Open Access Article
Open Access ArticleRapid antibiotic susceptibility testing using resazurin bulk modified screen-printed electrochemical sensing platforms†
Benjamin
Crane
,
Jack P.
Hughes
,
Samuel J.
Rowley Neale
 ,
Mamun
Rashid
,
Patricia E.
Linton
,
Mamun
Rashid
,
Patricia E.
Linton
 ,
Craig E.
Banks
,
Craig E.
Banks
 * and
Kirsty J.
Shaw
* and
Kirsty J.
Shaw
 *
*
Faculty of Science and Engineering, Manchester Metropolitan University, Chester Street, Manchester, M1 5GD, UK. E-mail: C.Banks@mmu.ac.uk; k.shaw@mmu.ac.uk
First published on 26th July 2021
Abstract
Urinary tract infections (UTIs) are one of the most common types of bacterial infection. UTIs can be associated with multidrug resistant bacteria and current methods of determining an effective antibiotic for UTIs can take up to 48 hours, which increases the chances of a negative prognosis for the patient. In this paper we report for the first time, the fabrication of resazurin bulk modified screen-printed macroelectrodes (R-SPEs) demonstrating them to be effective platforms for the electrochemical detection of antibiotic susceptibility in complicated UTIs. Using differential pulse voltammetry (DPV), resazurin was able to be detected down to 15.6 μM. R-SPEs were utilised to conduct antibiotic susceptibility testing (AST) of E. coli (ATCC® 25922) to the antibiotic gentamicin sulphate using DPV to detect the relative concentrations of resazurin between antibiotic treated bacteria, and bacteria without antibiotic treatment. Using R-SPEs, antibiotic susceptibility was determined after a total elapsed time of 90 minutes including the inoculation of the artificial urine, preincubation and testing time. The use of electrochemistry as a phenotypic means of identifying an effective antibiotic to treat a complicated UTI offers a rapid and accurate alternative to culture based methods for AST with R-SPEs offering an inexpensive and simpler alternative to other AST methods utilising electrochemical based approaches.
Introduction
Urinary tract infections (UTIs) are one of the most common types of infection caused by bacteria;1 there is also evidence to suggest that the number of hospital admissions due to UTIs in the UK is increasing.2 UTIs can be broadly split into two categories: uncomplicated and complicated UTIs. Uncomplicated UTIs are typically associated with individuals who do not have any structural or neurological issues of the urinary tract and are otherwise healthy,3 while complicated UTIs are infections that occur in individuals who are structurally or immunologically compromised in some way, with examples being renal failure or immunosuppression.4The majority of uncomplicated and complicated UTIs are caused by Escherichia coli (E. coli).1,5 The E. coli associated with complicated UTIs can be either single- or multidrug-resistant which limits effective antibiotic treatment options.3 The principle issue with progressive colonisation of the urinary tract by antibiotic resistant bacteria is that it may lead to the infection of the kidneys which can progress to bacteraemia.1 Bacteraemia results in urosepsis which accounts for 25% of all adult sepsis cases and carries a morality rate of 25 to 60%.6 This highlights the importance of identifying effective antibiotics to treat UTIs, especially regarding complicated UTIs.
Following the diagnosis of a UTI through the presentation of clinical symptoms,7–9 or the use of a dipstick,10 antibiotic susceptibility testing (AST) is performed in order to assess the type of antibiotic a pathogenic bacteria may be susceptible to. Whilst AST is being conducted, it is common for patients to be treated with general broad-spectrum antibiotics until the results are available.11 This can lead to further deterioration in cases caused by strains resistant to such antibiotics, potentially leading to poor clinical outcomes for patients.12 Additionally this practice in prescribing a potentially ineffective antibiotic is a major contributor to the further development of antibiotic resistance in pathogenic bacterial strains.13,14
AST can be phenotypic or genotypic. Phenotypic testing directly determines antibiotic susceptibility and typically uses a culture-based methodology to monitor the response in bacterial growth to different antibiotics. Although accurate, culture-based methods are time consuming, taking between one to three days to sufficiently grow the bacterial culture and for AST to be conducted.15,16 Genotypic testing offers an alternative and utilises amplification and identification of known genes, which confer antibiotic resistance, to accurately identify which antibiotics would be ineffective against a bacterial strain type. One of the limitations of genotypic testing is that it relies on a time consuming pre-incubation step which can take up to four hours to grow the bacterial population in addition to the testing time.16 Additionally, it is impossible to identify all of the resistance genes which are numerous and subject to frequent changes due to mutations,17 in addition the presence of a resistance gene may not confer phenotypic resistance making genotypic testing potentially unreliable.18 A faster method of conducting AST could potentially help reduce or eliminate the prescription of ineffective broad-spectrum antibiotics.
