Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Iron-catalysed enantioselective carbometalation of azabicycloalkenes†
Laksmikanta
Adak‡
abc,
Masayoshi
Jin‡
 abd,
Shota
Saito
ab,
Tatsuya
Kawabata
ab,
Takuma
Itoh
ab,
Shingo
Ito
abd,
Shota
Saito
ab,
Tatsuya
Kawabata
ab,
Takuma
Itoh
ab,
Shingo
Ito
 abe,
Akhilesh K.
Sharma
abe,
Akhilesh K.
Sharma
 ab,
Nicholas J.
Gower
ab,
Paul
Cogswell
ab,
Jan
Geldsetzer
ab,
Nicholas J.
Gower
ab,
Paul
Cogswell
ab,
Jan
Geldsetzer
 ab,
Hikaru
Takaya
ab,
Hikaru
Takaya
 ab,
Katsuhiro
Isozaki
ab,
Katsuhiro
Isozaki
 *ab and
Masaharu
Nakamura
*ab and
Masaharu
Nakamura
 *ab
*ab
aInternational Research Center for Elements Science, Institute for Chemical Research (ICR), Kyoto University, Uji, Kyoto 611-0011, Japan
bDepartment of Energy and Hydrocarbon Chemistry, Graduate School of Engineering, Kyoto University, Nishikyo-ku, Kyoto 615-8510, Japan. E-mail: masaharu@scl.kyoto-u.ac.jp
cDepartment of Chemistry, Indian Institute of Engineering Science and Technology, Shibpur, Botanic Garden, Howrah 711103, India
dProcess Technology Research Laboratories, Pharmaceutical Technology Division, Daiichi Sankyo Co., Ltd., 1-12-1 Shinomiya, Hiratsuka, Kanagawa 254-0014, Japan
eDivision of Chemistry and Biological Chemistry, School of Physical and Mathematical Sciences, Nanyang Technological University, 21Nanyang Link 637371, Singapore
First published on 5th July 2021
Abstract
The first enantioselective carbometalation reaction of azabicycloalkenes has been achieved by iron catalysis to in situ form optically active organozinc intermediates, which are amenable to further synthetic elaborations. The observed chiral induction, along with the DFT and XAS analyses, reveals the direct coordination of the chiral phosphine ligand to the iron centre during the carbon–carbon and carbon–metal bond forming step. This new class of iron-catalysed asymmetric reaction will contribute to the synthesis and production of bioactive molecules.
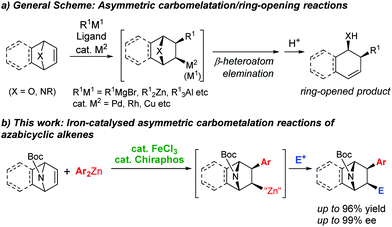
Carbometalation reactions, the 1,2-addition of organometallic species to alkenes or alkynes, are a powerful synthetic tool for carbon–carbon (C–C) bond formation.1 In particular, the transition-metal-catalysed asymmetric carbometalation of oxa- and azabicyclic alkenes is an effective strategy for the enantioselective synthesis of chiral building blocks for various natural products.2 Lautens and co-workers have extensively studied the asymmetric transformations of bicyclic alkenes catalysed by rhodium3 and palladium,2b,4 where the enantioselective carbometalation brings about desymmetrisation of the meso-substrates.5 Subsequent ring-opening reactions of the carbometalation intermediates give optically active products bearing multiple stereocentres. Copper6 and iridium7 catalysts can also affect the asymmetric transformations of oxa- and azabicyclic alkenes (Scheme 1a).
