The preparation of bifunctional hybrid nano-flowers and their application in the enzyme-linked immunosorbent assay for Helicobacter pylori detection†
Tiantian
Wang
a,
Xiangguang
Li
 *a,
Lili
Chen
a,
Youhuan
Zhang
a,
Yujun
Zheng
a,
Linjin
Yu
a,
Zhiyu
Ye
b,
Huaqian
Wang
a,
Xiping
Cui
a and
Suqing
Zhao
*a,
Lili
Chen
a,
Youhuan
Zhang
a,
Yujun
Zheng
a,
Linjin
Yu
a,
Zhiyu
Ye
b,
Huaqian
Wang
a,
Xiping
Cui
a and
Suqing
Zhao
 *a
*a
aDepartment of Pharmaceutical Engineering, School of Biomedical and Pharmace-utical Sciences, Guangdong University of Technology, Guangzhou 510006, People's Republic of China. E-mail: xgli@gdut.edu.cn; sqzhao@gdut.edu.cn
bCollege of Veterinary Medicine, South China Agricultural University, Guangzhou 510642, People's Republic of China
First published on 7th November 2020
Abstract
As the infection by Helicobacter pylori (H. pylori, HP) remains for a lifetime and may induce diseases such as gastric cancer, it is vital to detect and diagnose it. A new non-invasive indirect enzyme-linked immunosorbent assay (iELISA) method based on nano-flowers (NFs) is very advantageous for the sensitive detection of HP. Furthermore, the established iELISA method based on the organic–inorganic bifunctional hybrid nano-flowers including rabbit polyclonal antibody of HP labeled with peroxidase from horseradish (R-HP-Ab–HRP@Cu2+ NFs) showed linearity with HP at a concentration of 0–105 CFU mL−1 (R2 = 0.9997). Moreover, the limit of detection (LOD) reached 50 CFU mL−1, and not only was the detection sensitivity 20 times higher than that based on rabbit polyclonal antibody of HP labeled with peroxidase from horseradish (R-HP-Ab–HRP) but also the stability of R-HP-Ab–HRP in NFs was improved. In addition, the OD450 nm value was still linearly related to the concentration of HP at a range of 0–105 CFU mL−1 (R2 = 0.9952) with a LOD of 50 CFU mL−1 in an artificial saliva system. This study provided a sensitive, low-cost and convenient method for the non-invasive detection of HP.
Introduction
Helicobacter pylori (H. pylori, HP) is a microaerobic, Gram-negative bacterium that colonizes the human stomach. It is estimated that about 50% of mankind are infected, with infection rates in some developing countries reaching as high as 90%.1 It has been listed as a Class I pathogenic factor for gastric cancer.2 Individuals who have been exposed to HP infection for a long time will suffer from gastric diseases of different degrees, such as gastritis, peptic gastric ulcer, and some even to the degree of gastric cancer and gastric mucosa-associated lymphoid tissue lymphoma, making HP infection the third leading cause of cancer deaths woldwide.3 HP mainly colonizes the human gastric mucosa, and its infection is insidious.4 In addition, HP can also be found in the mouth causing oral problems such as bad breath. Furthermore, the infection is not restricted by age, and even an infant may be infected. Therefore, it is particularly important to detect HP non-invasively at an early stage to give a guideline for treatment.5,6Currently, the invasive methods used for the detection of HP in clinical reports mainly include the bacterial culture method and rapid urease test (RUT). These generally depend on human samples containing live bacteria and involve painful sampling processes which are difficult to be accepted.7,8 The non-invasive types mainly include the 13C/14C isotope breath test, 15N urine ammonia discharge test, molecular biology techniques and ELISA method. We have presented a comparative analysis of the existing non-invasive detection methods of HP in Table S1.† The ELISA method avoids the limitations of tested population, professional technology and high-cost equipments.9,10 In addition, the detection process of ELISA is simple and low-cost, the sample collection is convenient, and the sample itself is diverse and easily accepted by the public.11
With the establishment of the detection methods, a variety of detection media materials have emerged, including immunomagnetic beads, quantum dots, etc.12 In recent years, a special material called nano-flowers (NFs) has attracted widespread attention. It has a unique flower-like structure and excellent performance in loading subjects. Such flower-like nanoparticles generally take metal ions as the framework. In recent years, the most studied are copper (Cu2+),13–17 calcium (Ca2+),18,19 zinc (Zn2+),20 cobalt (Co2+)21 and manganese (Mn2+),22–24 which are naturally complexed with different functional proteins to obtain NFs with novel functions, such as glucose oxidase (GOx), HRP enzyme, protein with HRP enzyme activity (hemoglobin (Hb)) and hemin, all of which have catalytic properties. Some researchers have prepared NFs with nano-enzyme activity or combined the components with specific recognition, such as antigen and antibody combinations, streptavidin and biotin combinations, ConA and Gram-negative bacteria combination, etc.,25,26 so as to construct bifunctional hybrid NFs with specific recognition and catalytic properties.
With the enhancements in detection standards, improvement of detection sensitivity has gradually become a direction of interest for researchers. E. Casarin et al.27 reported that monoclonal 1G10 anti-bovine IgG1 antibody was biotinylated and integrated with the avidin-nucleic-acid-nanoassembly (ANANAS) reagents for indirect IBR diagnosis, thus increasing the sensitivity of 1G10 mAb-based conventional ELISA five-fold without decrease in the specificity. Due to the special complexation structure of NFs, enzyme and protein performance in NFs can be significantly improved through ion interaction. In addition, their unique petal-like structure greatly increases the surface area, which is more conducive to the exposure of functional sites for biochemical reactions. Thus, on the basis of these NFs, all kinds of biosensors have been developed, such as electrochemical biosensors,28–33 glucose sensors,26,34,35 DNA and miRNA sensors,36–38 micro-sensors based on the smartphones,25 and so on, which are widely used in the detection and analysis of various types of biological pathogenic bacteria, a variety of biomarkers of diseases and so on. Nowadays, the improvement of the detection sensitivity is a big issue for the early detection and prevention of various diseases, and poor detection sensitivity is seen as the biggest obstacle for the ultimate application of various methods in clinical practices.