Resazurin is a redox indicator that has a documented history of use in cell viability assays.19 Resazurin can be biologically reduced by metabolically active aerobic bacteria to form resorufin, and then reduced again to form dihydroresorufin.16,20 Conventional resazurin AST assays confirm the presence of live cells based off the detection of resorufin21–23 and can be carried out optically (using absorbance or fluorescence) or electrochemically. For example, Avesar et al., created a biosensor containing a stationary nanolitre droplet array for measuring production of the fluorescent resorufin product at 30 minutes time intervals for AST of different bacteria strains.23
The electrochemical mechanism of resazurin has been extensively studied in both aqueous solutions and ionic liquids using glassy carbon electrodes.20 An electrochemically based approach for resazurin based phenotypic AST offers a more sensitive method as resazurin can be detected directly rather than relying on an extended incubation period to enable resorufin concentrations to increase to detectable levels. Rapid AST has been demonstrated using organic redox-active crystalline layers on pyrolytic graphite sheets which enables accurate determination on minimum inhibitory concentrations (MIC) of antibiotics in as little as 60 mins.25 Microfabricated systems, for example, a three-dimensional interdigitated electrode array with impedimetric transducer, have shown how integration of the components required for AST can be achieved.19 While resazurin is heavily used in such systems for AST, other indicators are also available, such as ferricyanide which is reduced to ferrocyanide during bacterial respiration and has been investigated for amperometric assays.26
Screen-printed macroelectrodes (SPEs) present a variety of useful qualities such as being versatile, highly reproducible with the ability to be mass produced and can be utilised in either surface modified or bulk modified configurations26–28 For example, gold SPEs, modified with hydrogels containing antibiotics and growth media showed bacterial growth could be monitored using both electrochemical impedance spectroscopy (EIS) and differential pulse voltammetry (DPV).29 SPEs have been utilised as part of a surface modified resazurin based detection method however,30 the use of SPEs as a platform for resazurin based AST has not yet been fully explored. The use of a simple method for the bulk-modification of SPEs that is part of the SPE fabrication process would reduce the time and complexity in the steps required for the produce of a set of modified SPEs whilst maintaining effectivity. Consequently, in this paper, we report for the first time, the bulk modification of SPEs with resazurin which are applied to the AST of UTI causative bacteria. Fig. 1 shows a schematic overview of the phenotypic AST using an electrochemical based sensing methodology.
 | ||
| Fig. 1 An overview schematic of phenotypic AST using an electrochemical based sensing methodology. Resazurin can be reduced intracellularly by metabolically active bacteria or electrochemically at the surface of the working electrode which allows the antibiotic susceptible and antibiotic resistant bacteria to be determined.20,24 | ||
Experimental section
All chemicals used in this work were of analytical grade, some of the resulting solutions that were made up required sterilisation treatment before use as detailed below. Resazurin solutions were made every day prior to use. All solutions were prepared using water or dissolved in a suitable supporting electrolyte solution, using water purified using a Millipore Milli-Q system (Type 1 Ultrapure water, 18.2 MΩ cm at 25 °C). Potassium phosphate monobasic (KH2PO4), potassium chloride (KCl), surine, gentamicin sulphate and resazurin sodium salt were all obtained from Sigma-Aldrich (Gillingham, UK). Nutrient broth (CM0001) and nutrient agar (CM0003) were obtained from Thermo Scientific (Oxoid, UK). A HI 2209 pH meter obtained from Hanna instruments (Leighton Buzzard, UK) was utilised.For overnight cultures, single strength nutrient broth was also made up according to manufacturer's instructions (Oxoid, 13 g to 1 L distilled water) and autoclaved. Artificial urine broth was made up using an equal volume of surine and double strength nutrient broth which was adjusted to a pH of 6. Artificial urine was autoclaved at 121 °C for 20 minutes to sterilise the solution before use. A stock solution of the antibiotic gentamicin sulphate was made up at a concentration of 96 mM in sterile deionised water before being filter sterilised using a 45 nm syringe filter. All experiments were performed at room temperature without oxygen removal.
E. coli (ATCC® 25922) was grown on nutrient agar from a stock kept in freezer storage and incubated at 37 °C for 24 hours. Overnight cultures were prepared from these plates using one colony taken from the plates to inoculate 10 mL of single strength nutrient broth and incubated at 37 °C for a further 24 hours.
Electrochemical measurements
Electrochemical measurements utilised an EmStat3 potentiostat controlled by PSTrace 5.5 software utilising an EmStat three pin SPE connector as the interface between the electrode and the potentiostat all sourced from PalmSens (The Netherlands, Randhoeve). The electrochemical measurements were utilised using a three-electrode configuration, having a graphite working electrode acting as both the counter and reference electrode. Cyclic voltammetry was conducted using a potential range of 0 to −0.65 V, an E-step of 0.005 V and the following scan rates: 0.005, 0.01, 0.015, 0.025, 0.050, 0.075, 0.100, 0.150 and 0.200 V s−1. Differential pulse voltammetry (DPV) was carried out using a potential range of 0 V to −1.4 V, an E-step of 0.001 V, a T-pulse of 0.05 s, an E-pulse of 0.05 V and a scan rate of 0.01 V s−1. A buffered supporting electrolyte solution (BSES) made up of 1.5 mM KH2PO4 and 0.1 M KCl was made up for the purpose of testing the unmodified/bare SPEs using cyclic voltammetry.In order to test the R-SPEs using artificial urine and to conduct the limit of detection experiments a micro-dropping (μDrop) methodology was used to emulate the type of volume that would be used for the AST method. μDrop involved pipetting a 60 μL volume of the chosen sample onto the SPE to cover the working, counter, and reference electrodes completely.