The enantioselective carbometalation of azabicyclic alkenes without ring-opening is also of significant synthetic interest, as it can provide direct access to the azabicyclo[2.2.1]heptane skeleton of alkaloid derivatives, such as epibatidine and epiboxidine (Scheme 1b).8 Nevertheless, the catalytic asymmetric addition of organometallic species (i.e., carbon nucleophiles) to azabicyclic alkenes without the ring-opening remains virtually unexplored.9
Asymmetric iron catalyses have emerged rapidly in organic synthesis,10 while their use in enantioselective carbometalation remains limited to the highly strained cyclopropene substrates.5b This can be attributed to the unstable coordination of chiral ligands with the iron centre, of which the oxidation states often fluctuate during the catalytic cycle. Indeed, Bedford and coworkers discovered that phosphine ligands do not coordinate to the iron centre in the iron-catalysed Negishi coupling.11 On the other hand, we have observed evident asymmetric induction in iron-bisphospine-catalysed enantioselective cross-coupling reactions,12 and an acceleration effect of a chelate phosphine in the diastereoselective carbometalation of oxa- and azabicyclic alkenes with arylzinc reagents.9b These conflicting observations have led us to attempt an enantioselective carbometalation under iron catalysis.
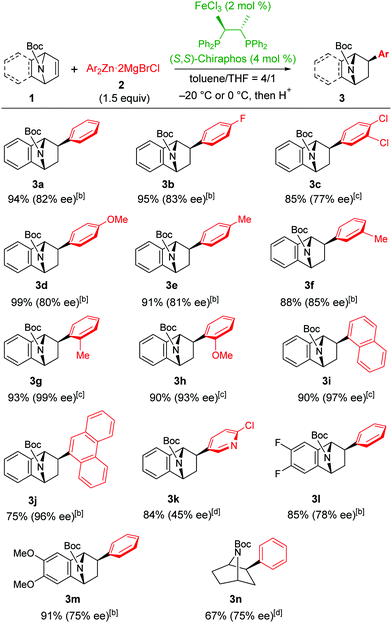
The study began with chiral phosphine ligand screening based on our success in iron-catalysed enantioselective cross-coupling reactions.12 We eventually found (S,S)-chiraphos as an optimal ligand for the carbometalation of azabicycloalkene with a phenylzinc reagent by using catalytic amounts of FeCl3 (details are shown in the ESI†).
Table 1 displays the scope of the reaction under the optimised conditions: the reaction of 1a with para- and meta-substituted arylzinc reagents gave the corresponding products 3a–3f in 85–99% yield with good enantioselectivities (77–85% ee).13 When an o-tolylzinc reagent was employed, the enantioselectivity increased dramatically to give 3g in 93% yield with 99% ee. Other sterically hindered arylzinc reagents such as o-methoxyphenyl-, 1-naphthyl-, and 9-phenanthrylzinc reagents also provided the corresponding products (3h–3j) with high enantioselectivities (93–97% ee). The heteroaromatic 4-chloro-3-pyridylzinc reagent can also participate in the carbometalation to give 3k in 84% yield with relatively low enantioselectivity (45% ee). The steric factor of aryl nucleophiles had a substantial impact on the enantioselectivity, suggesting that the spatial interaction of the aryl group and the alkene substrate leads to mutual orientation of the two reactants in the stereochemistry-determining carbometalation step.
The electronic factors of alkene substrates seemed not to affect this carbometalation reaction: substrates having electron-withdrawing fluoro groups or electron-donating methoxy groups provided the corresponding products 3l and 3m in excellent yields (85% and 91%, respectively) and good enantioselectivities (78% and 75% ee, respectively). On the other hand, the reaction with an aliphatic azabicyclic alkene 1n became sluggish and did not proceed at 0 °C: the expected product 3n was obtained in 67% yield with 75% ee at an increased reaction temperature. As this reaction's enantioselectivity is comparable to that of other substrates, the fused benzene ring has no significant effect on the enantioselectivity.
