A microfluidic chip for on-line derivatization and application to in vivo neurochemical monitoring†
Alec C.
Valenta
 a,
Cara I.
D'Amico
b,
Colleen E.
Dugan
a,
James P.
Grinias
a,
Cara I.
D'Amico
b,
Colleen E.
Dugan
a,
James P.
Grinias
 a and
Robert T.
Kennedy
a and
Robert T.
Kennedy
 *ab
*ab
aDepartment of Chemistry, University of Michigan, 930 N. University Avenue, Ann Arbor, Michigan 48109, USA. E-mail: rtkenn@umich.edu
bDepartment of Pharmacology, University of Michigan, 1150 W. Medical Center Drive, Ann Arbor, Michigan 48109, USA
First published on 14th December 2020
Abstract
Microfluidic chips can perform a broad range of automated fluid manipulation operations for chemical analysis including on-line reactions. Derivatization reactions carried out on-chip reduce manual sample preparation and improve experimental throughput. In this work we develop a chip for on-line benzoyl chloride derivatization coupled to microdialysis, an in vivo sampling technique. Benzoyl chloride derivatization is useful for the analysis of small molecule neurochemicals in complex biological matrices using HPLC-MS/MS. The addition of one or more benzoyl groups to small, polar compounds containing amines, phenols, thiols, and certain alcohols improves reversed phase chromatographic retention, electrospray ionization efficiency, and analyte stability. The current derivatization protocol requires a three-step manual sample preparation, which ultimately limits the utility of this method for rapid sample collection and large sample sets. A glass microfluidic chip was developed for derivatizing microdialysis fractions on-line as they exit the probe for collection and off-line analysis with HPLC-MS/MS. Calibration curves for 21 neurochemicals prepared using the on-chip method showed linearity (R2 > 0.99), limits of detection (0.1–500 nM), and peak area RSDs (4–14%) comparable to manual derivatization. Method temporal resolution was investigated both in vitro and in vivo showing rapid rise times for all analytes, which was limited by fraction length (3 min) rather than the device. The platform was applied to basal measurements in the striatum of awake rats where 19 of 21 neurochemicals were above the limit of detection. For a typical 2 h study, a minimum of 120 pipetting steps are eliminated per animal. Such a device provides a useful tool for the analysis of small molecules in biological matrices which may extend beyond microdialysis to other sampling techniques.
Introduction
Reaction automation is an attractive alternative to manual reagent dispensing for reducing sample handling and improving assay throughput. Microfluidics is well-suited for this function because it enables rapid and precise manipulation of low-volume samples. Automated reactions on microfluidic devices have been developed to address a variety of challenges including volume-limited biological assays,1 reaction screening,2 and drug discovery.3 On-chip reactions have been also been used for pre4 and post-column5,6 derivatizations to enable fluorescence detection for microchip capillary electrophoresis in fully integrated analytical systems. In this work, we describe a microfluidic chip capable of continuous on-line derivatization of a sampling stream for off-line analysis using high performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS). To demonstrate its utility, the device was applied to on-line derivatization of in vivo microdialysate for neurochemical monitoring in awake rats. This platform demonstrates the advantages of incorporating microfluidic sample preparation into traditional analytical workflows.Tracking concentration dynamics of chemicals in vivo is important for understanding their biological function and testing pharmacological interventions. In vivo measurements in the central nervous system are particularly difficult because of the relative inaccessibility of the brain and frequent need to combine chemical and behavioral monitoring.7,8 Microdialysis sampling is widely used for obtaining such measurements because a broad range of neurochemicals can be monitored simultaneously.9 In microdialysis, a semi-permeable membrane is inserted into the brain and perfused with artificial cerebrospinal fluid (aCSF). Dialysate is collected from the probe outlet at timed intervals for analysis.
HPLC-MS/MS has emerged as a technique well-suited for analysis of in vivo microdialysate.10–15 While methods have been developed for on-line HPLC-MS/MS, poor chromatographic retention of highly polar, unmodified neurochemicals limits analyte scope and temporal resolution.16,17 Pre-column benzoyl chloride (BzCl) derivatization, first utilized in conjunction with HPLC for UV detection of polyamines18,19 and sugars,20–22 significantly improves reversed-phase retention of small, polar analytes. For HPLC-MS, benzoylation also improves electrospray ionization and fragmentation efficiency.23 Together, such benefits enable high-sensitivity measurements and help make BzCl derivatization a valuable tool for neurochemical analysis.24–26 Up to 24 neurochemicals have been monitored with a 2 min separation using UHPLC-MS/MS and BzCl derivatization.27 Illustrating its utility, such methods have been applied to a variety of applications.11,28–34
The benzoylation reaction is rapid, with reactions complete in <10 s of vortexing at room temperature. Although rapid, protocols require addition of chemicals in three separate steps: sodium carbonate to buffer sample, BzCl to label, and internal standards for quantification. The reactivity of BzCl with protic solvents precludes mixing of these solutions prior to derivatization. Derivatization is best performed immediately after fraction collection to prevent oxidation of catecholamines and indoleamines. Although labeling is trivial for small sample sets, derivatizing large sample sets often encountered in microdialysis can be cumbersome. For example, collecting 3 min fractions across a 2 h experiment requires 120 pipetting steps (excluding calibration standards). The same experiment performed with 1 min fractions requires 360 pipetting steps, or 1 reagent addition every 20 s. Derivatizing at this rate creates the potential for error, can interfere with behavioral experiments, and reduces throughput because collecting and rapidly derivatizing samples from multiple animals simultaneously becomes impractical without risking analyte degradation in backlogged samples.
Here, we describe a microfluidic chip for sequential reagent addition of sodium carbonate, BzCl, and internal standard to the dialysate stream, eliminating the need for multiple pipetting steps for each fraction. Derivatized fractions are collected for off-line analysis by LC-MS/MS. The method was applied to monitor 21 widely studied neurotransmitters, neuromodulators, and metabolites in vivo using a 2 min gradient separation. Modifications were made to the existing BzCl derivatization workflow for reduction of precipitate within the device and improved analyte signal for low level neurochemicals. Such improvements may be adapted to other biological sampling techniques including on-chip cell and tissue-based secretion platforms. Ultimately, this device provides the possibility for future adaptation of BzCl derivatization to fully automated workflows for in vivo monitoring of a broad range of chemicals within interstitial fluid compartments.