Fabrication of the screen-printed macroelectrodes (SPEs) and resazurin bulk modified screen-printed macroelectrodes (R-SPEs)
The SPEs were fabricated by screen-printing a carbon-graphite ink (product code: C2000802P2; Gwent Electronic Materials Ltd, UK) layer onto a polyester substrate (Autostat, 250 μm thickness) using a DEK 248 screen-printing machine (DEK, Weymouth, UK). This layer was then cured using a fan oven set to 60 °C for a duration of 30 minutes. This defines the working electrode (3.1 mm), counter and reference electrodes as well as the connection points for the SPE to be interfaced with the EmStat three pin SPE connector to facilitate electrochemical readings. After curing, the connections were sealed with a dielectric paste (product code: D2070423D5; Gwent Electronic Materials Ltd, UK) to isolate the working area of the SPE made up by the working, counter, and reference electrode from the connection points. Then the SPEs were cured again at 60 °C for 30 minutes before being ready to use.The R-SPEs were made by modifying the carbon-graphitic ink via modification with resazurin. This was carried out using a weight percentage of MP to MI, where MP is the mass of particulate (the mass of resazurin) and MI is the total mass of the ink including the base graphitic ink and the mass of the particulate.31–34 This was thoroughly mixed into the ink and screen-printed on top of the carbon-graphite working electrode. The equation (MP/MI) × 100 was used to formulate the 10 wt% R-SPEs. A 10 wt% of resazurin was used to ensure consistent printing of the analyte in the fabrication process and to test viability of the proposed electrochemical AST system.
Detecting bacterial growth in response to different antibiotic concentrations
To establish that differences in growth rate can be detected in E. coli at different concentrations of gentamicin using electrochemical methods, an overnight culture was adjusted to an optical density of 0.7 at 600 nm post incubation using single strength nutrient broth as the diluent. After which 12.5 μL of the standardised solution was added to a total volume of 500 μL artificial urine in a bijou to produce an initial bacterial concertation of 1.76 × 107 CFU mL−1. A CFU mL−1 count of ≥105 is taken as confirmation of A UTI,10 an initial count of 1.76 × 107 CFU mL−1 was used to simulate a severe infection. The resulting inoculated solution was then vortexed and preincubated at 37 °C for 70 minutes on a shake plate bringing the bacterial population to exponential growth phase (see ESI Fig. S1†) and producing a bacterial concentration of 1.75 × 108 CFU mL−1, the antibiotic was then added. The inoculated artificial urine was vortexed again, and the μDrop method was used. Bacteria were freely suspended in the artificial urine and not immobilised on the electrodes. The chosen electrochemical method (cyclic voltammetry or differential pulse voltammetry) was then used to generate a resazurin reduction peak at hour zero. Between readings, the resazurin infused artificial urine was incubated at 37 °C. Electrochemical measurements were taken every hour for four hours.Statistical analysis
Statistical analysis was conducted using R (Version 3.5.3) with the R studio add-on (Version 1.2.5033) using 95% confidence limits. Normality of a dataset was assessed using a Shapiro–Wilk test. When comparing two data sets normally distributed data was analysed with a T-test or a Wilcoxon rank test if normality was not determined.Results and discussion
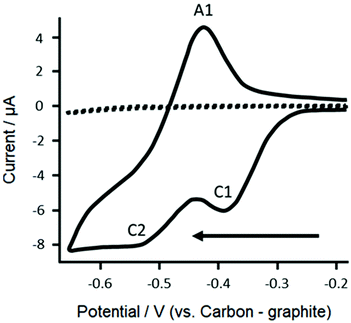
First, cyclic voltammetric responses for 1 mM resazurin (in pH 6 buffered supporting electrolyte solution (BSES)) were explored to determine the electrochemical mechanism using bare/unmodified screen-printed electrochemical sensing platforms. As shown within Fig. 2, voltammetric responses gives rise to two electrochemical reduction peaks and a single oxidation peak. From previous work on glassy carbon electrodes,20 we attribute the cathodic peak at C1 corresponding to the electrochemical irreversible reduction of resazurin to resorufin, while C2 corresponds to the electrochemical quasi-irreversible reduction of resorufin to dihydroresorufin and the anodic peak at A1 corresponds to the electrochemical oxidation of dihydroresorufin to resorufin. The height of the resazurin reduction peak height (IH, C1) was explored as a function of scan rate where a plot of IHvs. the square-root of scan rate was found to be linear. Further analysis in the form of a plot of log peak current versus log scan rate (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ip/μA = 0.43 μA/V s−1 + 0.77 μA) indicated a gradient of 0.4 close to the theoretically expected 0.5 for a diffusion-controlled process.