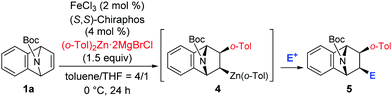
Trapping of the carbometalation intermediate with various electrophiles showed the stereospecific nature of the carbometalation/trapping sequence.5,9b The reaction of 1a with o-tolylzinc reagent gave optically active organozinc intermediate 4, which underwent electrophilic trapping with CD3CO2D to give deuterated product 5a in 96% yield with 99% ee and >99% cis-selectivity (entry 1, Table 2). Similarly, when trapped with iodine as the electrophile, product 5b was obtained in 84% yield with 99% ee and a diastereomeric excess of 94% (entry 2, Table 2).14
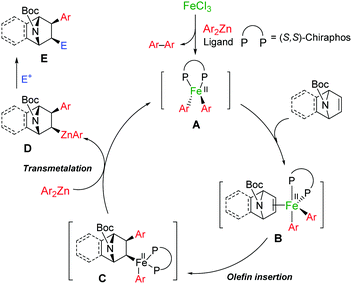
Preliminary mechanistic studies on the mixture of the iron salt, (S,S)-chiraphos, and aryl zinc reagent by the combination of X-ray absorption spectroscopy (XAS) and DFT-calculations show that the diaryl iron(II) species is the most likely intermediate responsible for this enantioselective carbometalation reaction. The direct coordination between the chiral phosphine ligand and iron centre inferred by the fact of chiral induction is also supported by the XAS analysis and the DFT calculations (the experimental and computational details are described in the ESI†).15Fig. 1 shows a plausible mechanism for the present carbometalation reaction. The catalytic cycle starts with diaryl iron(II)–(S,S)-chiraphos complex A, which is generated by the reduction of FeCl3 with an excess organozinc reagent (>3.0 equivalents) in the presence of (S,S)-chiraphos. The XAS and DFT analyses reveal that the geometry of A is tetrahedral. An azabicyclic alkene coordinates to the intermediate likely in an exo-fashion to give intermediate B. Enantioselective olefin insertion proceeds to form carboferration intermediate C. Subsequent transmetalation with the organozinc reagent leads to optically active organozinc intermediate D and regenerates iron(II) species A. Upon the sequential addition of electrophiles to the reaction mixture, intermediate D undergoes trapping to provide final product E. The sharp contrast between Bedford's and our observations can be attributed to the difference of the redox behaviours of the iron centre in cross-coupling and carbometalation; the latter reaction maintains iron(II) oxidation states during the catalytic cycle and the bisphosphine ligand predominantly coordinated to the iron centre, rather than to the zinc centre.16,17
In summary, we have developed the first enantioselective carbometalation reactions between various azabicycloalkenes and arylzinc reagents, which proceed under mild conditions by using a readily available FeCl3 and (S,S)-chiraphos catalytic system. Trapping experiments reveal the formation of a densely-functionalised optically active organozinc intermediate. XAS and DFT studies provided evidence for the direct coordination of the chiral phosphine ligand to the iron(II) centre, even in the presence of an excess zinc species that can undergo competitive coordination of the phosphine ligands. The present findings demonstrate the potential of iron-catalysed stereoselective C–C bond formations for synthesising complex chiral molecules of biological relevance. Further mechanistic studies on the detailed multi-spin reaction pathway and the origin of the asymmetric induction are currently underway.
This work was funded in part by a grant from JSPS through the ‘Funding Program for Next Generation World-Leading Researchers (NEXT Program)’, initiated by the Council for Science and Technology Policy, Core Research for Evolutional Science and Technology (CREST 1102545), ALCA from the Japan Science and Technology Agency (JST), and the JSPS Core-to-Core Program' Elements Function for Transformative Catalysis and Materials'. JSPS KAKENHI Grant Number 20H02740 also supported this work. L. A., J. G., and A. K. S. are grateful for a research fellowship from JSPS and the MEXT project 'Integrated Research Consortium on Chemical Sciences'. L. A. also thanks SERB, DST, Govt. of India (Project: SRG/2020/001350) and WBDST-BT for sanctioned Government Order [Memo No: 1854 (Sanc.)/ST/P/S&T/15G-7/2019]. This work was supported by the International Collaborative Research Program of Institute for Chemical Research, Kyoto University (grant #2019-20 and #2020-16). FAB-/ESI-MS and elemental analyses were supported by the JURC at ICR, Kyoto University. The synchrotron radiation experiments were performed at the BL14B2 (2015A0121, 2015B0121, 2016A0121, and 2016B0121) and BL02B1 (2016A0114) of SPring-8 with the approval of the Japan Synchrotron Radiation Research Institute (JASRI). The computations were performed at the Research Center for Computational Science, Okazaki, Japan.