The objective of this study was to provide a convenient and low-cost method based on indirect enzyme-linked immunosorbent assay (iELISA) and NFs for the sensitive detection of HP. This method can complete a sample test within 4 h. Moreover, including the preparation of antibodies, testing a sample costs much less than $1. With copper ions as the basic frame and anti-HP purified rabbit polyclonal antibodies (R-HP-Ab) doped with the HRP enzyme as the loaded subjects, we prepared an organic–inorganic bifunctional hybrid NF (R-HP-Ab–HRP@Cu2+) through natural complexation. The prepared NFs were applied to detect HP by iELISA for the first time, which could simplify the two steps required in the traditional ELISA processes of incubating antibodies and re-incubating enzyme-labeled secondary antibody into one step. The results showed that the detection sensitivity of the NF-based iELISA detection system increased 20 times compared with those of free enzyme-labeled antibodies.
Experimental
Materials and reagents
Columbia blood agar medium was purchased from Solarbio Biotechnology (Beijing, China). New Zealand white rabbits were obtained from Guangdong Medical Animal Center (Foshan, China). Peroxidase from horseradish (HRP, >300 units per mg), copper chloride (98%) and chemicals used in HRP enzymatic labeling of antibodies were obtained from Macklin Biochemical Technology (Shanghai, China). All other chemicals were acquired from Sigma-Aldrich (St Louis, MO, USA). All reagents were of analytical quality.HP strain and cultivation
HP SS1 was a gift from Southern Medical University (Guangzhou, China) and cultivated as previously described with minor modification.12 Briefly, HP was cultured in an anaerobic incubator with a microaerobic air bag and sterile PBS (0.01 M, pH 7.4) in Columbia blood agar plates, and placed in a 37 °C constant temperature incubator for 3 days. Then, they were scraped down to obtain the HP PBS suspension. The bacteria were obtained by centrifugation (7000g, 15 min) and inactivated with 1% formaldehyde solution for 24 h. After that, wet bacteria were washed with PBS 3 times, and the concentration of the bacteria was adjusted to 3 × 109 CFU mL−1 according to McFarland method.Preparation and purification of R-HP-Ab
All animal procedures were performed according to the Guidelines for Care and Use of Laboratory Animals of Guangdong University of Technology (Guangzhou, China) and experiments were approved by the Animal Ethics Committee of Guangdong University of Technology (Guangzhou, China).The New Zealand white rabbit was immunized with HP whole bacteria as the immunogen. Serum was collected after achieving the immune effect.39,40 Then, an appropriate amount of serum was taken out and purified by the saturated ammonium sulfate two-step method (50%, 33%)41 (refer to the ESI† for specific operation methods). Finally, the purified R-HP-Ab was stored and cryopreserved at −20 °C.
Preparation of HRP-labeled R-HP-Ab
R-HP-Ab was labeled with HRP by the sodium periodate method (refer to the ESI† for the detailed experimental procedure). According to the above methods, the proportion of HRP and antibody added in the reaction system was optimized. Specifically, HRP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) R-HP-Ab = 5
R-HP-Ab = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2.5
1, 2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5 and 1
2.5 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 and then, five kinds of HRP-labeled R-HP-Ab (R-HP-Ab–HRP) were prepared through the HRP-labeling process.
5 and then, five kinds of HRP-labeled R-HP-Ab (R-HP-Ab–HRP) were prepared through the HRP-labeling process.
Synthesis and characterization of NFs
Performance of R-HP-Ab–HRP@Cu2+ NFs
NFs were successfully prepared according to the above method, and further analysis and verification of the bioactive molecules of each component were needed. Therefore, the performances of HRP and R-HP-Ab in NFs were respectively verified (refer to the ESI† for the detailed preparation steps).Stability of R-HP-Ab–HRP@Cu2+ NFs under different conditions
The formation of NFs would affect the stability of R-HP-Ab–HRP under different conditions, including storage period, temperature and acid–base stability.44 A series of experiments were conducted to evaluate the stability of NFs43 (refer to the ESI† for the detailed preparation steps).Optimization of iELISA conditions based on R-HP-Ab–HRP@Cu2+ NFs or R-HP-Ab–HRP
Different conditions in the iELISA process had a certain influence on the test results. In order to improve the sensitivity of detection, this study optimized the following conditions: type and concentration of the sealing fluid, incubation time and concentration of R-HP-Ab–HRP@Cu2+ NFs/R-HP-Ab–HRP, and color development time (refer to the ESI† for the detailed preparation steps).Establishment and comparison of detection methods based on R-HP-Ab–HRP@Cu2+ NFs or R-HP-Ab–HRP
The synthetic method of R-HP-Ab–HRP@Cu2+ NFs or R-HP-Ab–HRP used for HP detection is delineated in Scheme 1A. Moreover, the R-HP-Ab–HRP@Cu2+ NFs or R-HP-Ab–HRP-based iELISA steps for HP detection are delineated in Scheme 1B. According to the above optimization conditions, a total of 100 μL of 101–108 CFU mL−1 HP was added to a 96-well plate for each well and 100 μL of PBS was used as the blank control, and the 96-well plate was kept at 37 °C for 1 h and then at 4 °C overnight. Then, the plate was washed with PBST 3 times, pat-dried with absorbent paper and then 270 μL of skimmed milk powder (1%) was added to each well and incubated at 37 °C for 1 h. After the plate was washed 3 times with PBST and pat-dried with absorbent paper, 100 μL of R-HP-Ab–HRP@Cu2+ NFs (200 μg ml−1) or R-HP-Ab (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2000) was added and kept at 37 °C for 70 min. Next, the plate was washed with PBST 3 times, pat-dried with absorbent paper, and 100 μL of color-rendering liquid containing TMB and H2O2 was added to each well, and the solution reacted at 37 °C for 40 min. Next, 50 μL of termination fluid was added to each well to terminate the reaction. The OD450 was measured within 3–5 min (n = 3).
