Open Access Article
Open Access ArticleDigital printing of selective and reversible ion optodes on fabrics: toward smart clothes for epidermal chemical sensing†
Brock
Brady
 ,
Renjie
Wang
,
Renjie
Wang
 ,
Rosemary
Cheong
and
Xuewei
Wang
,
Rosemary
Cheong
and
Xuewei
Wang
 *
*
Department of Chemistry, Virginia Commonwealth University, 1001 W. Main St., Richmond, VA 23283, USA. E-mail: wangx11@vcu.edu
First published on 8th September 2021
Abstract
While wearable chemical sensors often rely on electrochemical techniques, optical chemical sensors coupled with a smartphone or a miniaturized camera represent an attractive approach to the monitoring of sweat composition. In this paper, we modify real sports fabrics such as polyester–spandex fabrics with rational combinations of sensing chemicals including a pH indicator, an ion exchanger, and an ionophore via one-step inkjet printing. Highly selective and fully reversible pH optodes as well as Na+- and K+-selective optodes are obtained only when the most hydrophobic sensing chemicals are used (e.g., sodium ionophore VIII vs. sodium ionophore VI). These sensors exhibit large color-based responses that can be readily identified by naked eye or analyzed via an iPhone app. Their dynamic ranges well cover the physiological sweat concentrations of the analytes. Compared to most other sensors created on garments, our fabric-based optodes are cost-effective, mass-reproducible by the digital printing technology currently used in the textile industry, and do not significantly compromise the essential properties of fabrics such as flexibility, stretchability, wickability, and breathability.
Wearable sensing technology has experienced incredible growth over the past years. While there are many wearable devices currently on the market, they are largely limited to monitoring mobility and vital signs. Wearable chemical sensors are an emerging technology that provides real-time and non-invasive metabolic and physiological information at molecular levels via biofluids such as sweat.1–4 Electrolytes and pH are among the most popular analytes of wearable chemical sensors as their abnormalities are associated with a wide range of disorders.3,4 Because ion-selective electrodes (ISEs) are the gold standard technique for measuring ionic species in blood/serum/plasma/urine in clinical and hospital settings, current wearable sensors predominantly rely on ISEs for electrolyte and pH monitoring. However, several limitations hinder the application of ISEs in decentralized settings. First, individual calibration is performed right before the first use of an ISE because the standard electric potential varies among ISEs that are identically manufactured.5,6 Second, ISEs suffer from drifts in electric potential during intermittent or continuous measurements, thereby necessitating frequent calibration and correction to maintain accuracy.6,7 Third, conditioning (soaking) of the dry-stored ISEs for 0.5–48 h is required to stabilize the sensors before they can be used for multiple measurements.5,6,8 The recently proposed calibration-free or conditioning-free ISEs are still in their early stages of research and not yet adopted in wearable devices.7 Therefore, there is a considerable demand for maintenance-free electrolyte/pH sensors suited for decentralized sweat chemical monitoring.
As the optical counterpart of ISEs, ion-selective optodes (ISOs) are another type of highly selective sensor for pH and electrolytes.9–11 Instead of measuring the electromotive force of a two-electrode potentiometric system, absorbance or fluorescence of an indicator dye is used to determine the concentration of the ionic analyte in the sample. In classical ISOs, a lipophilic pH indicator (also known as a chromoionophore) along with an ionophore and an ion exchanger are impregnated into a plasticized polymer phase to detect electrolytes such as Na+, K+, Ca2+, Mg2+, and Cl− in the sample. Similar optodes without the ionophore allow for the detection of pH. We recently discovered that these hydrophobic sensing chemicals readily adsorb on hydrophilic substrates such as cellulose paper, enabling ion sensing in the absence of a plasticized polymer matrix.12–15 The optode cocktail without the plasticized polymer becomes inkjet-printable because of its ideal viscosity and surface tension. As a result, ISOs can be fabricated in an unprecedentedly precise and reproducible fashion, making batch calibration possible.13 The elimination of the plasticized polymer (typically PVC) also dramatically reduces the hydrophobicity of the sensor and facilitates spreading of the aqueous sample.14 These features make the inkjet-printed plasticizer-free optodes highly suited for wearable sensing applications compared to traditional ion optodes.