Conflicts of interest
The authors declare no conflict of interest.Notes and references
- (a) D. S. Müller and I. Marek, Chem. Soc. Rev., 2016, 45, 4552–4566 RSC; (b) D. Didier, P.-O. Delaye, M. Simaan, B. Island, G. Eppe, H. Eijsberg, A. Kleiner, P. Knochel and I. Marek, Chem. – Eur. J., 2014, 20, 1038–1048 CrossRef CAS; (c) K. Murakami and H. Yorimitsu, Beilstein J. Org. Chem., 2013, 9, 278–302 CrossRef CAS PubMed; (d) A. Pérez-Luna, C. Botuha, F. Ferreira and F. Chemla, New J. Chem., 2008, 32, 594–606 RSC.
- (a) K. Fagnou in Modern Rhodium-Catalyzed Organic Reactions, ed. P. A. Evans, Wiley-VCH, Weinheim, 2005, pp. 173–190 Search PubMed; (b) M. Lautens, K. Fagnou and S. Hiebert, Acc. Chem. Res., 2003, 36, 48–58 CrossRef CAS PubMed; (c) M. Lautens, K. Fagnou, M. Taylor and T. Rovis, J. Organomet. Chem., 2001, 624, 259–270 CrossRef CAS; (d) S. V. Kumar, A. Yen, M. Lautens and P. J. Guiry, Chem. Soc. Rev., 2021, 50, 3013–3093 RSC.
- (a) L. Zhang, C. M. Le and M. Lautens, Angew. Chem., Int. Ed., 2014, 53, 5951–5954 CrossRef CAS PubMed; (b) G. C. Tsui and M. Lautens, Angew. Chem., Int. Ed., 2012, 51, 5400–5404 CrossRef CAS PubMed; (c) J. Zhu, G. C. Tsui and M. Lautens, Angew. Chem., Int. Ed., 2012, 51, 12353–12356 CrossRef CAS PubMed; (d) Y. Cho, V. Zunic, H. Senboku, M. Olsen and M. Lautens, J. Am. Chem. Soc., 2006, 128, 6837–6846 CrossRef CAS PubMed; (e) M. Lautens and K. Fagnou, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 5455–5460 CrossRef CAS; (f) F. Menard and M. Lautens, Angew. Chem., Int. Ed., 2008, 47, 2085–2088 CrossRef CAS PubMed; (g) J. Panteleev, F. Menard and M. Lautens, Adv. Synth. Catal., 2008, 350, 2893–2902 CrossRef CAS; (h) J. Bexrud and M. Lautens, Org. Lett., 2010, 12, 3160–3163 CrossRef CAS PubMed.
- (a) M. Lautens, S. Hiebert and J.-L. Renaud, J. Am. Chem. Soc., 2001, 123, 6834–6839 CrossRef CAS; (b) M. Lautens and C. Dockendorff, Org. Lett., 2003, 5, 3695–3698 CrossRef CAS PubMed; (c) S. Cabrera, R. Gómez Arrayás and J. C. Carretero, Angew. Chem., Int. Ed., 2004, 43, 3944–3947 CrossRef CAS PubMed; (d) S. Cabrera, R. Gómez Arrayás, I. Alonso and J. C. Carretero, J. Am. Chem. Soc., 2005, 127, 17938–17947 CrossRef CAS PubMed; (e) H. A. McManus, M. J. Fleming and M. Lautens, Angew. Chem., Int. Ed., 2007, 46, 433–436 CrossRef; (f) T. Ogura, K. Yoshida, A. Yanagisawa and T. Imamoto, Org. Lett., 2009, 11, 2245–2248 CrossRef CAS PubMed; (g) X.-J. Huang, D.-L. Mo, C.-H. Ding and X.-L. Hou, Synlett, 2011, 943–946 CAS; (h) B. Fan, S. Li, H. Chen, Z. Lu, S. Liu, Q. Yang, L. Yu, J. Xu, Y. Zhou and J. Wang, Adv. Synth. Catal., 2013, 355, 2827–2832 CrossRef CAS; (i) K.-L. Huang, C. Guo, L.-J. Cheng, L.-G. Xie, Q.-L. Zhou, X.-H. Xu and S.-F. Zhu, Adv. Synth. Catal., 2013, 355, 2833–2838 CrossRef CAS.