2000) was added and kept at 37 °C for 70 min. Next, the plate was washed with PBST 3 times, pat-dried with absorbent paper, and 100 μL of color-rendering liquid containing TMB and H2O2 was added to each well, and the solution reacted at 37 °C for 40 min. Next, 50 μL of termination fluid was added to each well to terminate the reaction. The OD450 was measured within 3–5 min (n = 3).
 | ||
| Scheme 1 (A) Synthesis process of the R-HP-Ab–HRP hybrid nano-flower. (B) Schematic illustration of the comparison of iELISA for HP detection based on the dual-functional hybrid nano-flowers. | ||
Results and discussion
Preparation and HRP-labeling of R-HP-Ab
The concentration of R-HP-Ab obtained after dialysis was calibrated with the BCA kit. The P/N value was calculated as a ratio of positive sample OD450 nm to negative control OD450 nm. Generally, P/N > 2.1 is defined as a positive value. The titers before and after purification were 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8
8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 192
192![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (Fig. S1A†) and 1
000 (Fig. S1A†) and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 128
128![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (Fig. S1B†), respectively. Then R-HP-Ab was identified by SDS-PAGE. The molecular weight of the complete R-HP-Ab is 150 kDa which consists of symmetric two heavy chains and two light chains. When SDS-PAGE is performed, this antibody will denature and break into 50 kDa heavy chains and 25 kDa light chains. As shown in Fig. 1, the purified R-HP-Ab (lane 1) was almost free of heterozygosity compared with that before purification (lane 2). In other words, the electrophoretic bands on the SDS-PAGE map are impurity bands except for the bands indicated by the red arrows.
000 (Fig. S1B†), respectively. Then R-HP-Ab was identified by SDS-PAGE. The molecular weight of the complete R-HP-Ab is 150 kDa which consists of symmetric two heavy chains and two light chains. When SDS-PAGE is performed, this antibody will denature and break into 50 kDa heavy chains and 25 kDa light chains. As shown in Fig. 1, the purified R-HP-Ab (lane 1) was almost free of heterozygosity compared with that before purification (lane 2). In other words, the electrophoretic bands on the SDS-PAGE map are impurity bands except for the bands indicated by the red arrows.
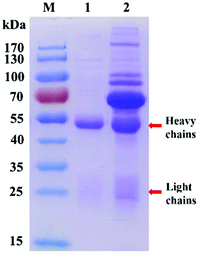 | ||
| Fig. 1 SDS-PAGE of rabbit polyclonal antibodies before and after purification. Lane M: marker; lane 1: purified; lane 2: before purification. | ||
Five kinds of R-HP-Ab–HRP were detected by SDS-PAGE and iELISA, and the optimized results were assessed by titer detection. In Fig. 2A, the bands of HRP and R-HP-Ab can be seen clearly and evenly from lane 3 with HRP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) R-HP-Ab = 1
R-HP-Ab = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The following HRP
1. The following HRP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) R-HP-Ab = 1
R-HP-Ab = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5 and 1
2.5 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 showed clear R-HP-Ab and shallow HRP bands. However, the bands of HRP
5 showed clear R-HP-Ab and shallow HRP bands. However, the bands of HRP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) R-HP-Ab = 5
R-HP-Ab = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 2.5
1 and 2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 showed only shallow HRP bands and hardly showed any R-HP-Ab bands. In addition, their labeling centrifugation process and dialysis process were significantly abnormal compared with others, with a large amount of soft yellow material floating on the top during the centrifugation process and a large amount of yellow precipitates during the dialysis process. These phenomena indicated that excess HRP may eventually lead to the denaturation and precipitation of system proteins. As shown in Fig. 2B, HRP
1 showed only shallow HRP bands and hardly showed any R-HP-Ab bands. In addition, their labeling centrifugation process and dialysis process were significantly abnormal compared with others, with a large amount of soft yellow material floating on the top during the centrifugation process and a large amount of yellow precipitates during the dialysis process. These phenomena indicated that excess HRP may eventually lead to the denaturation and precipitation of system proteins. As shown in Fig. 2B, HRP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) R-HP-Ab = 1
R-HP-Ab = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5 had similar binding titers with the highest titer of 1
2.5 had similar binding titers with the highest titer of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 64
64![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000. Therefore, HRP
000. Therefore, HRP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) R-HP-Ab = 1
R-HP-Ab = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5 were selected as a marker ratio in this study. It was further optimized and selected in the following test.
2.5 were selected as a marker ratio in this study. It was further optimized and selected in the following test.