Ip/μA = 0.43 μA/V s−1 + 0.77 μA) indicated a gradient of 0.4 close to the theoretically expected 0.5 for a diffusion-controlled process.
Next, the effect of pH was explored, where the voltammetric profiles were explored as a function of pH. A plot the resazurin reduction peak position (Ep) using the voltametric peak at C1 was plotted vs. pH. Noting that the pKa of resazurin is 6.71 (at 25 °C),35 a linear trend was observed after which with an increase in pH causing a shift in Ep to a more negative value. The shift in the Ep value is due to the participation of protons in the electrochemical reduction of resazurin to resorufin which can be described with the following equation:
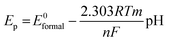 | (1) |
Fig. 3A shows typical DPV responses of both the R-SPEs and bare SPEs. Both electrode types produce a single cathodic peak (C1), which is likely due to the resazurin and resorufin reduction peak have become amalgamated which has been previously observed when using DPV and gold working, counter and reference electrodes to detect resazurin in artificial urine where the bulk of the reduction peak height corresponds to resazurin reduction.16 In comparing the DPV as shown in Fig. 3, note that in the case of the R-SPEs compared to the bare SPEs, both are different electrochemical systems. In the later, resazurin is added into the solution such that the concentration is high resulting in a large voltammetric signal. However, in the case of the latter, the peak is smaller and not as well-defined due to the fact that the resazurin is lower in concentration within the bulk of the SPE and likely a dual processes of dissolution and surface electrochemistry is in operation. That said, the R-SPE simplify the system.
Next, DPV was explored within artificial urine. Artificial urine was used in place of a BSES to better emulate the conditions of a urine sample and thus gain better insight into how sensitive DPV is in detecting resazurin in real world urine samples. Artificial urine of pH 6 was used as the normal pH range of urine is pH 4–8 with an average skewed towards acidic values,38 therefore pH 6 was chosen as the physiological pH value. DPV was chosen as the electroanalytical technique due to its sensitivity,37 and rapid speed of analysis.
Fig. 3B shows the DPV peak height response of bare SPEs to a range of resazurin concentrations (0.0156–1 mM) using artificial urine and μDrop. A plot of analytical signal (Ip, C1) vs. resazurin concentration revealed that as the concentration was increased the magnitude of the analytical signal increased (see ESI Fig. S2†). The lowest tested concentration (LTC) of resazurin was 15.6 μM. Plotting the reduction peak height as a function of concentration produced a nonlinear trend, as such the following equations were used to estimate the limit of detection:39
| LOB = Meanblank + 1.645 × SDblank | (2) |
| LOD = LOB + 1.645 × SDLTC | (3) |
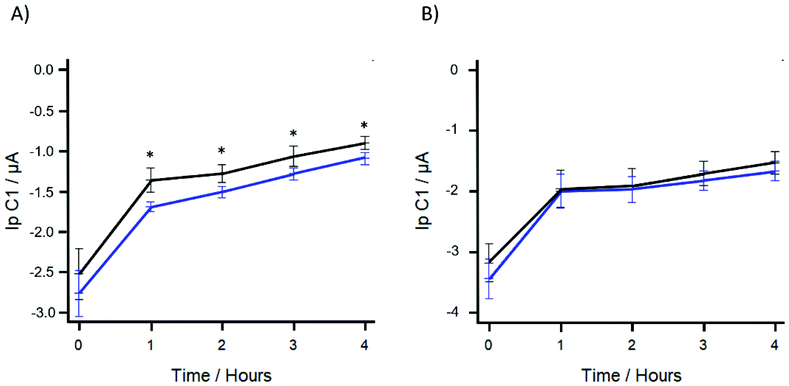
To determine the viability of using R-SPEs in combination with DPV as a method of conducting AST, R-SPEs were used to determine the antibiotic susceptibility of E. coli to gentamicin sulphate. Gentamicin sulphate concentrations in the range of 0.194–2.71 μM were used, with the antibiotic being added after the initial 70-minute preincubation step. After antibiotic loading, the artificial urine was added to the R-SPEs via μDrop and DPV was applied to produce an initial resazurin reduction peak. Subsequent readings were taken every hour, for up to four hours. This timing window was chosen to determine how quickly a significant difference in the resazurin reduction peak values could be determined to establish feasibility for use in a point of care setting. Statistically significant results were obtained at antibiotic concentrations of 1.36 μM and above. Fig. 4A shows the DPV peak height response of the R-SPEs using 1.55 and 0 μM concentrations of gentamicin sulphate. The trend shows that the DPV peak height response of the antibiotic loaded artificial urine is higher than the control as significantly less resazurin has been biologically reduced.