- (a) M. Nakamura, M. Arai and E. Nakamura, J. Am. Chem. Soc., 1995, 117, 1179–1180 CrossRef CAS; (b) M. Nakamura, A. Hirai and E. Nakamura, J. Am. Chem. Soc., 2000, 122, 978–979 CrossRef CAS.
- (a) F. Bertozzi, M. Pineschi, F. Macchia, L. A. Arnold, A. J. Minnaard and B. L. Feringa, Org. Lett., 2002, 4, 2703–2705 CrossRef CAS; (b) W. Zhang, L.-X. Wang, W.-J. Shi and Q.-L. Zhou, J. Org. Chem., 2005, 70, 3734–3736 CrossRef CAS PubMed.
- (a) R. Lou, J. Liao, L. Xie, W. Tang and A. S. C. Chan, Chem. Commun., 2013, 49, 9959–9961 RSC; (b) Y. Long, D. Yang, Z. Zhang, Y. Wu, H. Zeng and Y. Chen, J. Org. Chem., 2010, 75, 7291–7299 CrossRef CAS PubMed.
- (a) T. F. Spande, H. M. Garraffo, M. W. Edwards, H. J. C. Yeh, L. Pannell and J. W. Daly, J. Am. Chem. Soc., 1992, 114, 3475–3478 CrossRef CAS; (b) C. Qian, T. Li, T. Y. Shen, L. Libertine-Garahan, J. Eckman, T. Biftu and S. Ip, Eur. J. Pharmacol., 1993, 250, R13–R14 CrossRef CAS PubMed; (c) B. Badio, H. M. Garraffo, C. V. Plummer, W. L. Padgett and J. W. Daly, Eur. J. Pharmacol., 1997, 321, 189–194 CrossRef CAS; (d) J. C. Namyslo and D. E. Kaufmann, Synlett, 1999, 804–806 CrossRef CAS.
- (a) Only one example of a cobalt-catalysed asymmetric addition of silylacetylenes to azabicyclic alkenes has been reported recently, see: T. Sawano, K. Ou, T. Nishimura and T. Hayashi, Chem. Commun., 2012, 48, 6106–6108 RSC; (b) We have reported a racemic carbozincation of oxa- and aza-bicycloalkenes under iron catalysis S. Ito, T. Itoh and M. Nakamura, Angew. Chem., Int. Ed., 2011, 50, 454–457 CrossRef CAS PubMed.
- Selected books and reviews: (a) A. Casnati, M. Lanzi and G. Cera, Molecules, 2020, 25, 3889 CrossRef CAS PubMed; (b) A. Piontek, E. Bisz and M. Szostak, Angew. Chem., Int. Ed., 2018, 57, 11116–11128 CrossRef CAS PubMed; (c) A. Fürstner, ACS Cent. Sci., 2016, 2, 778–789 CrossRef PubMed; (d) I. Bauer and H.-J. Knölker, Chem. Rev., 2015, 115, 3170–3387 CrossRef CAS PubMed; (e) J. Legros and B. Figadere, Nat. Prod. Rep., 2015, 32, 1541–1555 RSC; (f) E. Nakamura, T. Hatakeyama, S. Ito, K. Ishizuka, L. Ilies and M. Nakamura, Org. React., 2014, 83, 1–209 CAS; (g) K. Gopalaiah, Chem. Rev., 2013, 113, 3248–3296 CrossRef CAS PubMed; (h) C. Bolm, J. Legros, J. Le Paih and L. Zani, Chem. Rev., 2004, 104, 6217–6254 CrossRef CAS PubMed.
- A. M. Messinis, S. L. J. Luckham, P. P. Wells, D. Gianolio, E. K. Gibson, H. M. O’Brien, H. A. Sparkes, S. A. Davis, J. Callison, D. Elorriaga, O. Hernandez-Fajardo and R. B. Bedford, Nat. Catal., 2019, 2, 123–133 CrossRef CAS.
- We reported the iron-catalysed enantioselective Kumada–Tamao–Corriu and Suzuki–Miyaura coupling reactions of α-chloroesters. see: (a) T. Iwamoto, C. Okuzono, L. Adak, M. Jin and M. Nakamura, Chem. Commun., 2019, 55, 1128–1131 RSC; (b) M. Jin, L. Adak and M. Nakamura, J. Am. Chem. Soc., 2015, 137, 7128–7134 CrossRef CAS PubMed.