Synthesis and characterization of R-HP-Ab–HRP@Cu2+ NFs
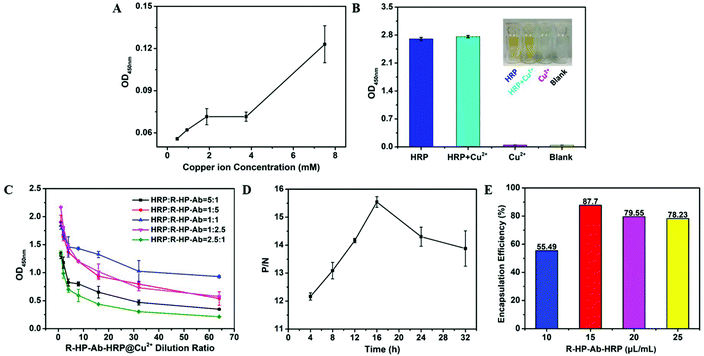
As shown in Fig. S2,† both Zn2+ and Mn2+ had significant negative effects on HRP activity, while Cu2+ or Ca2+ did not. When Ca2+ was used as a metal skeleton to form NFs, the resulting NFs appeared as a fluid paste during the washing processes, which seriously affected the detection. Fortunately, Cu2+ did not have the above problem and was selected for further study.Firstly, the concentration of copper ions should be optimized to minimize their side effects on detection. The results showed that a concentration of copper ions less than 4 mM had little effect on the OD values in our detection system (Fig. 3A). Moreover, the presence of Cu2+ (60 mM) had no side effects on the enzyme activity of HRP (Fig. 3B), and thus could meet the needs of this study. Then, iELISA was used to detect the performance of 5 different kinds of R-HP-Ab–HRP@Cu2+ NFs at different concentrations. As shown in Fig. 3C, increasing the dilution ratios of R-HP-Ab–HRP@Cu2+ NFs in the detection system resulted in a gradual decrease of the OD450 nm values of these five different kinds of R-HP-Ab–HRP. Moreover, the OD450 nm values decreased the most slowly in the HRP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) R-HP-Ab = 1
R-HP-Ab = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 system, and the values were always higher than those of the others. Therefore, the NFs formed with R-HP-Ab–HRP at HRP
1 system, and the values were always higher than those of the others. Therefore, the NFs formed with R-HP-Ab–HRP at HRP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) R-HP-Ab = 1
R-HP-Ab = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and copper ions at 2.4 mM were selected for further experiments. The iELISA results showed that NFs formed by natural complexation for 16 h had the best detection performance, but the P/N ratio decreased after 16 h (Fig. 3D). According to the results of SEM (Fig. S3†) and TEM (Fig. S4†), the NF petals exhibited a good stretch state at 16 h, while they showed a compact state at 48 h. Therefore, we speculate that the growth process of NFs starts from the formation of a complexation structure between the protein (R-HP-Ab–HRP) and metal ions (Cu2+), and then the petals gradually accumulate and grow on the basis of the “crystal nucleus”. With the increase of growth time of NFs, the petals are stacked layer by layer, which makes the structure of the NFs more and more compact and the active sites difficult to reveal, thus reducing the biological activity of the NFs. Our results showed that (Fig. S6†) the NFs that grew for 48 h had bigger sizes than those that grew for 16 h. However, the larger size of the NFs should lead to a decrease in the P/N ratio (Fig. 3D), when applying to detect HP.
1 and copper ions at 2.4 mM were selected for further experiments. The iELISA results showed that NFs formed by natural complexation for 16 h had the best detection performance, but the P/N ratio decreased after 16 h (Fig. 3D). According to the results of SEM (Fig. S3†) and TEM (Fig. S4†), the NF petals exhibited a good stretch state at 16 h, while they showed a compact state at 48 h. Therefore, we speculate that the growth process of NFs starts from the formation of a complexation structure between the protein (R-HP-Ab–HRP) and metal ions (Cu2+), and then the petals gradually accumulate and grow on the basis of the “crystal nucleus”. With the increase of growth time of NFs, the petals are stacked layer by layer, which makes the structure of the NFs more and more compact and the active sites difficult to reveal, thus reducing the biological activity of the NFs. Our results showed that (Fig. S6†) the NFs that grew for 48 h had bigger sizes than those that grew for 16 h. However, the larger size of the NFs should lead to a decrease in the P/N ratio (Fig. 3D), when applying to detect HP.
In addition, the encapsulation rate reached a peak of 87.70% when 15 μL of R-HP-Ab–HRP was added to the 1 mL system (Fig. 3E).
As shown in Fig. 4, the bifunctional organic–inorganic nanocomposites prepared in this study presented a flower-like shape. The petal structure could be clearly seen from the edge of each NF in Fig. 4(A and B). The morphology characterized by SEM showed that the NFs had a loose and multi-lobed structure with a size of about 5 μm (Fig. 4C and D).
Moreover, the EDS experiment revealed the distribution of six typical elements including C, O, N, P, S and Cu in the R-HP-Ab–HRP@Cu2+ NFs (Fig. S5†). Furthermore, these elements were uniformly dispersed in the R-HP-Ab–HRP@Cu2+ NFs, confirming that R-HP-Ab and HRP were uniformly doped in the NFs. This should be a reason for the increased stability of proteins in NFs.
In addition to SEM, DLS was also applied to measure the size of the NFs. The results (Fig. S6†) showed that the equivalent diameter of the NFs was about 1500 nm which was smaller than that indicated by SEM, the reason being that the petaloid and nonspherical structure of the NFs presents a larger relative surface area indicated by SEM and a smaller equivalent diameter indicated by DLS. This property is conducive to the exposure of their active sites and provides a basis for excellent performance in improving detection sensitivity.