Methods
Chemicals and reagents
All chemicals were purchased from Sigma Aldrich (Saint Louis, MO) unless otherwise noted. Acetonitrile was purchased from Honeywell Research Chemicals through VWR (Radnor, PA). NaCl, KCl, MgSO4, CaCl2, and sulfuric acid were purchased from Fisher Scientific (Fairlawn, NJ). 3,4-Dihydroxyphenylacetic acid (DOPAC), 3-methoxytyramine (3-MT), sodium phosphate dibasic and sodium tripolyphosphate were purchased from Acros Organics (Geel, Belgium). Serotonin (5-HT) was purchased from Alfa Aesar (Ward Hill, MA). D4-acetylcholine (ACh) and choline (Ch) were purchased from CDN Isotopes (Pointe-Claire, Quebec). Stock solutions of 2 mM tyrosine (Tyr); 5 mM adenosine (Ado); 10 mM 5-HT, norepinephrine (NE), dopamine (DA), 3-MT, epinephrine (Epi); 20 mM histamine (Hist), γ-aminobutyric acid (GABA), aspartate (Asp), homovanillic acid (HVA), glycine (Gly), glutamate (Glu), DOPAC, phenylalanine (Phe), serine (Ser); 25 mM ACh; 40 mM (taurine) Tau; 100 mM glutamine (Gln), glucose (Glc); and 1 M Ch were prepared in HPLC grade water. A calibration standard mixture was prepared by diluting stocks into aCSF consisting of 145 mM NaCl, 2.68 mM KCl, 1.4 mM CaCl2, 1.0 mM MgSO4, 1.55 mM Na2HPO4, and 0.45 mM NaH2PO4 adjusted to pH of 7.4 with 0.1 M NaOH. Calibration curves were prepared in aCSF with 250 μM ascorbic acid for the following concentration ranges: 0.1–50 nM for 5-HT, NE, DA, 3-MT, and Epi; 0.25–125 nM for ACh; 0.5–250 nM for Hist; 1–500 nM for GABA and Ado; 0.002–1 μM for Asp; 0.0025–1.25 μM for HVA and Tyr; 0.01–5 μM for Glu, Gly, Phe, Ch, and DOPAC; 0.02–10 μM for Tau; 0.025–12.5 for Ser, 0.05–25 μM for Gln, and 0.25–125 μM for Glc.All-glass chip fabrication
Microfluidic devices were fabricated from D263 glass slides (Telic Company, Valencia, CA). Photolithography and hydrofluoric acid etching were modified from previous protocols.35–39 Briefly, slides were baked at 70 °C for 10 min, then coated with AZ1505 photoresist (Integrated Micro Materials, Argyle, TX). After exposure and development, slides were etched with 49% hydrofluoric acid: hydrochloric acid: water, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 to a depth of 85 μm. Following the wet etch, access holes were drilled with 1 mm drill bits. Slides were then washed for 20 min in piranha solution and 40 min in heated RCA solution. Post-wash, slides were aligned under a stream of water and bonded at 560 °C for 8 h. Captite Bonded-port connectors (LabSmith, Livermore, CA) were secured over each drill hole with Loctite Marine Epoxy (Loctite, Düsseldorf, Germany). Prior to device operation, channels were derivatized with 2% trichloro(1H,1H,2H,2H-perfluorooctyl)silane dissolved in perfluorodecalin (PFD). The devices were left to dry at 70 °C overnight then flushed with PFD to remove excess silanes. PFD was flushed from chips with nitrogen and allowed to dry overnight at 70 °C.
2 to a depth of 85 μm. Following the wet etch, access holes were drilled with 1 mm drill bits. Slides were then washed for 20 min in piranha solution and 40 min in heated RCA solution. Post-wash, slides were aligned under a stream of water and bonded at 560 °C for 8 h. Captite Bonded-port connectors (LabSmith, Livermore, CA) were secured over each drill hole with Loctite Marine Epoxy (Loctite, Düsseldorf, Germany). Prior to device operation, channels were derivatized with 2% trichloro(1H,1H,2H,2H-perfluorooctyl)silane dissolved in perfluorodecalin (PFD). The devices were left to dry at 70 °C overnight then flushed with PFD to remove excess silanes. PFD was flushed from chips with nitrogen and allowed to dry overnight at 70 °C.
On-chip mixing analysis
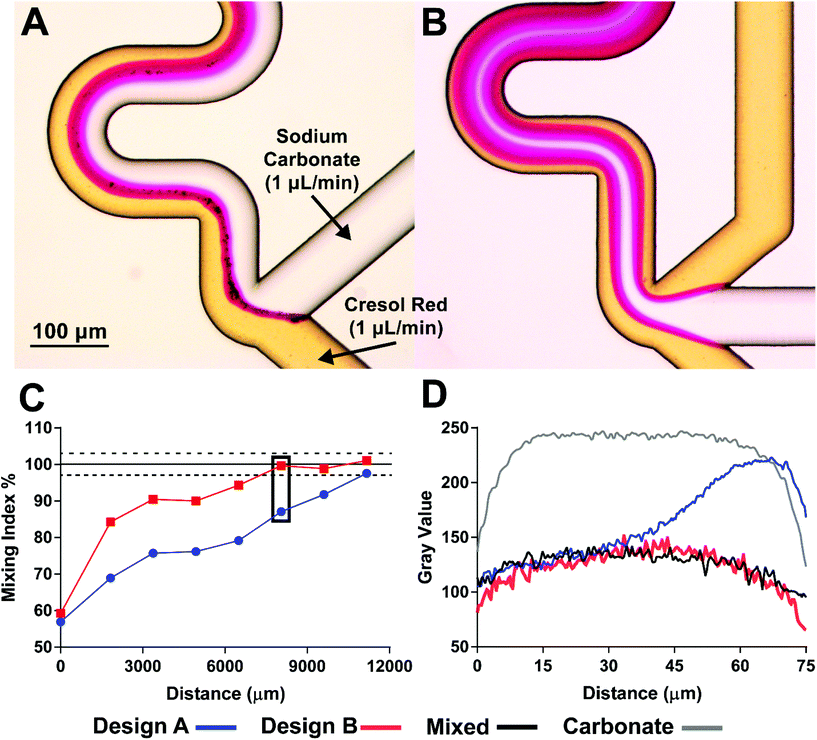
For on-chip mixing visualization at different mixing geometries, cresol red pH indicator was mixed with sodium carbonate (both at 1 μL min−1). Mixing was observed as a color change from yellow (neutral pH) to magenta (basic pH). Color analysis was performed using ImageJ (version 1.52, NIH). The gray scale intensity profile of radial line scans were used to compare each geometry to complete mixing. Mixing index was defined as the ratio of the average pixel intensity of a color profile across the mixing channel and the average pixel intensity of a fully mixed channel, expressed as a percentage.UHPLC-MS/MS assay for Bz-labeled neurochemicals
All analysis was done using a Thermo Fisher Vanquish UHPLC interfaced with a Thermo Fisher TSQ Quantum Ultra triple-quadrupole mass spectrometer. Briefly, 5 μL of sample was injected onto a Phenomenex Kinetex C18 column (2.1 mm × 100 mm, 1.7 μm). Mobile phase A consisted of 10 mM ammonium formate and 0.15% formic acid. Mobile phase B was pure acetonitrile. The method flow rate was 600 μL min−1 and the gradient was as follows: initial, 5% B; 0.01 min, 19% B; 0.68 min, 26% B; 1.055 min, 75% B; 1.805–2.18 min, 100% B; 2.28–3 min, 5% B. The pressure range across each gradient was 350–800 bar. The autosampler was set to 25 °C while the column was held at 30 °C in still air mode. ESI was used in positive mode with a spray voltage of 2.5 kV. Vaporizer and transfer capillary temperatures were 400 °C. Sheath gas was set to 10 and auxiliary gas was set to 5. The skimmer offset was −1 V and tube lens values were tuned for each individual analyte. The TSQ Quantum Ultra was operated in MRM mode. Total analysis time was 4 min.On-chip calibration curves
All reagents were prepared the day of the experiment. Reagent 1 (C/TP): 100 mM sodium carbonate/100 mM sodium tripolyphosphate in H2O. Reagent 2 (BzCl): 0.5% BzCl in acetonitrile (v/v). Reagent 3 (IS): 13C6-Bz derivatized internal standards in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 acetonitrile
1 acetonitrile![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O with 1% H2SO4 (v/v). Off-line derivatization was performed by pipetting standards and reagents into autosampler vials with ∼5 s of vortexing between each reagent addition as previously described.29 For on-chip derivatization, reagents and standards were loaded into 1 mL glass syringes (Hamilton Company, Reno, NV) and driven by two separate fusion 400 syringe pumps (Chemyx, Strafford, TX). Standards were flowed at 1 μL min−1 while reagents were flowed at 0.5 μL min−1 to give a ratio of addition of 2