One intriguing way to interface wearable sensors with the human body is direct functionalization of garments such as compression tops with sensor components. Thus, users do not suffer from the discomfort of wearing extra gadgets to monitor their sweat composition. Currently, most smart (sensing) garments rely on electrochemical sensors that are quite complicated and hardly mass-produced.16 In this paper, we for the first time fabricated colorimetric ion-selective optodes on sports fabrics such as a Nike Dri-FIT shirt made of polyester–spandex. Since digital printing has been widely used in the textile industry for dyeing purposes, the simple replacement of traditional dye inks with optode inks will create sensors onto clothing. In contrast to previously reported Na+- and F−-selective optodes on cellulose paper for disposable applications,12,13 the fabric-based pH, Na+, and K+ optodes are characterized with a focus on their reversibility and response time. More hydrophobic ionophores are necessitated to attain robust chemical adsorption on the fabric and therefore acceptable reversibility in multiple measurements. We also demonstrated that fabric properties such as surface charge, polarity, and solvent compatibility play a role in the color and response of the optodes.
To create wearable pH optodes, a dye that exhibits a very low water solubility and strong adsorption on the substrate should be employed. A variety of lipophilic Nile Blue derivatives have been developed as pH indicators in ion-selective optodes and are commercially available (Chromoionophores I, II, III, and VII from Sigma). They exhibit different basicities and allow for pH detection in different ranges.17 Preliminary experiments revealed that Chromoionophore III with a pKa of 12.0 (determined in methanol)17 has the best sensitivity and dynamic range for the determination of sweat pH (4.5–7.5). Fig. 1 shows the colorimetric response of the Chromoionophore III-based optodes on Whatman filter paper (Grade 5), polyester–spandex cloth (88/12) from Testfabrics, and Nike Dri-FIT cloth that also uses polyester–spandex (92/8). The optodes change their color from bluish to orangish/brownish due to the gradual deprotonation of Chromoionophore III as pH increases. The color differs on different substrates probably due to the solvatochromic property of the Nile Blue chromophore18 and the different polarities of the polymer fibers. Unlike the raw fabric from Testfabrics, Nike Dri-FIT has additives such as whitening and wicking agents which may also interact with the dye and affect the optode color. The hue value of each photo was extracted via a color analysis app, Color Mate, and used to construct calibration curves. According to the images and the hue-based calibration curves (Fig. S1†), the dynamic range is shifted toward higher pH on the fabrics compared to filter paper, which is ascribed to the different densities of anionic sites of the fabrics and paper. While cellulose has few carboxylate groups as an impurity, polyester (polyethylene terephthalate) has a significant number of terminal carboxylate groups. This is why the zeta potential of the polyester fabric is much lower than that of the filter paper (−60 vs. −35 mV at pH 6).13,19 The anionic sites possessed by polyester serve as cation exchangers to attract protons to the sensing layer, thereby requiring more basic solutions to deprotonate the Chromoionophore III and leading to a dynamic range at higher pH.
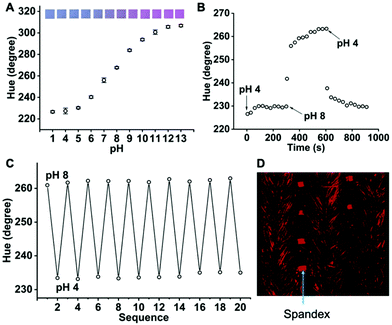
Although Chromoionophore III has good sensitivity to pH changes, we found that this dye is not a suitable indicator for Na+ and K+ optodes because only very high concentrations of Na+ (>0.1 M) and K+ (>0.01 M) can induce appreciable responses. Instead, Chromoionophore I with a lower pKa (10.6)17 enables the creation of optodes that respond to Na+ and K+ at physiological sweat concentrations (see below). Chromoionophore I can also be used to detect sweat pH when combined with a hydrophobic cation exchanger, sodium tetrakis[3,5-bis(trifluoromethyl)phenyl]borate (NaTFPB). In addition, the cost of Chromoionophore I is much lower than that of Chromoionophore III. Therefore, we used Chromoionophore I to create all pH and electrolyte optodes on fabrics for the remainder of the paper. Since Nike Dri-FIT shirts are commonly used exercise garments, it was chosen as the primary testing fabric. As shown in Fig. 2A, a dynamic range of pH 4 to 11 and a linear range of pH 5 to 9 were obtained on Nike Dri-FIT fabric modified with Chromoionophore I and NaTFPB at a molar ratio of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Using pH 6.0 to 7.0 as an example range, a pH change of 0.2 can be readily detected by this method (Fig. S2†). The 90% response time of the pH optode is less than 2.5 min for both the increase and decrease of pH (Fig. 2B). Excellent reversibility between pH 4 and 8 was obtained in 10 rounds of tests, which agrees with the fact that the protonation/deprotonation process of the dye is fully reversible (Fig. 2C).