HRP and R-HP-Ab performance of R-HP-Ab–HRP@Cu2+ NFs
As shown in Fig. 5A, both R-HP-Ab–HRP@Cu2+ NFs and HRP@Cu2+ NFs could catalyze TMB color development in the presence of H2O2 with an absorption wavelength at 650 nm, while R-HP-Ab@Cu2+ NFs had no absorption wavelength at 650 nm. When these NFs were applied to the detection system, only the bifunctional hybrid R-HP-Ab–HRP@Cu2+ NFs containing both R-HP-Ab and HRP showed positive results (Fig. 5B). Collectively, the R-HP-Ab–HRP@Cu2+ NFs showed the performances of R-HP-Ab and HRP.Stability of R-HP-Ab–HRP@Cu2+ NFs under different conditions
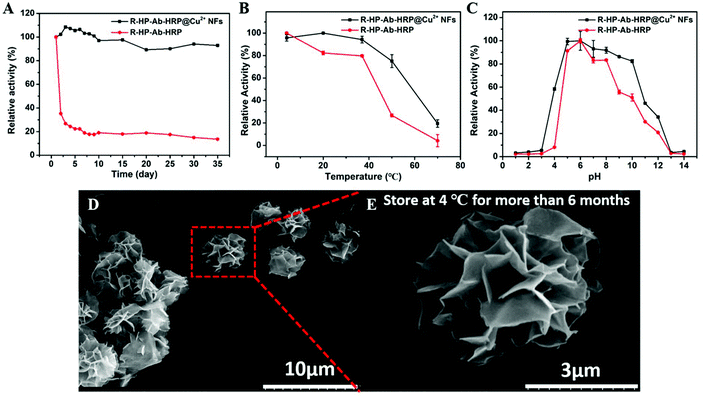
The R-HP-Ab–HRP@Cu2+ NFs could still maintain more than 90% storage stability within 35 days, while R-HP-Ab–HRP showed a sharp decrease in activity on the second day and the activity was about 35% of the original activity (Fig. 6A). It could be concluded that the NF structure was beneficial for the improvement of storage stability. Temperature activity tests showed that the relative activity of R-HP-Ab–HRP@Cu2+ NFs could still be 80% at 50 °C, while that of R-HP-Ab–HRP was only about 20% (Fig. 6B). This indicated that the NF structure had stronger resistance to temperature. Moreover, R-HP-Ab–HRP@Cu2+ NFs also showed stronger resistance to pH than R-HP-Ab–HRP (Fig. 6C).As shown in Fig. 6D and E, NFs can maintain an excellent flower-like structure for more than 6 months at 4 °C, which may improve the stability of R-HP-Ab–HRP@Cu2+ NFs under different conditions. Furthermore, when Cu2+ is used as an inorganic metal matrix to form organic–inorganic hybrid NFs, protein molecules form a complex with copper ions which become nucleation sites for the primary crystals of copper phosphate. Then, the interaction between proteins and copper ions leads to the growth of micron-sized particles with nanoscale features and petal-like shape. We surmise that this was because R-HP-Ab–HRP was denatured, but R-HP-Ab–HRP combined with NFs was not denatured by the effect of NFs under different conditions. Compared with a free enzyme, the activity and stability are enhanced when the enzyme is used as a protein component of the hybrid NFs.45 This should be due to the protection of the NFs against R-HP-Ab–HRP denaturation.
Optimization of iELISA conditions based on R-HP-Ab–HRP@Cu2+ NFs or R-HP-Ab–HRP
As shown in Fig. S7A and B,† in the presence of excess HP, the OD450 values gradually increased with increasing concentrations of R-HP-Ab–HRP@Cu2+ NFs, whereas increasing the dilution ratios led to decreasing OD450 values of R-HP-Ab–HRP. It is generally believed that the optimal control OD450 values should be within the range of 0.8–1.2. Therefore, the concentrations of R-HP-Ab–HRP@Cu2+ NFs and R-HP-Ab–HRP for detection were selected to be 200 μg ml−1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2000, respectively.
2000, respectively.
Chromogenic time is another vital factor for ELISA. The results showed that the OD450 values increased rapidly before 40 min and then reached a stable level during iELISA based on both R-HP-Ab–HRP@Cu2+ NFs and R-HP-Ab–HRP (Fig. S7C and D†). Therefore, 40 min was selected as the chromogenic time for further studies.
In order to lower the OD450 nm values of the background, we chose skimmed milk, BSA and casein as the sealing fluids. As shown in Fig. S8,† iELISA based on either R-HP-Ab–HRP@Cu2+ NFs (A) or R-HP-Ab–HRP (B) had the lowest OD450 nm values of the background by using skimmed milk as the sealing fluid, when compared with the others. Therefore, skimmed milk was chosen as the sealing fluid. Additionally, 1% skimmed milk showed the lowest OD450 nm values of the negative control (Fig. S8A,† black dotted line) and the highest OD450 nm values of the positive sample (Fig. S8A,† solid black line) in R-HP-Ab–HRP@Cu2+ NF-based iELISA. Therefore, 1% skimmed milk was selected as the optimum sealing fluid in R-HP-Ab–HRP@Cu2+ NF-based iELISA. Similarly, 1% skimmed milk was selected as the optimum sealing fluid in R-HP-Ab–HRP-based iELISA (Fig. S8B,† black lines). In addition, as shown in Fig. S8C and D,† the presence of the sealing fluid is important for the detection system.