H2O with 1% H2SO4 (v/v). Off-line derivatization was performed by pipetting standards and reagents into autosampler vials with ∼5 s of vortexing between each reagent addition as previously described.29 For on-chip derivatization, reagents and standards were loaded into 1 mL glass syringes (Hamilton Company, Reno, NV) and driven by two separate fusion 400 syringe pumps (Chemyx, Strafford, TX). Standards were flowed at 1 μL min−1 while reagents were flowed at 0.5 μL min−1 to give a ratio of addition of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (standard
1 (standard![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C/TP
C/TP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BzCl
BzCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IS). Fractions were collected at 3 min intervals (7.5 μL total volume) in vials for off-line UHPLC-MS/MS analysis. Each concentration was perfused through the system for 15 min before collecting 5 fractions to generate both blanks and a 6-point calibration curve. All fluidic switching was done using a 6-port microbore HPLC switching valve (VICI, Houston, TX) for uninterrupted flow.
IS). Fractions were collected at 3 min intervals (7.5 μL total volume) in vials for off-line UHPLC-MS/MS analysis. Each concentration was perfused through the system for 15 min before collecting 5 fractions to generate both blanks and a 6-point calibration curve. All fluidic switching was done using a 6-port microbore HPLC switching valve (VICI, Houston, TX) for uninterrupted flow.
Microdialysis probe construction
Briefly, custom concentric microdialysis probes were constructed for implantation into the rat striatum. An inlet (40/110, ID/OD) and outlet (75/150) fused silica capillary were glued into a 13.5 mm (25 G) piece of stainless steel hypodermic sheath tubing (Small Parts Inc., Logansport, In). The inlet capillary was cut to a length of 4 mm extending beyond the sheath and a regenerated cellulose dialysis membrane (18 kDa MWCO, Spectrum Life Sciences, LLC. Rancho Dominquez, CA) was placed over the inlet and inserted into the sheath, yielding a 4 mm probe active area. Before in vivo use, the probe was rinsed with 70% ethanol, then flushed extensively with sterile aCSF.In vitro concentration step-changes
Chip temporal response to concentration changes was explored through in vitro step-change experiments. A microdialysis probe perfused with aCSF at 1 μL min−1 was placed in a stirred vial containing aCSF and connected to the inlet of the derivatization chip. System dead time and analyte residence time on-chip were visualized by microdialysis sampling of cresol red pH indicator and mixing with sodium carbonate buffer on-chip. The color change was observed at the first reagent junction and monitored until it reached the device outlet. Analyte residence time within the chip was measured to be 1.64 ± 0.02 min (excluding inlet/outlet ports). For step change experiments, the microdialysis probe was placed in a stirred vial of aCSF at a flow rate of 1 μL min−1 connected to the device. All reagents were flowed at 0.5 μL min−1. Once stable flow was established the chip was equilibrated for 30 min before fraction collection. To approximate an in vivo microdialysis experiment, 3 min fractions were collected at the outlet of the device. After collection of 5 blank fractions, a standard mixture was spiked into the stirred vial repeatedly to create sharp concentration step changes in the vial. Five 3 min fractions were collected at each concentration and after the high-point the probe was quickly moved to a fresh vial of aCSF to collect another five blank samples.In vivo microdialysis
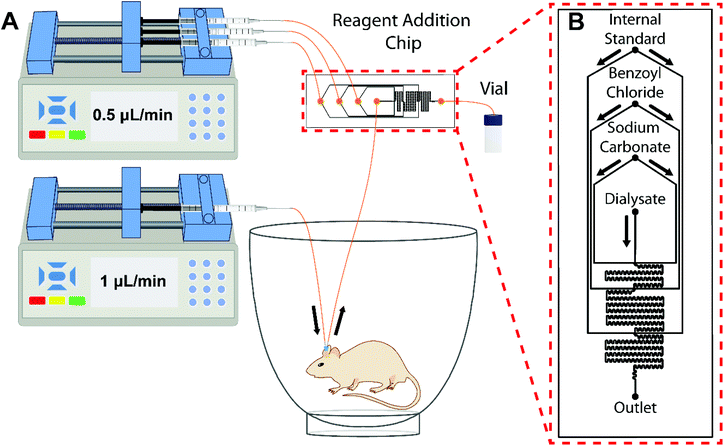
In vivo neurochemical measurements were performed in male Sprague Dawley rats (300–350 g). Rats were anesthetized using 1–5% isoflurane and mounted to a stereotaxic instrument (David Kopf Instruments, Tujunga, CA). A 4 mm microdialysis probe was implanted into the striatum using the following coordinates with reference to bregma: +1 mm anterior, ±3 mm lateral, and −6.75 mm ventral to the surface of the brain. For basal measurements in awake rats, three stainless steel skull screws (Plastics Once Inc., Roanoke, VA) were used to mount the probe to the brain. Dental cement (A-M Systems, Sequim, WA) was used to encase the probe and skull screws to secure the probe. Rats were tethered to a Raturn (Bioanalytical Systems, Inc., West Lafeyette, IN) and perfused continuously with aCSF at 0.5 μL min−1 during the 24-h recovery period. Two hours prior to fraction collection a fresh syringe of aCSF (250 μM ascorbic acid) was perfused at 1 μL min−1 and 30 min prior to collection the reagents and dialysate were flowed through the chip. Three min basal fractions were collected for 30 min and averaged. Fig. 1 shows the experimental setup for on-chip derivatization in awake rats. Temporal response was investigated in anesthetized rats using 75 mM K+ aCSF retrodialysis. The probe was connected to the chip and flowed at 1 μL min−1 for 15 min before fraction collection. A total of 10 (30 min) baseline samples were collected followed by two 3 min 75 mM K+ stimulations separated by 30 min to allow for return to baseline. All procedures were approved by the Institutional Animal Care & Use Committee (IACUC) of the University of Michigan in accordance with Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) guidelines.Results and discussion
Microfluidic chip design
Key considerations when designing a microfluidic device for BzCl derivatization coupled on-line to microdialysis include backpressure, mixing rate, temporal resolution, reagent compatibility, and durability. Device backpressure must be kept low to prevent dialysis membrane rupturing. On-chip mixing rate must be sufficient to ensure the labeling reaction proceeds to completion at microdialysis-compatible flow rates (∼1 μL min−1). Ideally, the device will have little impact on temporal resolution and, therefore, preserve transient concentration changes for analysis. Finally, compatibility with all reagents is required for continuous operation during experiments lasting >2 h.
