Ni crossover catalysis: truth of hydrogen evolution in Ni-rich cathode-based lithium-ion batteries†
Abstract
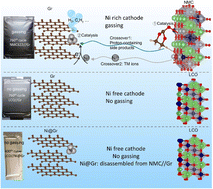
Hydrogen in Ni-rich cathode-based batteries is always accompanied by capacity decay and safety risks. However, insights into the H2 evolution have puzzled the battery community for decades. In general, solvent reduction on the anode side is considered the reason. However, we have found that it contradicts some experimental results. Herein, we experimentally demonstrate the clear pathway of H2 evolution, which we call “double crossover–double catalysis” (DC–DC). The first “catalysis” occurs on the cathode side, where Ni catalyzes solvent decomposition, forming proton-containing side products. The “double crossover” indicates that the side products and dissolved nickel ions both cross to the anode side, where the nickel ion is reduced to the Ni metal catalyst. The second “catalysis” is that the Ni metal on the anode catalyzes the reduction of the proton-containing side-products, forming H2. This study emphasizes the catalytic effect of Ni on both electrodes and establishes a “DC–DC” pathway for H2 evolution in LIBs, shedding light on the hindrance of H2 evolution in Ni-rich cathode-based batteries.


 Please wait while we load your content...
Please wait while we load your content...