Palladium hydride with high-index facets for enhanced methanol oxidation†
Abstract
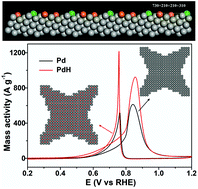
Nobel metal catalysts with high-index facets feature a high density of steps and kink sites, which bring about high activity but could be unstable during the electrocatalytic process. Doping with interstitial hydrogen atoms is a unique and effective way to regulate the electronic structure of the host materials. The formation of hydride also helps to stabilize the active sites on the surface of catalysts. Herein, we demonstrate the conformal doping of H atoms into the Pd nanostructure with preferential exposure of {730} facets, forming concave nanocubes of palladium hydride. Compared to the palladium counterparts, the palladium hydride catalysts show enhanced activity and stability in electrocatalytic methanol oxidation, and the structural differences between the Pd and PdH catalysts are revealed by XRD and X-ray photoelectron spectroscopy. Our work presents a powerful strategy for designing durable catalysts with high performance by combining high-index facet with interstitial atom doping.


 Please wait while we load your content...
Please wait while we load your content...