Finally, the incubation time of R-HP-Ab–HRP@Cu2+ NFs and R-HP-Ab–HRP was optimized. As shown in Fig. S9,† the OD450 nm values increased rapidly before 70 min and then reached a stable level during iELISA based on both R-HP-Ab–HRP@Cu2+ NFs and R-HP-Ab–HRP. However, the OD450 nm values of the negative control group showed a sudden rise after 70 min. Similar results were shown in other reports.25 However, we are unable to figure out the exact reason for this phenomenon. Thus, further studies are greatly needed. Therefore, 70 min was selected as the incubation time for further studies.
Establishment and comparison of iELISA detection methods based on R-HP-Ab–HRP@Cu2+ NFs and R-HP-Ab–HRP
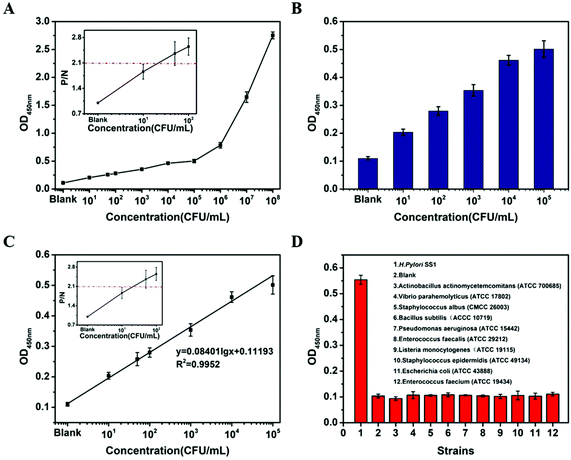
Detection curves were established according to the OD450 nm values of the samples containing HP from 101 CFU mL−1 to 108 CFU mL−1, and detection limits were determined at the same time (Fig. S10A and C†). The P/N value was calculated as a ratio of positive sample OD450 nm to negative control OD450 nm. Generally, P/N > 2.1 is defined as a positive value. So, the limit of detection (LOD) is defined as the concentration of HP in the sample which was just enough to make P/N > 2.1. In addition, the linear relationship between the OD450 nm values and the HP concentrations in iELISA based on R-HP-Ab–HRP@Cu2+ NFs ranged from 0–105 CFU mL−1 HP (R2 = 0.9997), with a LOD of 50 CFU mL−1 (Fig. S10B†). As shown in Fig. S10C,† iELISA based on R-HP-Ab–HRP had no significant linear range at the concentration of 0–108 CFU mL−1 HP, and its LOD was 103 CFU mL−1. The detection sensitivity based on R-HP-Ab–HRP@Cu2+ NFs was greatly advanced and it was 20 times higher than that of R-HP-Ab–HRP, which provided evidence of good amplification of the detection signal by the NF structure. In order to evaluate the applicability of the established method to a complex biological matrix, human artificial saliva was introduced. After adding different concentrations of HP in artificial saliva, the detection results still exhibited an obvious OD450 nm value from low to high (Fig. 7A). The OD450 nm value was still linearly related to the concentration of HP at a range of 0–105 CFU mL−1 (R2 = 0.9952), with a LOD of 50 CFU mL−1 (Fig. 7B and C). Moreover, we noted that the OD450 nm value showed a sudden increase and moved away from the linear range when the concentration of HP was beyond 105 CFU mL−1 (Fig. 7A and S10A, C†). We speculate that there may be a threshold for bacterial detection using ELISA, beyond which a tipping point may occur. Besides, we also detected the specificity of the proposed method to other 10 bacteria that colonize the gastrointestinal tract. The results showed that the OD450 nm values of the other 10 bacteria were almost the same as that of the blank, which indicated no cross-reaction with other bacteria in the established iELISA for HP based on R-HP-Ab–HRP@Cu2+ NFs (Fig. 7D).In order to further prove the reliability of the established iELISA, samples containing different concentrations of HP were prepared for recovery tests. The recoveries were between 94.73% and 107.71% with relative standard deviation (RSD) values varying from 2.30% to 5.99% (Table 1). Therefore, the established iELISA was applicable for HP determination.
| Sample number | Added (CFU mL−1) | Found (CFU mL−1) | Recovery (%) | RSD (%) (n = 3) |
|---|---|---|---|---|
| 1 | 105 | 105.39 | 107.71 | 2.34 |
| 2 | 104 | 103.91 | 97.84 | 5.99 |
| 3 | 103 | 102.89 | 96.31 | 2.30 |
| 4 | 102 | 101.89 | 94.73 | 3.71 |
| 5 | 101 | 101.03 | 103.39 | 3.98 |
We have made a comparison to better illustrate the advantages of this method over other methods (Table S1†). In the existing clinical detection methods, the 13C/14C isotope breath test is radioactive and is not recommended for pregnant women or children; the 15N urine ammonia discharge test is restricted by metabolic pathways and should not be used in patients with severe liver and kidney injuries; molecular biology techniques are unstable and need professionals; and the serotonin test involves hysteresis. In addition, these non-invasive tests for clinical HP that have been reported are only qualitatively positive/negative but cannot be quantified. This study provided a sensitive, timely, low-cost and convenient method for the non-invasive and painless detection of HP at a range of 0–105 CFU mL−1 (R2 = 0.9952), with a LOD of 50 CFU mL−1, which is based on the traditional reliable ELISA technology and applicable to all individuals. Furthermore, this study achieved a lower detection limit than our previously report, and may provide a potential method for improving quantitative tests in clinical practices.