Fig. 1 illustrates the workflow for an in vivo microdialysis experiment with on-line BzCl derivatization and off-line fraction collection. To match the manual derivatization protocol reagents were delivered sequentially into the device at a volume ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (dialysate
1 (dialysate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sodium carbonate
sodium carbonate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BzCl
BzCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) internal standard mixture). For 3 min fractions at a microdialysis flow rate of 1 μL min−1 (regent flow rates of 0.5 μL min−1), the total sample volume collected was 7.5 μL. While the device could be operated at different flow rates, further investigate would be required to determine is mixing requirements and backpressure limits were met. Channels were fabricated with a width of 75 μm and depth of 85 μm, the smallest dimensions compatible with probe backpressure limits, yielding an estimated total chip volume of 3.6 μL. Such narrow channels minimize chip volume and longitudinal dispersion of concentration profiles within the device which can impact method temporal resolution. Device backpressure was conservatively estimated to be <0.75 psi, and was not expected, or observed, to cause bleeding through the membrane.
internal standard mixture). For 3 min fractions at a microdialysis flow rate of 1 μL min−1 (regent flow rates of 0.5 μL min−1), the total sample volume collected was 7.5 μL. While the device could be operated at different flow rates, further investigate would be required to determine is mixing requirements and backpressure limits were met. Channels were fabricated with a width of 75 μm and depth of 85 μm, the smallest dimensions compatible with probe backpressure limits, yielding an estimated total chip volume of 3.6 μL. Such narrow channels minimize chip volume and longitudinal dispersion of concentration profiles within the device which can impact method temporal resolution. Device backpressure was conservatively estimated to be <0.75 psi, and was not expected, or observed, to cause bleeding through the membrane.
For successful operation, the device substrate must be compatible with dialyzed cerebrospinal fluid, reagents that are 100% aqueous and 100% acetonitrile, and pH range of 2–10. Both PDMS-glass and all-glass devices were evaluated for compatibility with BzCl derivatization. Although PDMS-glass devices are cheaper and more easily fabricated,40 we found them unsuitable because rapid precipitation of reagents limited devices to 1–2 min of operation prior to clogging. Such precipitation was preventable only through the removal of BzCl. PDMS-based devices have been shown to suffer from slight acetonitrile permeability41 and significant surface fouling from hydrophobic compounds.42 Therefore, we hypothesize that adsorption of BzCl to the surface and increasing local concentrations of BzCl due to PDMS absorbing acetonitrile are both factors contributing to device clogging. All-glass reagent addition chips were next tested and yielded a significant reduction in visible precipitate at the BzCl junction and no clogging during 80 min of continuous aCSF infusion (Fig. S1†). This reduction may be attributed to a combination of glass surface polarity and impermeability to acetonitrile. Without the presence of hydrophobic surface groups, all-glass devices are potentially less prone to the BzCl adsorption. Such reduction enables chip operation across relevant timescales for in vivo neurochemical measurements.
Reducing carbonate precipitation
When combined with aCSF on glass chips, sodium carbonate initiated a steady buildup of salt on the channel walls which was preventable through the removal of Mg2+ and Ca2+ from the aCSF. With respective Ksp of 3.36 × 10−9 and 6.82 × 10−6, CaCO3 and MgCO3 were concluded to be the likely source of precipitate. Precipitation was addressed through the addition of sodium triphosphate, a commonly used chelating agent,43 to the sodium carbonate reagent stream. Triphosphate was chosen for its ability to bind tightly to divalent cations, solubility in a wide pH range, and tolerance to moderate amounts of acetonitrile. Sodium triphosphate was dissolved in the carbonate reagent in large molar excess (100 mM) to the CaCl2 (1.4 mM) and MgSO4 (1 mM) in aCSF to prevent precipitation of carbonate salts. Fig. S2† shows the effect of sodium triphosphate on reducing precipitation following 2 h of continuous operation.On-chip mixing
Although BzCl did not precipitate within all-glass devices during reagent addition to an aCSF stream, a distinct phase boundary was seen between water and acetonitrile at the BzCl junction (Fig. S1†). Moreover, addition of neurochemicals led to precipitation on channel walls (Fig. S3†). Not only is this precipitate a concern for device clogging, but loss of analyte through adsorption to the device walls can also impact sensitivity and temporal response. This precipitation is believed to occur, in part, because of poor on-chip mixing. When laminar flow persists between acetonitrile and water phases following benzoyl chloride reagent addition, analytes may undergo benzoylation at the solvent interface and precipitate due to poor solubility in H2O. At the range of Reynolds numbers found in microfluidic devices (generally < 100) laminar flow dominates and mixing requires input of energy, specialized geometries, or some combination of both.44Several reagent addition geometries were explored to improve passive mixing.45 The two best-performing geometries utilized a sharp turn into serpentine mixers at a Y-junction junction with either a single (Fig. S4-A†) or dual-addition junction with each reagent split into parallel channels (Fig. S4-B†). Mixing performance was evaluated using sodium carbonate and cresol red pH indicator.46 As cresol red mixed with sodium carbonate, a color shift from yellow to magenta was observed (Fig. 2A and B). The completeness of mixing at sequential serpentines along the devices, termed “mixing index”, was defined