The reported MIC of gentamicin sulphate is 0.968 μM (see ESI Fig. S3†).40,41 MIC testing, however, relies on visual identification of microbial growth through liquid or agar based culturing not electrochemical detection of an redox marker. Gentamicin sulphate is an aminoglycoside type antibiotic and induces bacterial cell death via inhibition of protein synthesis.42 Although cell growth is inhibited at a concentration of 0.968 μM, the presence of residual protein synthesis can produce enzymes causing intracellular resazurin reduction.24 Therefore, a higher concentration of gentamicin sulphate than the MIC was required to inhibit the residual protein synthesis. For subsequent experiments, a concentration of 1.55 μM was used to ensure that bacterial protein synthesis was reliably inhibited. As part of a control we tested if gentamicin sulphate has a significant effect on the reduction of resazurin as the sulphate group may be electrochemically active in the applied potential range.43 Using the proposed method, the inhibitory concentration of gentamicin sulphate 1.55 μM was run against a control of 0 μM in sterile artificial urine using a one-hour time interval between electrochemical readings. This control experiment also served to determine if the significant difference that is observed between resazurin reduction peak heights between the gentamicin sulphate treated and the untreated artificial urine was due to bacterial metabolism and subsequent inhibition by the gentamicin, or just due to the potential electrochemical properties of the sulphate group. Fig. 4B shows the DPV response of R-SPEs using 1.55 and 0 μM concentrations of gentamicin sulphate in the absence of bacteria. No statistical significance was determined to occur at any of the time points. Therefore, at a dose of 1.55 μM the antibiotic gentamicin sulphate had no significant effect on the electrochemical reduction of resazurin and any statistically significance between the gentamicin sulphate treated and untreated artificial urine was caused by the metabolic activity of bacteria.
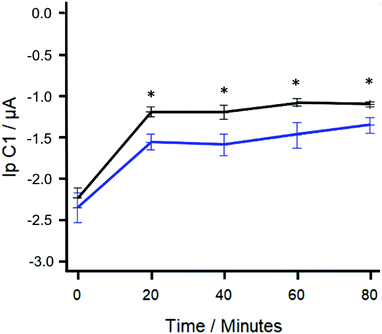
To determine if statistical significance could be determined faster and to minimise any effect that residual protein synthesis may have on the concentration of resazurin, the use of a shorter time interval between DPV readings was explored. Fig. 5 shows the difference in the DPV responses using R-SPEs and gentamicin sulphate concentrations of 1.55 and 0 μM using 20-minute time intervals. A statistically significant difference (paired T test; t = 6.3626, df = 4, p-value = 0.003128) between the resazurin reduction peak heights generated was found after 90-minutes of total elapsed time (70-minute preincubation + 20-minute time interval). Using this proposed method, the most rapid means of determining statistical significance using R-SPEs was to take a reading 20-minute after the 70-minute preincubation step.
Reported in the literature there have been other studies utilising resazurin or electrochemical AST methods (Table 1). The proposed method is significantly faster in terms of time to analysis than the disk diffusion assay which is the current gold standard for culture-based AST as well as the resazurin assay. The time to analysis is also comparable, or faster than many of the other electrical and electrochemical methods. The current limitation is that the proposed method has only been tested versus E. coli and the antibiotic gentamicin sulphate, however future work is being undertaken to test the method against other bacteria and antibiotics. The method is compatible with any bacteria that can synthesise dehydrogenase enzymes to biologically reduce resazurin,24 which are ubiquitous amongst aerobic bacteria, giving the proposed method a high potential for versatility in AST. Although the presented time to analysis is slower than some of the methods contemporaries, the simplicity in the fabrication and performance of the R-SPEs makes them a powerful analytical tool.