- The absolute configuration of 3,4-dichlorophenyl-substituted product 3c was determined by X-ray single crystal analysis (Fig. S2, ESI†). Similar configurations are expected for the other products owing to the similarity of the 1H NMR coupling constants and the chiral HPLC retention times.
- The absolute configuration of iodo-substituted product 5b was determined by X-ray single crystal analysis (Fig. S3, ESI†).
- The formation of diaryl iron(II) species with the bisphosphine ligand is confirmed by various methods: by Mössbauer spectroscopy: (a) S. L. Daifuku, M. H. Al-Afyouni, B. E. R. Snyder, J. L. Kneebone and M. L. Neidig, J. Am. Chem. Soc., 2014, 136, 9132–9143 CrossRef CAS PubMed; (b) S. L. Daifuku, J. L. Kneebone, B. E. R. Snyder and M. L. Neidig, J. Am. Chem. Soc., 2015, 137, 11432–11444 CrossRef CAS PubMed ; by XAS and DFT: ; (c) R. Agada, H. Takaya, H. Matsuda, N. Nakatani, K. Takeuchi, T. Iwamoto, T. Hatakeyama and M. Nakamura, Bull. Chem Soc. Jpn., 2019, 92, 381–390 CrossRef ; by XRD:; (d) E. J. Hawrelak, W. H. Bernskoetter, E. Lobkovsky, G. T. Yee, E. Bill and P. J. Chirik, Inorg. Chem., 2005, 44, 3103–3111 CrossRef CAS PubMed; (e) J. M. Hoyt, M. Shevlin, G. W. Margulieux, S. W. Krska, M. T. Tudge and P. J. Chirik, Organometallics, 2014, 33, 5781–5790 CrossRef CAS; (f) C.-L. Sun, H. Krause and A. Fürstner, Adv. Synth. Catal., 2014, 356, 1281–1291 CrossRef CAS.
- (a) L. Adak, S. Kawamura, G. Toma, T. Takenaka, K. Isozaki, H. Takaya, A. Orita, H. C. Li, T. K. M. Shing and M. Nakamura, J. Am. Chem. Soc., 2017, 139, 10693–10701 CrossRef CAS PubMed; (b) T. Hatakeyama, T. Hashimoto, K. K. A. D. S. Kathriarachchi, T. Zenmyo, H. Seike and M. Nakamura, Angew. Chem., Int. Ed., 2012, 51, 8834–8837 CrossRef CAS PubMed; (c) S. Kawamura, T. Kawabata, K. Ishizuka and M. Nakamura, Chem. Commun., 2012, 48, 9376–9378 RSC; (d) T. Hatakeyama, Y. Okada, Y. Yoshimoto and M. Nakamura, Angew. Chem., Int. Ed., 2011, 50, 10973–10976 CrossRef CAS PubMed; (e) T. Hatakeyama, T. Hashimoto, Y. Kondo, Y. Fujiwara, H. Seike, H. Takaya, Y. Tamada, T. Ono and M. Nakamura, J. Am. Chem. Soc., 2010, 132, 10674–10676 CrossRef CAS PubMed.
- Computational DFT studies show that certain bulky bisphosphines coordinate to the iron centre even when the oxidation state of the iron fluctuates in the catalytic cycle: (a) A. K. Sharma and M. Nakamura, Molecules, 2020, 25(16), 3612 CrossRef CAS PubMed; (b) A. K. Sharma, W. M. C. Sameera, M. Jin, L. Adak, C. Okuzono, T. Iwamoto, M. Kato, M. Nakamura and K. Morokuma, J. Am. Chem. Soc., 2017, 139, 16117–16125 CrossRef CAS PubMed; (c) H. Takaya, S. Nakajima, N. Nakagawa, K. Isozaki, T. Iwamoto, R. Imayoshi, N. J. Gower, L. Adak, T. Hatakeyama, T. Honma, M. Takagi, Y. Sunada, H. Nagashima, D. Hashizume, O. Takahashi and M. Nakamura, Bull. Chem. Soc. Jpn., 2015, 88, 410–418 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1cc02387j |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2021 |