as the average pixel intensity across the mixing channel diameter divided by the average pixel intensity of a fully mixed channel, expressed as a percentage (Fig. 2C). Faster mixing was observed in design B, with complete mixing observed at serpentine 10 (8053 μm post-junction) compared to serpentine 14 (11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 170 μm post-junction) in design A. For visualization, Fig. 2D shows radial color profiles for each device taken at serpentine 10 (black box), for complete mixing taken at serpentine 40 (black), and for unmixed carbonate taken before the junction (grey). Complete mixing was seen as a flat gray scale value across the diameter of the channel. Design A had two distinct regions across the channel diameter: well mixed (0–30 μm) and completely unmixed (55–70 μm) suggesting only partial mixing well past the junction. In contrast, Design B showed complete mixing at serpentine 10. The results indicate that design B enabled faster mixing, although design A may still represent a viable alternative. Improved mixing in design B is attributed to doubling the surface area for solution contact and, therefore, shortening the distance for diffusion between reagent streams.47
170 μm post-junction) in design A. For visualization, Fig. 2D shows radial color profiles for each device taken at serpentine 10 (black box), for complete mixing taken at serpentine 40 (black), and for unmixed carbonate taken before the junction (grey). Complete mixing was seen as a flat gray scale value across the diameter of the channel. Design A had two distinct regions across the channel diameter: well mixed (0–30 μm) and completely unmixed (55–70 μm) suggesting only partial mixing well past the junction. In contrast, Design B showed complete mixing at serpentine 10. The results indicate that design B enabled faster mixing, although design A may still represent a viable alternative. Improved mixing in design B is attributed to doubling the surface area for solution contact and, therefore, shortening the distance for diffusion between reagent streams.47
Improving reaction yield
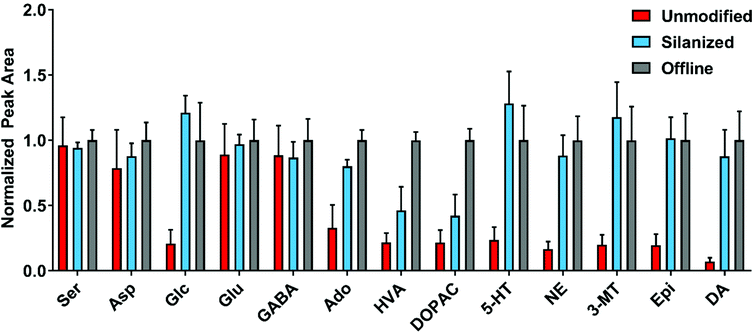
Despite prevention of visible precipitate at the BzCl junction, a substantial loss in analyte peak area was observed when comparing on-chip and benchtop derivatization (Fig. 3). This loss in signal may result from analyte degradation, incomplete benzoylation, or loss of analyte to surface adsorption within the device. The effect of device surface chemistry on analyte signal was investigated through the use of glass surface silanization. Fluoroalkyl silane surface modifications have been used to prevent surface fouling in microfluidic devices for a variety of applications.48–52 1H,1H,2H,2H-Perfluorooctyl-trichlorosilane was chosen to reduce analyte adsorption to the channel walls. Fig. 3 shows a large improvement in analyte peak areas in devices with surface modification. Increases in analyte signal were observed in 11 of 21 analytes and were particularly notable for low concentration monoamine neurotransmitters including 5-HT, NE, Epi, and DA. We, therefore, hypothesize that analyte surface adsorption within the device was a major factor in reducing peak areas although prevention of reactions on the glass surface may also play a role.Device validation
Calibration curves (Fig. S5†) were generated for all 21 analytes using on-chip derivatization by delivering standards dissolved in aCSF into the chip with a syringe pump and a 6-port valve for switching between concentrations. Fractions were collected at 3 min intervals (n = 5). Figures of merit for each analyte are summarized in Table 1. Limits of detection (LODs) were calculated using the limit of blank method.53 LODs below 1 nM were achieved for Hist, Ado, 5-HT, NE, 3-MT, Epi, and DA. Overall, LODs, linearity, and percent relative standard deviation (peak area ratio) were comparable to previous reports for manual benzoylation.23,29| Analyte | RSD (%) | Fit (R2) | LoD (nM) | Basal concentration (nM) |
|---|---|---|---|---|
| Relative standard deviation, linearity (R2), and limits of detection were calculated from 6-point on-chip calibration curves (n = 5) prepared in aCSF. All analytes were benzoylated except for choline and acetylcholine. RSD values were calculated using the level 4 calibration standard. Basal concentrations were determined by averaging 10 baseline samples collected from the striatum of awake at 1 μL min−1 (n = 3).a Below limit of detection in the striatum. | ||||
| Choline | 6 | 0.9999 | 50 | 160 ± 30 |
| Acetylcholine | 6 | 0.9979 | 2 | 4 ± 1 |
| Taurine | 10 | 0.9998 | 80 | 250 ± 100 |
| Histamine | 4 | 0.9989 | 0.7 | 3 ± 1 |
| Serine | 5 | 0.9998 | 160 | 8800 ± 1200 |
| Glutamine | 7 | 0.9997 | 20 | 34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ± 4000 000 ± 4000 |
| Aspartate | 7 | 0.9996 | 20 | 40 ± 15 |
| Glycine | 11 | 0.9997 | 130 | 9600 ± 2500 |
| Glucose | 5 | 0.9986 | 500 | 530![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ± 60 000 ± 60 |
| Glutamate | 6 | 0.9990 | 10 | 250 ± 70 |
| GABA | 6 | 0.9998 | 1 | 60 ± 15 |
| Adenosine | 8 | 0.9993 | 0.9 | 7 ± 3 |
| Phenylalanine | 10 | 0.9993 | 6 | 660 ± 130 |
| HVA | 4 | 0.9997 | 2 | 1100 ± 470 |
| Tyrosine | 7 | 0.9968 | 105 | |
| DOPAC | 11 | 0.9983 | 6 | 1200 ± 190 |
| 5-HT | 14 | 0.9963 | 0.1 | 1 ± 0.05 |
| Norepinephrine | 11 | 0.9970 | 0.1 | 1 ± 0.06 |
| 3-MT | 9 | 0.9996 | 0.1 | 3 ± 0.6 |
| Epinephrine | 5 | 0.9999 | 0.3 | |
| Dopamine | 6 | 0.9985 | 0.4 | 6 ± 2 |
For in vivo device validation, microdialysis fractions were collected from the striatum of awake rats (n = 3). Basal concentrations in Table 1 are an average of 10 fractions collected from each animal. Of the 21 analytes, 19 were routinely measurable. As expected, Epi was below the limit of detection in the rat striatum.29
Evaluating temporal resolution
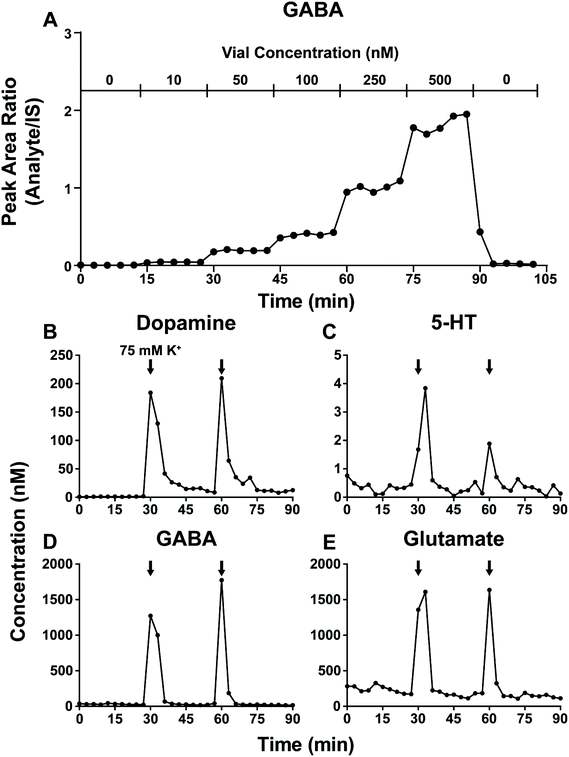