| Electrode material | Method | Bacteria tested | Antibiotics tested | Time to analysis (Minutes) | Advantages | Disadvantages | |
|---|---|---|---|---|---|---|---|
| Disk diffusion assay | N/A | Zones of inhibition44 | Any Isolates | Any | 960–1440 | Can be used with any culturable bacteria | Slow |
| Resazurin assay | N/A | Fluorescent detection of resorufin23 | Clinical isolates: | Freeze dried, multiplexed: | 330 | High throughput | Slow relative to electrochemical methods |
| E. coli | Ampicillin | ||||||
| K. pneumoniae | Ciprofloxacin | ||||||
| S. aureus | Colistin | ||||||
| A. baumannii | Erythromycin | ||||||
| C. freundii | Gentamicin | ||||||
| S. haemolyticus | |||||||
| Label free | Planar pyrolytic graphite | CV detection of pH change via redox-active crystalline layer25 | E. coli (K-12) | Added to solution: Ampicillin Kanamycin | 60 | No dye | Extensive modification process |
| Tantalum silicide | Impedance45 | E. coli ATCC 25922 | Added to solution: Ampicillin | 60–120 | High throughput | Specific to one bacterium | |
| N/A | Impedance46 | E. coli ATCC 25922 (Susceptible) & ATCC 700609 (Resistant) | Added to solution: Nalidixic acid | N/A | Fastest AST method discussed | Requires adaptation for non-motile bacteria | |
| Silver interdigitated carbon WE, CE & RE | Impedance47 | E. coli K-12 (ATCC 700891) | Added to solution: | < 90 | No dye to interfere with cellular processes. | Specific to one bacterium | |
| Methicillin-resistant SRM551 | Ampicillin Erythromycin | ||||||
| Ciprofloxacin | |||||||
| Methicillin | |||||||
| Daptomycin | |||||||
| Gentamicin | |||||||
| Labeled | Carbon WE, CE & RE | DPV detection of resazurin | E. coli ATCC 25922 | Added to solution: Gentamicin | 90 | Single step modification | Requires preincubation step. |
| Gold WE &CE, silver RE | Impedance or DPV detection of oxidation using potassium ferrocyanide29 | S. aureus (ATCC 29213) Methicillin-resistant S. aureus (ATCC 43300) | Added to hydrogel: Amoxicillin Oxacillin | < 45 | Low cost, reagents encapsulated in a hydrogel | Electrodes require maintenance | |
| Platinum WE, CE, silver, silver chloride RE | DPV detection of resazurin48 | E. coli ATCC 25922 | Added to solution: | 180 | Reusable biosensor | Electrode type incompatible with miniaturization | |
| K. pneumoniae ATCC 700603 | Ampicillin | ||||||
| Kanamycin | |||||||
| Tetracycline | |||||||
| Carbon WE & CE, silver, silver chloride RE | DPV detection of resazurin30 | S. gallinarum | Added to solution: Ofloxacin Penicillin | 60 | Single step modification | Modification process currently incompatible with bulk modification | |
| Gold WE, CE & RE. | DPV detection of resazurin16 | E. coli ATCC 700928 | Added to solution: | 30 | High throughput | Reagents must be added to reaction chambers manually | |
| K. pneumoniae ATCC 700603 | Ciprofloxacin | ||||||
| Ampicillin | |||||||
| Labeled | Platinum WE (3 mm) & CE, silver, silver chloride RE. | Amperometric detection of oxidation using potassium ferrocyanide49 | E. coli DH5α | Added to solution: | 180 | Use of a rotating WE to prevent loss of electrode sensitivity | Reliance on detection of a biological reduction product |
| Clinical sample, B. pseudomallei | Ciprofloxacin | ||||||
| Cefepime | |||||||
| Ceftazidime | |||||||
| Dicloxacillin | |||||||
| Co-trimoxazole | |||||||
| Carbon WE & CE, silver, silver chloride RE. | Cyclic voltammetry detection of didodecyldimethylammonium bromide50 | E. coli JM109 | Added to solution: | 120–300 | Single modification step | High variance in time to analysis | |
| Cefepime | |||||||
| Ampicillin | |||||||
| Amikacin | |||||||
| Erythromycin | |||||||
| Carbon WE & CE, silver, silver chloride RE | Amperometric detection of potassium ferricyanide and dichlorophenolindophenol51 | E. coli JM109 | Added to solution: | 20 | Antifouling coating | Several off sensor preparatory steps add 20 minutes to the analysis time. | |
| Bacitracin | |||||||
| D-Cycloserin | |||||||
| Erythromycin | |||||||
| Geneticin | |||||||
| Hygromycin | |||||||
| Kanamycin | |||||||
| Neomycin | |||||||
| Paromomycin | |||||||
| Rifampicin | |||||||
| Streptomycin | |||||||
| Trimethoprim | |||||||
| Vancomycin | |||||||
| Chloramphenicol | |||||||
| Nystatin | |||||||
| Carbenicillin | |||||||
| Cefotaxime | |||||||
| Nalidixic acid |
The proposed method offers an inexpensive and rapid alternative to other resazurin based electrochemical detection systems for antibiotic susceptibility testing with an application towards urinary tract infections. This proposed system utilises 10 wt% R-SPEs, the percentage of which can be varied to potentially further decrease the time taken to achieve statistical significance to further optimise the system and reduce cost; such work is currently being undertaken.
