Super-assembled silica nanoprobes for intracellular Zn(ii) sensing and reperfusion injury treatment through in situ MOF crystallization†
Abstract
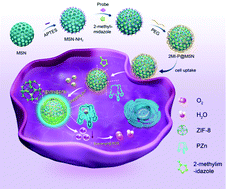
The production of excess free zinc ions (Zn2+) in cells has been identified as an important cause of cell injury or apoptosis after ischemia reperfusion. Thus, developing a nanosystem with multiple therapeutic functions to significantly eliminate multiple cell injury factors is of great interest. Here, a super-assembled nanosystem consisting of a polyethylene glycol (PEG) surface-modified mesoporous silica nanoparticle (MSN) encapsulating 2-methylimidazole (2MI) and a Zn2+ probe (PZn) was fabricated. The 2MI-P@MSN nanoassemblies showed a “turn-on” fluorescence signal at 476 nm toward zinc ions due to the presence of PZn. Besides, zeolitic imidazolate framework-8 (ZIF-8) could be assembled on the site intracellularly after 2MI chelating with free zinc ions. The experimental results revealed that 2MI-P@MSN exhibited excellent biocompatibility and non-cytotoxicity, and was able to provide satisfactory protection to OGD/R-treated cells based on zinc ion adsorption and the antioxidant effect of ZIF-8, which could effectively improve the survival rate of reperfusion injury cells from 52% to 73%. Notably, selective and quantitative sensing of Zn2+ was successfully carried out in the cells. This strategy highlights the potential of the detection, absorption and assembly of excess zinc ions simultaneously for cell therapy, which provides a promising therapeutic method for ischemic stroke, oxidative damage and diseases associated with zinc ion accumulation.
- This article is part of the themed collection: Analyst HOT Articles 2021


 Please wait while we load your content...
Please wait while we load your content...