The ability of microdialysis sampling to capture transient biological events is dictated by the temporal resolution of the system. Factors limiting temporal resolution include sample requirements for analysis (large sample requirements worsen temporal resolution) and broadening of sample concentration zones as they flow toward fraction collection.54 To assess the device temporal resolution, 3 min fractions were collected from a stirred vial while spiking in increasing analyte concentrations. Defining temporal resolution as the rise time from 10–90% analyte signal, the device has an in vitro temporal resolution of 3 min (Fig. 4A). This suggest that on-chip mixing and dead volume have negligible impacts on concentration profiles when collecting 3 min fractions. While rise time is rapid, a ∼20% carryover was observed in the first fraction after the returning to a blank aCSF solution. Two potential sources for carryover are: (1) analyte adsorption within the device and (2) mechanical carryover from switching between analyte solutions. Further investigations of the potential for higher temporal resolution with this approach are warranted since the on-line method should be especially useful for experiments that require collection of many fractions.The origin of carryover and utility of the device for measuring concentration changes in vivo were explored using high K+ retrodialysis. Elevated K+ concentration is known to cause rapid depolarization of neurons and release of many neurotransmitters. A series of 10 baseline samples were collected followed by a pair of 3 min 75 mM K+ stimulations spaced 30 min apart. A 6-port valve was used to place a 3 μL sample loop filled with 75 mM K+ aCSF in the flow path without disrupting flow to the probe. DA, 5-HT, GABA, and Glu all show a rapid rise in concentration in response to the 75 mM K+ but a delayed return to baseline similar to the step-change experiment (Fig. 4B–E). Similar responses can be seen for NE, 5-HT, Asp, Ado, and Ch (Fig. S6†). DA requires longer to return to baseline than Glu or GABA suggesting either differing biological responses or possibly carryover scaling with benzoylated analyte hydrophobicity.
Conclusions
On-line derivatization of microdialysis samples has several advantages over off-line sample preparation. By using automated labeling in a microfluidic chip, challenges including analyte degradation when exposed to air and difficulty in preparing small volume samples are overcome. Furthermore, because the reaction occurs continuously in flow, temporal resolution is no longer hampered by the need to manually add reagents to collected fractions. Reaction automation also limits disturbances to animals during experimentation which can impact both behavior and neurochemical measurements.55 The use of on-line derivatization also opens the door to a fully on-line system wherein derivatized samples are directly injected the HPLC for analysis. Several platforms have been developed for on-line analysis with microdialysis including those based on capillary electrophoresis,56–59 HPLC,60–64 biosensors65–67 and mass spectrometry.49 These methods offer near real time out but require fast analysis. For example, on-line methods for measuring 5-HT and DA with 1 min temporal resolution have been previously developed using fast capillary HPLC with electrochemical detection.54,68 It remains to be determined if temporal resolution can be improved to compete with these other methods while maintaining the broad analyte capability of the BzCl-HPLC-MS method. Achieving such temporal resolution would require more detailed evaluation of the broadening associated with the chip and evaluation of separation conditions for the analytes at the minute or less time scale. Achieving such improvements would be significant because although on-line methods have enabled measurement of a range of neurochemicals, no method has successfully been applied to more than a single or small group of analytes.69 BzCl derivatization offers broad analyte scope (including catecholamines, amino acids, sugars, nucleosides etc.).This work demonstrates the use of a novel all-glass microfluidic device for the benzoylation of neurochemicals directly coupled to in vivo microdialysis. BzCl derivatization serves as an example of a reaction which suffers from solubility issues at the microfluidic scale, but not at during benchtop, off-line derivatization. This device illustrates how challenges with reaction chemistry including precipitation can arise in the microfluidic environment but be overcome through the use of modifications such as surface treatment and additives. For example, Fig. S2† illustrates the use of sodium triphosphate chelation of Ca2+ chelate and Mg2+ and prevent large-scale precipitation at the sodium carbonate reagent addition. Furthermore, peak areas of low-level neurochemicals were markedly improved through perfluorosilane surface modification (Fig. 3). Calibration curves generated on-chip showed strong figures of merit including linearity, LoDs, and repeatability for 21 widely-studied neurochemicals. Such figures are comparable to previous reports of off-line BzCl derivatization.23,29 Basal concentrations of 19 of 21 analytes were measurable in the striatum of awake rats. Temporal response was investigated in vitro through step-change experiments and in vivo using 75 mM K+ retrodialysis in an anesthetized rat. Devices exhibited rapid rise times in response to concentration increases with moderate carryover seen when returning to blank/basal concentrations.
Development of on-chip BzCl derivatization represents a crucial step toward a platform for on-line derivatization and analysis of benzoylated neurochemicals in microdialysate. Future work may be aimed at performing faster separations to match sampling intervals, thus enabling automated analysis when experimental design permits animals to be near the instrument. The device may be applied not only to neurochemical monitoring, but also to sampling techniques in other interstitial fluid compartments and to cell-based microfluidic secretion studies. Lastly, this work presents a series of modifications to surface and reaction chemistries which may aid in the development of future microfluidic flow reaction devices.