Conclusions
We have demonstrated that R–SPEs can successfully be used for the basis of an electrochemical detection system for AST. The incorporation of resazurin via bulk modification of SPEs was successfully demonstrated as a viable delivery mechanism for resazurin in artificial urine. Using DPV, resazurin was able to be detected down to 15.6 μM. Using the R-SPEs we have successfully shown the ability to detect small changes in the concentration of resazurin caused by bacterial metabolism. In using SPEs antibiotic susceptibility was determined in 90 minutes. The method developed here, offers a novel means of conducting AST using cost-effective and mass-producible electrochemical sensing platforms for AST which is simpler and more cost effective than other reported methods.Author contributions
BC: formal analysis, investigation, methodology, visualisation, writing – original draft/review & editing; JPH: visualisation, writing – original draft; SJR-N: visualisation, writing – original draft; MR: conceptualisation, methodology, supervision, writing – original draft/review & editing; PEL: conceptualisation, methodology, supervision, writing – original draft/review & editing; CEB: conceptualisation, methodology, supervision, writing – original draft/review & editing; KJS: conceptualisation, methodology, project administration, supervision, writing – original draft/review & editing.Conflicts of interest
There are no conflicts to declare.References
- A. L. Flores-Mireles, J. N. Walker, M. Caparon and S. J. Hultgren, Nat. Rev. Microbiol., 2015, 13, 269–284 CrossRef CAS PubMed
.
- M. Bardsley, I. Blunt, S. Davies and J. Dixon, BMJ Open, 2013, 3, e002007 CrossRef PubMed
.
- T. Hooton, N. Engl. J. Med., 2012, 366, 1028–1037 CrossRef CAS PubMed
.
- P. Lichtenberger and T. Hooton, Curr. Infect. Dis. Rep., 2008, 10, 499–504 CrossRef PubMed
.
-
M. Grabe, R. Bartoletti, T. E. Bjerklund Johansen, T. Cai, M. Çek, B. Köves, K. G. Naber, R. S. Pickard, P. Tenke, F. Wagenlehner and B. Wullt, EAU: Urological Infections, 2015, pp. 1–85 Search PubMed
.
- B. C. Peach, G. J. Garvan, C. S. Garvan and J. P. Cimiotti, Gerontol. Geriatr. Med., 2016, 2, 1–7 Search PubMed
.
- L. G. Giesen, G. Cousins, B. D. Dimitrov, F. A. van de Laar and T. Fahey, BMC Fam. Pract., 2010, 11, 78 CrossRef PubMed
.
- M. S. Najar, C. L. Saldanha and K. A. Banday, Indian J. Nephrol, 2009, 19, 129–139 CrossRef CAS PubMed
.
- L. E. Nicolle, Clin. Geriatr. Med., 2016, 32, 523–538 CrossRef PubMed
.
- G. Schmiemann, E. Kniehl, K. Gebhardt, M. M. Matejczyk and E. Hummers-Pradier, Deutsches Ärzteblatt Int., 2010, 107, 361–367 Search PubMed
.
- F. M. E. Wagenlehner, M. Cek, K. G. Naber, H. Kiyota and T. E. Bjerklund-Johansen, World J. of Urol., 2012, 30, 59–67 CrossRef CAS PubMed
.
- S. Karve, K. Ryan, P. Peeters, E. Baelen, S. Rojas-Farreras, D. Potter and J. Rodríguez-Baño, J. Infect., 2018, 76, 121–131 CrossRef PubMed
.
- S. B. Levy and B. Marshall, Nat. Med. Suppl., 2004, 10, 122–129 CrossRef
.
- G. Raman, E. Avendano, S. Berger and V. Menon, BMC Infect. Dis., 2015, 15, 395 CrossRef PubMed
.
- M. A. Pfaller and R. N. Jones, Arch. Pathol. Lab. Med., 2006, 130, 767–778 CrossRef CAS PubMed
.
- J. D. Besant, E. H. Sargent and S. O. Kelley, Lab Chip, 2015, 15, 2799–2807 RSC
.
- M. C. Roberts, S. Schwartz and H. J. M. Aarts, Front. Microbiol., 2012, 3, 1–17 Search PubMed
.
- J. Davies and D. Davies, Microbiol. Mol. Biol. Rev., 2010, 74, 417–433 CrossRef CAS PubMed
.
- J. O'Brien, I. Wilson, T. Orton and F. Pognan, Eur. J. Biochem., 2000, 267, 5421–5426 CrossRef PubMed
.
- S. Khazalpour and D. Nematollahi, RSC Adv., 2014, 4, 8431–8438 RSC
.
- E. Montoro, D. Lemus, M. Echemendia, A. Martin, F. Portaels and J. C. Palomino, J. Antimicrob. Chemother., 2005, 55, 500–505 CrossRef CAS
.
- A. Mariscal, R. M. Lopez-Gigosos, M. Carnero-Varo and J. Fernandez-Crehuet, Appl. Microbiol. Biotechnol., 2009, 82, 773–783 CrossRef CAS
.
- J. Avesar, D. Rosenfeld, M. Truman-Rosentsvit, T. Ben-Arye, Y. Geffen, M. Bercovici and S. Levenberg, Proc. Natl. Acad. Sci. U. S. A., 2017, 114, E5787 CrossRef CAS PubMed
.
- J.
L. Chen, T. W. J. Steele and D. C. Stuckey, Biotechnol. Bioeng., 2017, 115, 351–358 CrossRef
.
- A. Bolotsky, R. Muralidharan, D. Butler, K. Root, W. Murray, Z. Liu and A. Ebrahimi, Biosens. Bioelectron., 2021, 172, 112615 CrossRef CAS
.
