Pyrolysis behaviors dominated by the reaction–diffusion mechanism in the fluorine-free metal–organic decomposition process
Abstract
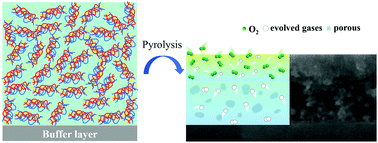
Owing to the advantages of its environmentally friendly process and superior high growth rate, the fluorine-free metal–organic decomposition (FF-MOD) is a promising route for the growth of REBa2Cu3O7−δ (REBCO, where RE is Y or other rare-earth elements) superconducting films. In this work, the pyrolysis behaviors dominated by the reaction–diffusion mechanism were investigated. Combining the atomic force microscopy and the nanoindentation analysis, we concluded that the strain generated by decomposition reactions plays a crucial role in defect formation. Due to the smooth release of the strain and enhancement of the mechanical properties, the defect-free film could be obtained under a fast process. However, an inhomogeneous microstructure along the film thickness was always found by cross-section observation, i.e., a dense layer at the top, while a porous layer was found at the bottom, being separated by a clear boundary. Accordingly, the one-dimensional reaction–diffusion model is proposed. In addition, the formation of such laminar structure is associated with different reaction mechanisms, depending on the diffusion of oxygen and evolved gases. Moreover, severe copper segregation was discerned in the dense layer, which would heavily affect the superconducting phase transformation. Remarkably, a high critical current density value exceeding 2 MA cm−2 (77 K, self-field) was achieved in the GdBa2Cu3O7 films grown on the CeO2 buffered technical substrates. This work provides deep insight into the pyrolysis behaviors of the FF-MOD route in view of the fabrication of REBCO superconducting films with high throughput.


 Please wait while we load your content...
Please wait while we load your content...