Author contributions
ACV designed devices, designed and carried out experiments, and wrote and revised the manuscript. CID and CED designed devices, carried out experiments, and revised the manuscript. JPG designed experiments and revised the manuscript. RTK designed and directed experiments and wrote and revised the manuscript.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
The authors thank Emory Payne and Brian Shay for input in device development. This work was supported by NIH grant R01EB003320. JPG was supported by NIH grand F32EB019800.References
- V. Houbart and M. Fillet, in Advances in Microfluidics - New Applications in Biology, Energy, and Materials Sciences, InTech, 2016 Search PubMed.
- E. M. Payne, D. A. Holland-Moritz, S. Sun and R. T. Kennedy, Lab Chip, 2020, 20, 2247–2262 RSC.
- P. S. Dittrich and A. Manz, Nat. Rev. Drug Discovery, 2006, 5, 210–218 CrossRef CAS PubMed.
- S. C. Jacobson, R. Hergenröder, A. W. Moore and J. M. Ramsey, Anal. Chem., 1994, 66, 4127–4132 CrossRef CAS.
- S. C. Jacobson, L. B. Koutny, R. Hergenröder, A. W. Moore and J. M. Ramsey, Anal. Chem., 1994, 66, 3472–3476 CrossRef CAS.
- D. J. Harrison, K. Fluri, N. Chiem, T. Tang and Z. H. Fan, Sens. Actuators, B, 1996, 33, 105–109 CrossRef CAS.
- K. N. Schultz and R. T. Kennedy, Annu. Rev. Anal. Chem., 2008, 1, 627–661 CrossRef CAS PubMed.
- M. Ganesana, S. T. Lee, Y. Wang and B. J. Venton, Anal. Chem., 2017, 89, 314–341 CrossRef CAS PubMed.
- Handbook of Microdialysis: Methods, Applications and Perspectives, ed. B. H. C. Westerink and T. I. F. H. Cremers, Academic Press, 1st edn, 2007 Search PubMed.
- P. Uutela, R. Reinilä, P. Piepponen, R. A. Ketola and R. Kostiainen, Rapid Commun. Mass Spectrom., 2005, 19, 2950–2956 CrossRef CAS PubMed.
- C. M. Patterson, J.-M. T. Wong, G. M. Leinninger, M. B. Allison, O. S. Mabrouk, C. L. Kasper, I. E. Gonzalez, A. Mackenzie, J. C. Jones, R. T. Kennedy and M. G. Myers, Endocrinology, 2015, 156, 1692–1700 CrossRef CAS PubMed.
- L. Zheng, X. E. Zhao, S. Zhu, Y. Tao, W. Ji, Y. Geng, X. Wang, G. Chen and J. You, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2017, 1054, 64–72 CrossRef CAS PubMed.
- R. E. Wilson, A. Jaquins-Gerstl and S. G. Weber, Anal. Chem., 2018, 90, 4561–4568 CrossRef CAS PubMed.
- C. Defaix, A. Solgadi, T. H. Pham, A. M. Gardier, P. Chaminade and L. Tritschler, J. Pharm. Biomed. Anal., 2018, 152, 31–38 CrossRef CAS PubMed.
- O. Bodner, I. Radzishevsky, V. N. Foltyn, A. Touitou, A. Valenta, I. F. Rangel, R. Panizzutti, R. T. Kennedy, J. M. Billard and H. Wolosker, J. Neurosci., 2020, 40(34), 6489–6502 CrossRef CAS PubMed.
- Q. Wang, J. Zhang, Z. Pi, Z. Zheng, J. Xing, F. Song, S. Liu and Z. Liu, Anal. Methods, 2014, 7, 45–52 RSC.
- M. Kim, J. Lee, C. H. Yang and S. Lee, Anal. Chim. Acta, 2016, 923, 55–65 CrossRef CAS PubMed.
- J. W. Redmond and A. Tseng, J. Chromatogr. A, 1979, 170, 479–481 CrossRef CAS.
- N. Seiler, J. Chromatogr. B: Biomed. Sci. Appl., 1986, 379, 157–176 CrossRef CAS.
- J. Lehrfeld, J. Chromatogr. A, 1976, 120, 141–147 CrossRef CAS.
- M. S. F. Ross, J. Chromatogr. A, 1977, 141, 107–119 CrossRef CAS.
- C. A. White, J. F. Kennedy and B. T. Golding, Carbohydr. Res., 1979, 76, 1–10 CrossRef CAS.
- P. Song, O. S. Mabrouk, N. D. Hershey and R. T. Kennedy, Anal. Chem., 2012, 84, 412–419 CrossRef CAS PubMed.
- W. H. Lee, T. Ngernsutivorakul, O. S. Mabrouk, J.-M. T. Wong, C. E. Dugan, S. S. Pappas, H. J. Yoon and R. T. Kennedy, Anal. Chem., 2016, 88, 1230–1237 CrossRef CAS PubMed.
- M. G. Fleszar, J. Wiśniewski, M. Krzystek-Korpacka, B. Misiak, D. Frydecka, J. Piechowicz, K. Lorenc-Kukuła and A. Gamian, Chromatographia, 2018, 81, 911–921 CrossRef CAS PubMed.
- J. Le, Z. Lin, L. Song, H. Wang and Z. Hong, J. Pharm. Biomed. Anal., 2019, 176, 112807 CrossRef CAS PubMed.
- J. P. Grinias, J.-M. T. Wong and R. T. Kennedy, J. Chromatogr. A, 2016, 1461, 42–50 CrossRef CAS PubMed.
- N. D. Hershey and R. T. Kennedy, ACS Chem. Neurosci., 2013, 4, 729–736 CrossRef CAS PubMed.
- J.-M. T. Wong, P. A. Malec, O. S. Mabrouk, J. Ro, M. Dus and R. T. Kennedy, J. Chromatogr. A, 2016, 1446, 78–90 CrossRef CAS PubMed.
- E. Kaplan, S. Zubedat, I. Radzishevsky, A. C. Valenta, O. Rechnitz, H. Sason, C. Sajrawi, O. Bodner, K. Konno, K. Esaki, D. Derdikman, T. Yoshikawa, M. Watanabe, R. T. Kennedy, J.-M. Billard, A. Avital and H. Wolosker, Proc. Natl. Acad. Sci. U. S. A., 2018, 115, 9628–9633 CrossRef CAS PubMed.
- A. Mohebi, J. R. Pettibone, A. A. Hamid, J. M. T. Wong, L. T. Vinson, T. Patriarchi, L. Tian, R. T. Kennedy and J. D. Berke, Nature, 2019, 570, 65–70 CrossRef CAS PubMed.