- A. García-Miranda Ferrari, S. J. Rowley-Neale and C. E. Banks, Talanta Open, 2021, 3, 100032 CrossRef
.
- A. L. Squissato, R. A. A. Munoz, C. E. Banks and E. M. Richter, ChemElectroChem, 2020, 7, 2211–2221 CrossRef CAS
.
- R. O. Kadara, N. Jenkinson and C. E. Banks, Electroanalysis, 2009, 21, 2410–2414 CAS
.
- S. Hannah, E. Addington, D. Alcorn, W. Shu, P. A. Hoskisson and D. K. Corrigan, Biosens. Bioelectron., 2019, 145, 111696 CrossRef CAS PubMed
.
- Y. Ren, J. Ji, J. Sun, F. Pi, Y. Zhang and X. Sun, J. Solid State Electrochem., 2020, 24, 1539–1549 CrossRef CAS
.
- J. P. Hughes, F. D. Blanco, C. E. Banks and S. J. Rowley-Neale, RSC Adv., 2019, 9, 25003–25011 RSC
.
- P. S. Adarakatti, M. Mahanthappa, J. P. Hughes, S. J. Rowley-Neale, G. C. Smith, S. Ashoka and C. E. Banks, Int. J. Hydrogen Energy, 2019, 44, 16069–16078 CrossRef CAS
.
- S. Rowley-Neale, C. W. Foster, G. Smith, D. A. C. Brownson and C. Banks, Sustain.e Energy Fuels, 2017, 1, 74–83 RSC
.
- E. M. Richter, D. P. Rocha, R. M. Cardoso, E. M. Keefe, C. W. Foster, R. A. Munoz and C. E. Banks, Anal. Chem., 2019, 91, 12844–12851 CrossRef CAS PubMed
.
- Resazurin 550-82-3, https://www.chemicalbook.com/ChemicalProductProperty_EN_CB5733831.htm, (accessed 16/02/2021, 2021).
-
R. G. Compton and C. E. Banks, Understanding Voltammetry, World Scientific (Europe), 2017 Search PubMed
.
- F. Scholz, ChemTexts, 2015, 1, 1–24 CrossRef CAS
.
-
M. J. Bono and W. C. Reygaert, in StatPearls, StatPearls Publishing, Treasure Island (FL), 2020 Search PubMed
.
- D. A. Armbruster and T. Pry, Clin. Biochem. Rev., 2008, 29(1), S49–S52 Search PubMed
.
- J. M. Andrews, J. Antimicrob. Chemother., 2001, 48, 5–16 CrossRef CAS PubMed
.
- H. Sun, C.-W. Chan, Y. Wang, X. Yao, X. Mu, X. Lu, J. Zhou, Z. Cai and K. Ren, Lab Chip, 2019, 19, 2915–2924 RSC
.
- K. M. Krause, A. W. Serio, T. R. Kane and L. E. Connolly, Cold Spring Harbor Perspect. Med., 2016, 6, 1–18 Search PubMed
.
- W. Su, L. Zhang, Y. Tao, G. Zhan, D. Li and D. Li, Electrochem. Commun., 2012, 22, 37–40 CrossRef CAS
.
- F. Arena, B. Viaggi, L. Galli and G. M. Rossolini, Pediatr. Infect. Dis. J., 2015, 34, 1128–1130 CrossRef PubMed
.
- S. Brosel-Oliu, O. Mergel, N. Uria, N. Abramova, P. van Rijn and A. Bratov, Lab Chip, 2019, 19, 1436–1447 RSC
.
- V. Kara, C. Duan, K. Gupta, S. Kurosawa, D. J. Stearns-Kurosawa and K. L. Ekinci, Lab Chip, 2018, 18, 743–753 RSC
.
- M. Safavieh, H. J. Pandya, M. Venkataraman, P. Thirumalaraju, M. K. Kanakasabapathy, A. Singh, D. Prabhakar, M. K. Chug and H. Shafiee, ACS Appl. Mater. Interfaces, 2017, 9, 12832–12840 CrossRef CAS PubMed
.
- P. Mishra, D. Singh, K. P. Mishra, G. Kaur, N. Dhull, M. Tomar, V. Gupta, B. Kumar and L. Ganju, J. Microbiol. Methods, 2019, 162, 69–76 CrossRef CAS PubMed
.
- K. Chotinantakul, W. Suginta and A. Schulte, Anal. Chem., 2014, 86, 10315–10322 CrossRef CAS PubMed
.
- Y. Chalenko, V. Shumyantseva, S. Ermolaeva and A. Archakov, Biosens. Bioelectron., 2012, 32, 219–223 CrossRef CAS PubMed
.
- T. S. Mann and S. R. Mikkelsen, Anal. Chem., 2008, 80, 843–848 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1an00850a |
| This journal is © The Royal Society of Chemistry 2021 |