, N. Almora-Barrios, M. Lledos, A. Guell-Bara, J. Cabezas-Gimenez, E. C. Escudero-Adan, A. Vidal-Ferran, S. Calero, M. Reynolds, C. Marti-Gastaldo and J. R. Galan-Mascaros, J. Am. Chem. Soc., 2019, 141, 14306–14316 CrossRef.
- M. C. Hennion and V. Pichon, Environ. Sci. Technol., 1994, 28, A576–A583 CrossRef.
- M. Salarian, A. Ghanbarpour, M. Behbahani, S. Bagheri and A. Bagheri, Microchim. Acta, 2014, 181, 999–1007 CrossRef CAS.
- X. J. Li, J. W. Xing, C. L. Chang, X. Wang, Y. Bai, X. P. Yan and H. W. Liu, J. Sep. Sci., 2014, 37, 1489–1495 CrossRef CAS.
- X. L. Liu, C. Wang, Q. H. Wu and Z. Wang, Anal. Chim. Acta, 2015, 870, 67–74 CrossRef CAS.
- B. Tang, J. H. Zhang, M. Zi, X. X. Chen and L. M. Yuan, Chirality, 2016, 28, 778–783 CrossRef CAS.
- J. Navarro-Sánchez, A. I. Argente-García, Y. Moliner-Martínez, D. Roca-Sanjuán, D. Antypov, P. Campíns-Falco, M. J. Rosseinsky and C. Martí-Gastaldo, J. Am. Chem. Soc., 2017, 139, 4294–4297 CrossRef.
- C. Marti-Gastaldo, J. E. Warren, M. E. Briggs, J. A. Armstrong, K. M. Thomas and M. J. Rosseinsky, Chem.–Eur. J., 2015, 21, 16027–16034 CrossRef CAS.
- Y. Gao, W. R. Du, A. J. Yu, X. L. Li, W. D. Zhao and S. S. Zhang, Anal. Methods, 2018, 10, 5811–5816 RSC.
- F. Beuerle and B. Gole, Angew. Chem., Int. Ed., 2018, 57, 4850–4878 CrossRef CAS.
- R. P. Bisbey and W. R. Dichtel, ACS Cent. Sci., 2017, 3, 533–543 CrossRef CAS.
- Q. R. Fang, Z. B. Zhuang, S. Gu, R. B. Kaspar, J. Zheng, J. H. Wang, S. L. Qiu and Y. S. Yan, Nat. Commun., 2014, 5, 1–8 Search PubMed.
- H. Furukawa and O. M. Yaghi, J. Am. Chem. Soc., 2009, 131, 8875–8883 CrossRef CAS.
- G. Liu, J. Sheng and Y. Zhao, Sci. China: Chem., 2017, 60, 1015–1022 CrossRef CAS.
- X. Han, Q. C. Xia, J. J. Huang, Y. Liu, C. X. Tan and Y. Cui, J. Am. Chem. Soc., 2017, 139, 8693–8697 CrossRef CAS.
- Y. W. Peng, Y. Huang, Y. H. Zhu, B. Chen, L. Y. Wang, Z. C. Lai, Z. C. Zhang, M. T. Zhao, C. L. Tan, N. L. Yang, F. W. Shao, Y. Han and H. Zhang, J. Am. Chem. Soc., 2017, 139, 8698–8704 CrossRef CAS.
- D. Bessinger, L. Ascherl, F. Auras and T. Bein, J. Am. Chem. Soc., 2017, 139, 12035–12042 CrossRef CAS.
- Q. R. Fang, J. H. Wang, S. Gu, R. B. Kaspar, Z. B. Zhuang, J. Zheng, H. X. Guo, S. L. Qiu and Y. S. Yan, J. Am. Chem. Soc., 2015, 137, 8352–8355 CrossRef CAS.
- C. R. DeBlase, K. E. Silberstein, T. T. Truong, H. D. Abruna and W. R. Dichtel, J. Am. Chem. Soc., 2013, 135, 16821–16824 CrossRef CAS.
- C. X. Yang, C. Liu, Y. M. Cao and X. P. Yan, Chem. Commun., 2015, 51, 12254–12257 RSC.
- L. H. Liu, C. X. Yang and X. P. Yan, J. Chromatogr. A, 2017, 1479, 137–144 CrossRef CAS.
- G. F. Liu, D. Zhang and C. L. Feng, Angew. Chem., Int. Ed., 2014, 53, 7789–7793 CrossRef CAS.
- H. L. Qian, C. X. Yang and X. P. Yan, Nat. Commun., 2016, 7, 1–7 Search PubMed.
- X. Hang, J. Huang, C. Yuan, Y. Liu and Y. Cui, J. Am. Chem. Soc., 2018, 140, 892–895 CrossRef.
- S. Zhang, Y. Zheng, H. An, B. Aguila, C.-X. Yang, Y. Dong, W. Xie, P. Cheng, Z. Zhang, Y. Chen and S. Ma, Angew. Chem., Int. Ed., 2018, 57, 16754–16759 CrossRef CAS.
- S. Xu, Y. Wang, W. Li and Y. Ji, J. Chromatogr. A, 2019, 1602, 481–488 CrossRef CAS.
- W. Wang, J. Wang, S. Zhang, P. Cui, C. Wang and Z. Wang, Talanta, 2016, 161, 22–30 CrossRef CAS.
- R. Q. Wang, X. B. Wei and Y. Q. Feng, Chem.–Eur. J., 2018, 24, 10979–10983 CrossRef CAS.
- Y. Wang, S. Zhuo, J. Hou, W. Li and Y. Ji, ACS Appl. Mater. Interfaces, 2019, 11, 48363–48369 CrossRef CAS.
- Y. Li, X. Lin, S. Qin, L. Gao, Y. Tang, S. Liu and Y. Wang, Chirality, 2020, 32, 1008–1019 CrossRef CAS.
- X. Kuang, Y. Ma, H. Su, J. Zhang, Y. B. Dong and B. Tang, Anal. Chem., 2014, 86, 1277–1281 CrossRef CAS.
- X. Bao, L. J. Broadbelt and R. Q. Snurr, Mol. Simul., 2009, 35, 50–59 CrossRef CAS.
- P. Z. Moghadam and T. Duren, J. Phys. Chem. C, 2012, 116, 20874–20881 CrossRef CAS.
- Z. Qiao, A. Torres-Knoop, D. Dubbeldam, D. Fairen-Jimenez, J. Zhou and R. Q. Snurr, AIChE J., 2014, 60, 2324–2334 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2021 |