1. Using pH 6.0 to 7.0 as an example range, a pH change of 0.2 can be readily detected by this method (Fig. S2†). The 90% response time of the pH optode is less than 2.5 min for both the increase and decrease of pH (Fig. 2B). Excellent reversibility between pH 4 and 8 was obtained in 10 rounds of tests, which agrees with the fact that the protonation/deprotonation process of the dye is fully reversible (Fig. 2C).
The blended fabric of polyester and spandex (>85% polyurethane) is one of the most popular materials used for high performance activewear. While polyester is durable and resistant to stretching and abrasion, spandex imparts excellent elasticity to the cloth. Interestingly, the dye is more concentrated in spandex according to the stacked confocal image (Fig. 2D). Both polyethylene terephthalate and polyurethane are not fully resistant to cyclohexanone, the organic solvent used in the sensing ink. Therefore, the dye may not only reside on fiber surfaces but also penetrate into these fibers with the aid of the solvent. With only about 10 L of cyclohexanone used per sq. cm of optode, fibers were clearly not dissolved according to the confocal image and observations from the naked eye. We did not further study the dye distribution and the reason for its higher affinity toward spandex. Nevertheless, the excellent pH response shown in Fig. 2 suggests that most dyes are readily accessible to analytes in the aqueous sample.
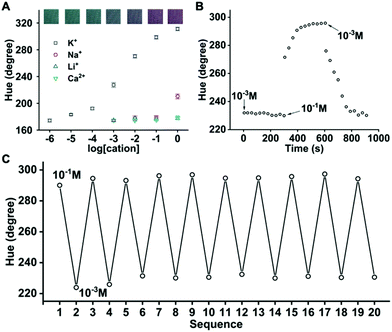
To create optodes for electrolyte cations, an ionophore specific to the cationic analyte needs to be printed along with the chromoionophore and the ion exchanger. According to the well-established mechanism of ion-selective optodes,10 the analyte cations transfer into the sensing phase and expel protons into the aqueous phase, resulting in the deprotonation of the pH-sensitive dye. A 100 mM Bis–Tris–HCl buffer at pH 6.5 is used as the background of all calibration solutions, which is close to the average sweat pH of 6.3. As shown in Fig. 3, a dynamic range of 10−5 to 1 M and a linear range of 10−4 to 10−2 M was obtained when the molar ratio of Chromoionophore I, NaTFPB, and potassium ionophore III is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
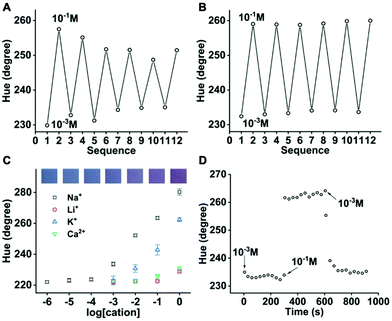
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3. This well covers the physiologically relevant range of 2 to 10 mM K+ in sweat. The K+ response is at least 1000 times more sensitive than those of other electrolytes including Na+, Li+, and Ca2+. This optode is also reversible and has a 90% response time of <2.5 min. When a commonly used ionophore, sodium ionophore VI,12,15 is printed along with Chromoionophore I and KTFPB to create Na+ optodes, the reversibility is unsatisfactory (Fig. 4A). The gradually decreased response is presumably attributed to the loss of ionophore. Sodium ionophore VIII shares the same crown ether-based ion binding moiety with sodium ionophore VI but bears one more C12 chain. The enhanced hydrophobicity is supposed to suppress the leaching of ionophore and may improve optode reversibility. As shown in Fig. 4B, the reversibility is indeed significantly better with sodium ionophore VIII. The fabric-based sensor using sodium ionophore VIII exhibits a linear range of 10−4 to 1 M, covering the sweat Na+ concentration of 10−2 to 10−1 M. The response is >10 times more sensitive than K+ and >1000 times more sensitive than Li+ and Ca2+. To confirm that K+ will not interfere with the Na+ detection, the Na+ optode was tested in 10 mM NaCl in the Bis–Tris–HCl buffer with different concentrations of KCl. The hue increase is only 2.1° when K+ increases from the lower end (1 mM) to the higher end (10 mM) of its physiological concentration in sweat. The 90% response time of this sensor is <1 min (Fig. 4D), shorter than that of the K+ optode on the Nike Dri-FIT fabric. The difference in response time may be attributed to the liquid state of sodium ionophore VIII and the solid-state of potassium ionophore III at room temperature.