Conclusions
A type of organic–inorganic bifunctional nano-flower (NF) was synthesized by means of antibody–enzyme coupling and hybridizing with copper ions through a self-assembly process. The NFs had the characteristics of better temperature stability, acid–base stability and storage stability than free R-HP-Ab–HRP. Furthermore, the NFs were first applied in the detection of gastrointestinal bacteria by iELISA, and the synthesis process of the NFs and the influencing factors of iELISA were optimized systematically. Specifically, the established iELSIA relied on the recognition of the antibodies to HP and the catalytic ability of the HRP enzyme towards TMB with H2O2 in the synthesized R-HP-Ab–HRP@Cu2+ NFs. The results indicated that the NFs could increase the sensitivity of R-HP-Ab–HRP 20 times when they were applied in HP detection by using iELISA. In summary, this study has broad prospects for improving the detection sensitivity of bacteria by using ELISA.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was financially supported by the Research and Development Program in Key Areas of Guangdong Province, P.R. China (No. 2019B020209009). The authors thank Dr Ying Zhang from the School of Foreign Languages at Guangdong University of Technology for her help in language editing.References
- M. D. Falco, A. Lucariello, S. Iaquinto, V. Esposito, G. Guerra and A. D. Luca, J. Cell. Physiol., 2015, 230, 1702–1707 CrossRef.
- A. Jafarzadeh, T. Larussa, M. Nemati and S. Jalapour, Microb. Pathog., 2018, 116, 227–236 CrossRef CAS.
- D. Elham, J. Abdollah, N. Maryam, B. Arezoo, H. A. Mehdi, K. Arezu, K. Nadia, G. Motahareh and M. Moghaddameh, Cytokine, 2020, 126, 154928 CrossRef.
- G. Somayyeh, F. Tahereh, A. Masoud, F. Nastaran, V. Farzam and R. Z. Mohamad, Microb. Pathog., 2017, 110, 100–106 CrossRef.
- L. F. Zhang, Z. H. Chen, X. J. Xia, J. S. Chi, H. Li, X. M. Liu, R. Li, Y. X. Li, D. Liu, D. L. Tian, H. Wang, G. F. Petroski, G. C. Flaker, H. Hao, Z. G. Liu and C. X. Xu, Atherosclerosis, 2019, 291, 71–77 CrossRef CAS.
- L. Yang, J. M. Zhang, J. J. Xu, X. X. Wei, J. J. Yang, Y. Liu, H. Li, C. Y. Zhao, Y. Wang, L. Zhang and Z. T. Gai, Front. Cell. Infect. Microbiol., 2019, 9, 00375 CrossRef CAS.
- R. Pellicano, A. Smedile, A. Ponzetto, M. Berrutti, M. Astegiano, G. Saracco, C. De Angelis, A. Repici, A. Morgando, M. L. Abate, S. Fagoonee and M. Rizzetto, Panminerva Med., 2005, 47, 191–194 CAS.
- P. Yañez, A. M. La-Garza, G. Pérez-Pérez, L. Cabrera, O. Muñoz and J. Torres, Arch. Med. Res., 2000, 31, 415–421 CrossRef.
- J. C. Thijs, A. A. Van-Zwet, W. J. Thijs, H. B. Oey, A. Karrenbeld, F. Stellaard, D. S. Luijt, B. C. Meyer and J. H. Kleibeuker, Am. J. Gastroenterol., 1996, 91, 2125–2129 CAS.
- S. K. Ogata, E. Kawakami, F. R. Patrício, M. Z. Pedroso and A. M. Santos, Sao Paulo Med. J., 2001, 119, 67–71 CrossRef CAS.
- P. Daniel, M. K. Peter, B. Valentine and W. Karoline, World J. Gastroenterol., 2019, 25, 4629–4660 CrossRef.
- L. L. Chen, X. G. Li, T. D. Zhou, T. T. Wang, X. P. Cui, Y. S. Chen, C. G. Zhang and S. Q. Zhao, Analyst, 2019, 144, 4086–4092 RSC.
- Y. C. Liu, J. Y. Chen, M. Y. Du, X. X. Wang, X. H. Ji and Z. K. He, Biosens. Bioelectron., 2017, 92, 68–73 CrossRef CAS.
- Z. X. Li, Y. F. Zhang, Y. C. Su, P. K. Ou-Yang, J. Ge and Z. Liu, Chem. Commun., 2014, 50, 12465–12468 RSC.
- D. S. Kong, R. Jin, X. Zhao, H. X. Li, X. Yan, F. M. Liu, P. Sun, Y. Gao, X. S. Liang, Y. H. Lin and G. Y. Lu, ACS Appl. Mater. Interfaces, 2019, 11, 11857–11864 CrossRef CAS.
- M. Ariza-Avidad, A. Salinas-Castillon and L. F. Capitán-Vallvey, Biosens. Bioelectron., 2016, 77, 51–55 CrossRef CAS.
- C. Altinkaynak, I. Yilmaz, Z. Koksal, H. Özdemir, I. Ocsoy and N. Özdemir, Int. J. Biol. Macromol., 2016, 84, 402–409 CrossRef CAS.
- T. S. Yan, F. Cheng, X. J. Wei, Y. D. Huang and J. M. He, Carbohydr. Polym., 2017, 170, 271–280 CrossRef CAS.
- R. F. Ye, C. Z. Zhu, Y. Song, J. H. Song, S. F. Fu, Q. Lu, X. Yang, M. J. Zhu, D. Du, H. Li and Y. H. Lin, Nanoscale, 2016, 10, 18980–18986 RSC.
- B. L. Zhang, P. T. Li, H. P. Zhang, X. J. Li, L. Tian, H. Wang, X. Chen, N. Ali, Z. Ali and Q. Y. Zhang, Appl. Surf. Sci., 2016, 366, 328–338 CrossRef CAS.
