Novel ultra-thin two-dimensional structures of strontium chloride
Abstract
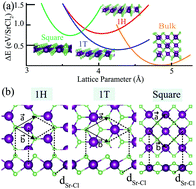
By performing density functional theory-based calculations, possible stable ultra-thin crystal structures of SrCl2 are investigated. Phonon calculations reveal that, among the possible crystal structures, three different phases; namely 1H, 1T, and square, are dynamically stable. In addition, ab initio molecular dynamics calculations show that these three phases are thermally stable up to well above room temperature. Another important stability factor of crystals, the chemical inertness against abundant molecules in the atmosphere, such as N2, O2, H2O, and CO2, is also investigated. The analysis shows that SrCl2 single-layers are chemically stable against these molecules. Moreover, it is determined that in contact with H2O and CO2, ultra-thin SrCl2 sheets display unique electronic features, allowing them to be used in sensing applications. It is also shown that single layers of SrCl2 crystals, all having a wide electronic band gap, can form type-I and type-II vertical van der Waals heterostructures with well-known 2D materials such as MoS2, WSe2, and h-BN.


 Please wait while we load your content...
Please wait while we load your content...