3. This well covers the physiologically relevant range of 2 to 10 mM K+ in sweat. The K+ response is at least 1000 times more sensitive than those of other electrolytes including Na+, Li+, and Ca2+. This optode is also reversible and has a 90% response time of <2.5 min. When a commonly used ionophore, sodium ionophore VI,12,15 is printed along with Chromoionophore I and KTFPB to create Na+ optodes, the reversibility is unsatisfactory (Fig. 4A). The gradually decreased response is presumably attributed to the loss of ionophore. Sodium ionophore VIII shares the same crown ether-based ion binding moiety with sodium ionophore VI but bears one more C12 chain. The enhanced hydrophobicity is supposed to suppress the leaching of ionophore and may improve optode reversibility. As shown in Fig. 4B, the reversibility is indeed significantly better with sodium ionophore VIII. The fabric-based sensor using sodium ionophore VIII exhibits a linear range of 10−4 to 1 M, covering the sweat Na+ concentration of 10−2 to 10−1 M. The response is >10 times more sensitive than K+ and >1000 times more sensitive than Li+ and Ca2+. To confirm that K+ will not interfere with the Na+ detection, the Na+ optode was tested in 10 mM NaCl in the Bis–Tris–HCl buffer with different concentrations of KCl. The hue increase is only 2.1° when K+ increases from the lower end (1 mM) to the higher end (10 mM) of its physiological concentration in sweat. The 90% response time of this sensor is <1 min (Fig. 4D), shorter than that of the K+ optode on the Nike Dri-FIT fabric. The difference in response time may be attributed to the liquid state of sodium ionophore VIII and the solid-state of potassium ionophore III at room temperature.
Since the response time was obtained in bulk solutions with gentle agitation, it may not accurately reflect the response time in real perspiration analysis. Although Nike Dri-FIT fabric is designed to reduce sweat absorption into fibers by wicking away sweat, there may still be residual sweat that interferes with new sweat in continuous monitoring applications. Therefore, it is necessary to perform on-body tests in the future to evaluate the temporal resolution of the fabric-based optodes as wearable sensors for sweat analysis.
Fig. 5 shows the dynamic water contact angle results of the unmodified and modified Nike Dri-FIT fabric. As expected, the modification with hydrophobic sensing chemicals increases the surface hydrophobicity. However, water completely spreads on the pH and Na+ optodes within 2 s, representing a negligible reduction in the water wicking ability. The K+ optode is most hydrophobic probably due to the more rigid structure of potassium ionophore III, but complete spreading can still be achieved within 15–20 s, which is advantageous over the plasticized PVC optodes that are highly water-repelling.14 Overall, the fabric-based optodes still have a high degree of water wickability desired for epidermal sensing. Stretchability is a challenge for many smart garments based on conducting materials. In contrast, the adsorbed sensing chemicals on fabrics are supposed to withstand stretching. Using pH optodes as an example, 150% stretching of the sensing fabric by 50 times does not cause any drift in the colorimetric response (a hue value deviation of <2° for pH 4). An abrasion test was performed on a sodium ionophore VIII-based Na+ optode printed on Nike Dri-FIT fabric. The optode was submerged in a buffered 10 mM NaCl solution for 2.5 min to get an initial hue value. The optode was then rubbed 10 times to simulate friction between the user and wearable. After 10 times, the fabric was tested for hue value in the same solution. This procedure was repeated for 5 trials. Hue value was consistent over all 5 trials with a standard deviation of 0.7° in hue. The K+ and pH optodes also showed no signal drift in the abrasion test.
 | ||
| Fig. 5 Dynamic contact angles of water on original Nike Dri-FIT fabric and optodes on the same fabric. | ||
An intriguing feature of the inkjet-printed sensors compared to most other wearable sensors on cloth and other substrates are that the shape, size, and shade of the printed sensors are easy to tune. As shown in Fig. 6, sensors as small as 2 × 2 mm2 can be readily printed on the fabric. By adjusting the drop spacing and/or printing layers, the density of chemicals can be precisely controlled on the fabric.