- L. H. He, S. Zhang, H. F. Ji, M. H. Wang, D. L. Peng, F. F. Yan, S. M. Fang, H. Z. Zhang, C. X. Jia and Z. H. Zhang, Biosens. Bioelectron., 2016, 79, 553–560 CrossRef CAS.
- W. Y. Li, S. Y. Lu, S. J. Bao, Z. Z. Shi, Z. S. Lu, C. M. Li and L. Yu, Biosens. Bioelectron., 2018, 99, 603–611 CrossRef CAS.
- Z. H. Zhang, Y. C. Zhang, L. H. He, Y. Q. Yang, S. L. Liu, M. H. Wang, S. M. Fang and G. D. Fu, J. Power Sources, 2015, 284, 170–177 CrossRef CAS.
- Z. H. Zhang, Y. C. Zhang, R. R. Song, M. H. Wang, F. F. Yan, L. H. He, X. Z. Feng, S. M. Fang, J. H. Zhao and H. Z. Zhang, Sens. Actuators, B, 2015, 211, 310–317 CrossRef CAS.
- M. M. A. Zeinhom, Y. J. Wang, L. N. Sheng, D. Du, L. Li, M. J. Zhu and Y. H. Lin, Sens. Actuators, B, 2018, 261, 75–82 CrossRef CAS.
- R. F. Ye, C. Z. Zhu, Y. Song, Q. Lu, X. X. Ge, X. Yang, M. J. Zhu, D. Du, H. Li and Y. H. Lin, Small, 2016, 23, 3094–3100 CrossRef.
- E. Casarin, L. Lucchese, S. Grazioli, S. Facchin, N. Realdon, E. Brocchi, M. Morpurgo and S. Nardelli, PLoS One, 2016, 11, 0145912 CrossRef.
- Y. Samira, B. Ali, A. Saleheh and R. Masoud, Microchem. J., 2019, 149, 104000 CrossRef.
- H. M. Cao, D. P. Yang, D. X. Ye, X. X. Zhang, X.-en Fang, S. Zhang, B. H. Liu and J. L. Kong, Biosens. Bioelectron., 2015, 68, 329–335 CrossRef CAS.
- A. Fabiana, C. Stefano, S. Viviana and M. Danila, Microchim. Acta, 2016, 183, 2063–2083 CrossRef.
- W. Wen, X. Yan, C. Z. Zhu, D. Du and Y. H. Lin, Anal. Chem., 2017, 89, 138–156 CrossRef CAS.
- R. F. Ye, C. Z. Zhu, Y. Song, J. H. Song, S. F. Fu, Q. Lu, X. Yang, M. J. Zhu, D. Du, H. Li and Y. H. Lin, Nanoscale, 2016, 8, 18980–18986 RSC.
- L. H. He, S. Zhang, H. F. Ji, M. H. Wang, D. L. Peng, F. F. Yan, S. M. Fang, H. Z. Zhang, C. X. Jia and Z. H. Zhang, Biosens. Bioelectron., 2016, 79, 553–560 CrossRef CAS.
- Y. X. Fang, S. J. Wang, Y. Y. Liu, Z. F. Xu, K. Zhang and Y. Guo, Biosens. Bioelectron., 2018, 110, 44–51 CrossRef CAS.
- Y. X. Li, G. M. Xie, J. H. Qiu, D. D. Zhou, D. Gou, Y. Y. Tao, Y. Li and H. Chen, Sens. Actuators, B, 2018, 258, 803–812 CrossRef CAS.
- X. S. Yu, L. Z. Hu, H. He, F. Zhang, M. Wang, W. L. Wei and Z. N. Xia, Anal. Chim. Acta, 2019, 1057, 114–122 CAS.
- T. T. Wu, Y. M. Yang, Y. Cao, Y. C. Song, L. P. Xu, X. J. Zhang and S. T. Wang, ACS Appl. Mater. Interfaces, 2018, 10, 42050–42057 CrossRef CAS.
- K. S. Park, B. S. Batule, M. Chung, K. S. Kang, T. J. Park, M. I. Kim and H. G. Park, J. Mater. Chem. B, 2017, 5, 2201–2366 RSC.
- Y. R. Xiao, Q. Q. Hu, L. Y. Jiao, X. P. Cui, P. P. Wu, P. He, N. N. Xia, R. Lv, Y. X. Liang and S. Q. Zhao, Microb. Pathog., 2019, 137, 103741 CrossRef CAS.
- Y. S. Chen, Y. X. Liang, R. Lv, N. N. Xia, T. J. Xue and S. Q. Zhao, Microchem. J., 2019, 145, 532–538 CrossRef CAS.
- C. G. Zhang, Y. Y. Zhong, Q. Y. He, D. Shen, M. B. Ye, M. L. Lu, X. P. Cui and S. Q. Zhao, Food Anal. Methods, 2020, 13, 1129–1137 CrossRef.
- T. D. Tran and M. I. Kim, BioChip J., 2018, 12, 268–279 CrossRef CAS.
- Y. Y. Zhong, L. J. Yu, Q. Y. He, Q. Y. Zhu, C. G. Zhang, X. P. Cui, J. X. Zheng and S. Q. Zhao, ACS Appl. Mater. Interfaces, 2019, 11, 32769–32777 CrossRef CAS.
- M. Y. Hao, G. M. Fan, Y. Zhang, Y. Xin and L. Zhang, Int. J. Biol. Macromol., 2019, 126, 539–548 CrossRef CAS.
- J. Ge, J. D. Lei and R. N. Zare, Nat. Nanotechnol., 2012, 7, 428–432 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0an01533d |
| This journal is © The Royal Society of Chemistry 2021 |