- A. G. Zestos, C. Carpenter, Y. Kim, M. J. Low, R. T. Kennedy and M. E. Gnegy, ACS Chem. Neurosci., 2019, 10, 1960–1969 CrossRef CAS PubMed.
- P. Mascia, Q. Wang, J. Brown, K. M. Nesbitt, R. T. Kennedy and P. Vezina, Prog. Neuropsychopharmacol. Biol. Psychiatry, 2020, 99, 109864 CrossRef CAS PubMed.
- P. A. Malec, M. Oteri, V. Inferrera, F. Cacciola, L. Mondello and R. T. Kennedy, J. Chromatogr. A, 2017, 1523, 248–256 CrossRef CAS PubMed.
- D. J. Harrison, K. Fluri, K. Seiler, Z. Fan, C. S. Effenhauser and A. Manz, Science, 1993, 261, 895–897 CrossRef CAS PubMed.
- M. G. Roper, J. G. Shackman, G. M. Dahlgren and R. T. Kennedy, Anal. Chem., 2003, 75, 4711–4717 CrossRef CAS PubMed.
- P. C. Simpson, A. T. Woolley and R. A. Mathies, Biomed. Microdevices, 1998, 1, 7–25 CrossRef CAS.
- OpenWetWare contributors, Mathies: Glass Etching in BNC.
- E. T. Lagally, P. C. Simpson and R. A. Mathies, Sens. Actuators, B, 2000, 63, 138–146 CrossRef CAS.
- J. M. K. Ng, I. Gitlin, A. D. Stroock and G. M. Whitesides, Electrophoresis, 2002, 23, 3461–3473 CrossRef CAS.
- J. N. Lee, C. Park and G. M. Whitesides, Anal. Chem., 2003, 75, 6544–6554 CrossRef CAS PubMed.
- H. Zhang and M. Chiao, J. Med. Biol. Eng., 2015, 35, 143–155 CrossRef PubMed.
- W. Klenk, Herbert; Gotz, Peter; Siegmeier, Rainer; Mayr, Ullman's Encycl. Ind. Chem, 2012, pp. 325–360 Search PubMed.
- H. A. Stone, A. D. Stroock and A. Ajdari, Annu. Rev. Fluid Mech., 2004, 36, 381–411 CrossRef.
- E. S. Shanko, Y. van de Burgt, P. D. Anderson and J. M. J. den Toonder, Micromachines, 2019, 10, 731 CrossRef PubMed.
- G. G. Yaralioglu, I. O. Wygant, T. C. Marentis and B. T. Khuri-Yakub, Anal. Chem., 2004, 76, 3694–3698 CrossRef CAS PubMed.
- A. Hibara, M. Tokeshi, K. Uchiyama, H. Hisamoto and T. Kitamori, Anal. Sci., 2001, 17, 89–93 CrossRef CAS PubMed.
- M. J. Owen and D. E. Williams, J. Adhes. Sci. Technol., 1991, 5, 307–320 CrossRef CAS.
- P. Song, N. D. Hershey, O. S. Mabrouk, T. R. Slaney and R. T. Kennedy, Anal. Chem., 2012, 84, 4659–4664 CrossRef CAS PubMed.
- D. J. Steyer and R. T. Kennedy, Anal. Chem., 2019, 91, 6645–6651 CAS.
- J. Zhang, S. Yang, C. Chen, J. H. Hartman, P. H. Huang, L. Wang, Z. Tian, P. Zhang, D. Faulkenberry, J. N. Meyer and T. J. Huang, Lab Chip, 2019, 19, 984–992 RSC.
- S. Breisch, B. De Heij, M. Löhr and M. Stelzle, J. Micromech. Microeng., 2004, 14, 497–505 CrossRef CAS.
- D. A. Armbruster and T. Pry, Clin. Biochem. Rev., 2008, 29(Suppl 1), S49–S52 Search PubMed.
- J. Zhang, A. Jaquins-Gerstl, K. M. Nesbitt, S. C. Rutan, A. C. Michael and S. G. Weber, Anal. Chem., 2013, 85, 9889–9897 CrossRef CAS.
- H. M. Schellinck, D. P. Cyr and R. E. Brown, in Advances in the Study of Behavior, Academic Press, 2010, vol. 41, pp. 255–366 Search PubMed.
- S. Y. Zhou, H. Zuo, J. F. Stobaugh, C. E. Lunte and S. M. Lunte, Anal. Chem., 1995, 67, 594–599 CrossRef CAS PubMed.
- M. W. Lada and R. T. Kennedy, Anal. Chem., 1996, 68, 2790–2797 CrossRef CAS PubMed.
- C. M. Ciriacks and M. T. Bowser, Anal. Chem., 2004, 76, 6582–6587 CrossRef CAS.
- M. Wang, G. T. Roman, M. L. Perry and R. T. Kennedy, Anal. Chem., 2009, 81, 9072–9078 CrossRef CAS PubMed.
- T. Azekawa, A. Sano, K. Aoi, H. Sei and Y. Morita, J. Chromatogr. B: Biomed. Sci. Appl., 1990, 530, 47–55 CrossRef CAS.
- J. Kehr, J. Chromatogr. B: Biomed. Sci. Appl., 1998, 708, 27–38 CrossRef CAS.
- C. S. Chaurasia, C. E. Chen and C. R. Ashby, J. Pharm. Biomed. Anal., 1999, 19, 413–422 CrossRef CAS PubMed.
- H. M. Shackman, M. Shou, N. A. Cellar, C. J. Watson and R. T. Kennedy, J. Neurosci. Methods, 2007, 159, 86–92 CrossRef CAS PubMed.
- H. Yang, A. B. Thompson, B. J. McIntosh, S. C. Altieri and A. M. Andrews, ACS Chem. Neurosci., 2013, 4, 790–798 CrossRef CAS PubMed.
- W. G. Kuhr and J. Korf, J. Cereb. Blood Flow Metab., 1988, 8, 130–137 CrossRef CAS PubMed.
- M. G. Boutelle, L. K. Fellows and C. Cook, Anal. Chem., 1992, 64, 1790–1794 CrossRef CAS PubMed.
- T. Yao and G. Okano, Anal. Sci., 2008, 24, 1469–1473 CrossRef CAS PubMed.
- H. Gu, E. L. Varner, S. R. Groskreutz, A. C. Michael and S. G. Weber, Anal. Chem., 2015, 87, 6088–6094 CrossRef CAS PubMed.
- P. Nandi and S. M. Lunte, Anal. Chim. Acta, 2009, 651, 1–14 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0an01729a |
| This journal is © The Royal Society of Chemistry 2021 |