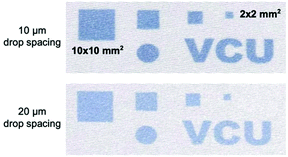 | ||
| Fig. 6 Different optode patterns printed on Nike Dri-FIT fabric using a drop spacing setting of 10 or 20 μm in the Dimatix Material Printer. | ||
In conclusion, we present for the first time a fully reversible, plasticizer-free ISOs applied to fabric via inkjet printing. Our pH, Na+, and K+ optodes have demonstrated excellent sensitivity, response time, and reversibility. Due to the pH fluctuation of sweat, chromoionophore-based electrolyte sensors can be problematic with real sweat samples because of the pH cross-sensitivity of the optodes. Calibration curves may need to be constructed for each pH so that pH correction can be performed with the aid of the pH sensor. A buffering system may also be introduced to the electrolyte sensing areas to control the local pH. Replacement of the pH-sensitive dye with a pH-independent solvatochromic dye is another possible direction.20 We believe our method presents a strong step towards fabricating smart garments using optical chemical sensors. In future work, we will explore more sensing chemistries, compare more polymer fabrics, extend the ionic analytes, and initiate on-body tests.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by Virginia Commonwealth University (Startup Grant for X. W.).Notes and references
- J. Kim, A. S. Campbell, B. E. F. de Ávila and J. Wang, Nat. Biotechnol., 2019, 37, 389–406 CrossRef CAS PubMed.
- L. Chang, Y. C. Wang, F. Ershad, R. Yang, C. Yu and Y. Fan, Trends Biotechnol., 2019, 37, 1175–1188 CrossRef CAS PubMed.
- J. R. Sempionatto, I. Jeerapan, S. Krishnan and J. Wang, Anal. Chem., 2020, 92, 378–396 CrossRef CAS PubMed.
- Y. Yang and W. Gao, Chem. Soc. Rev., 2019, 48, 1465–1491 RSC.
- Individual caibration and conditioning were performed: W. Gao, S. Emaminejad, H. Y. Y. Nyein, S. Challa, K. Chen, A. Peck, H. M. Fahad, H. Ota, H. Shiraki, D. Kiriya, D. H. Lien, G. A. Brooks, R. W. Davis and A. Javey, Nature, 2016, 529, 509–514 CrossRef CAS PubMed.
- According to the manual of Radiometer “ABL90 FLEX”, Instrumentation Laboratory “GEM 5000”, Seimens “RAPIDPoint 500”, and Nova Biomedical “Stat Profile Prime® CCS Comprehensive”, each electrode needs to be conditioned and calibrated prior to its first use and more calibrations are performed for subsequent measurements.
- C. R. Rousseau and P. Bühlmann, TrAC, Trends Anal. Chem., 2021, 140, 116277 CrossRef CAS.
- M. Guzinski, J. M. Jarvis, B. D. Pendley and E. Lindner, Anal. Chem., 2015, 87, 6654–6659 CrossRef CAS PubMed.
- G. Mistlberger, G. A. Crespo and E. Bakker, Annu. Rev. Anal. Chem., 2014, 7, 483–512 CrossRef PubMed.
- X. Xie and E. Bakker, Anal. Bioanal. Chem., 2015, 407, 3899 CrossRef CAS PubMed.
- X. Du and X. Xie, Sens. Actuators, B, 2021, 335, 129368 CrossRef CAS.
- X. Wang, Y. Qin and M. E. Meyerhoff, Chem. Commun., 2015, 51, 15176–15179 RSC.
- X. Wang, Q. Zhang, C. Nam, M. Hickner, M. Mahoney and M. E. Meyerhoff, Angew. Chem., 2017, 129, 11988–11992 CrossRef.
- X. Wang, M. Mahoney and M. E. Meyerhoff, Anal. Chem., 2017, 89, 12334–12341 CrossRef CAS PubMed.
- Q. Zhang, X. Wang, V. Decker and M. E. Meyerhoff, ACS Appl. Mater. Interfaces, 2020, 12, 25616–25624 CrossRef CAS PubMed.
- A. Hatamie, S. Angizi, S. Kumar, C. M. Pandey, A. Simchi, M. Willander and B. D. Malhotra, J. Electrochem. Soc., 2020, 167, 037546 CrossRef CAS.
- E. Bakker, M. Lerchi, T. Rosatzin, B. Rusterholz and W. Simon, Anal. Chim. Acta, 1993, 278, 211–225 CrossRef CAS.
- J. Jose and K. Burgess, Tetrahedron, 2006, 62, 11021–11037 CrossRef CAS.
- A. M. Grancaric, A. Tarbuk and T. Pusic, Color. Technol., 2005, 121, 221–227 CAS.
- X. Wang, Y. Zhou, V. Decker, M. Meyerhoff, M. Sun and Y. Cui, Anal. Methods, 2020, 12, 2547–2550 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental section, structures of sensing chemicals, Fig. S1. See DOI: 10.1039/d1an01349a |
| This journal is © The Royal Society of Chemistry 2021 |